Abstract
Molecular characterization of mechanisms by which human pattern recognition receptors (PRRs) detect danger signals has greatly expanded our understanding of the innate immune system. PRRs include Toll-like receptors (TLRs), nucleotide oligomerization domain-like receptors (NLRs), retinoic acid inducible gene-like receptors (RLRs) and C-type lectin receptors (CLRs). Characterization of the developmental expression of these systems in the fetus, newborn and infant is incomplete but has yielded important insights into neonatal susceptibility to infection. Activation of PRRs on antigen-presenting cells enhances co-stimulatory function, and thus PRRs agonists are potential vaccine adjuvants, some of which are already in clinical use. Thus study of PRRs has also revealed how previously mysterious immunomodulators are able to mediate their actions, including the vaccine adjuvant aluminum hydroxide (Alum) whose adjuvant activity depends on its ability to activate a cytosolic protein complex known as the Nacht Domain Leucine-Rich Repeat and PYD-Containing Protein 3 (NALP3) inflammasome leading to IL-1ß production. Progress in characterizing PRRs is thus informing and expanding the design of improved adjuvants. This review summarizes recent developments in the field of innate immunity with special emphasis on developmental expression in the fetus, newborn and infant and its implications for the design of more effective neonatal and infant vaccines.
Newborns and infants are at high risk of infection: Need for effective neonatal and infant vaccines
On a global basis, infections result in ~2 million deaths per year in those less than 6 months of age (WHO) (1). Common pathogens in neonates and/or infants include Grampositive bacteria (e.g., Group B Streptococcus, S. pneumoniae), Gram-negative bacteria (such as E. coli, B. pertussis), herpes simplex virus (HSV), respiratory syncitial virus (RSV) and rotavirus. This susceptibility highlights the medical need for effective vaccines for newborns and infants and the functional immunodeficiencies that must be overcome in designing vaccines that adequately protect the very young. As birth is the most reliable point of healthcare contact worldwide, vaccines that are active at birth are a major global health imperative (2). Design and development of such vaccines will require understanding of the developmental expression of innate immune pathways whose activation enhances the adaptive immune response.
Distinct aspects of neonatal innate immunity that pose challenges to effective vaccination early in life
The fetal immune system is heavily Th2-biased, presumably to avoid pro-inflammatory Th1-type alloimmune responses to maternal tissues that may trigger preterm birth or spontaneous abortion.
Birth triggers a dramatic shift in environment that places further demands on the function of the neonatal immune system, mediating the transition from a normally sterile intrauterine compartment to a foreign antigen (Ag)-rich outside world, including the first colonization of skin and gastrointestinal tract. In contrast to low levels of Th1-type cytokines (e.g. TNF, IL-12p70, IFN-γ), human neonatal plasma contains high levels of the Th2-type cytokine IL-6 in vivo at both birth and throughout the first week of life (3). IL-6 induces an acute phase response at birth that may serve to shield against bacterial infections and clear microbial products and/or PRR agonists.
The distinct polarization of fetal and early neonatal immune responses presents obstacles to effective neonatal immunization, including impaired antigen-presenting cell (APC) responses (e.g., impaired IFNγ production) to many (but not all) stimuli, a Th2 bias to immune responses, and impaired antibody (Ab) affinity maturation (4), as well as the potential inhibitory effect of maternal Abs (5).
Quantitative and qualitative differences exist between neonatal and adult APCs. Qualitative differences are evident in human monocytes in utero as assessed by flow cytometry indicating reduced expression levels of MHC class II molecules (6). Several mechanisms have been implicated in skewing neonatal APCs towards Th2-type responses, including: a) the production by placental tissues of TGF-β, progesterone and prostaglandin E2 that enhance Th2 cytokine production (7), and b) the presence in neonatal blood plasma of relatively high concentrations (~300 nM) of adenosine, an immunosuppressive endogenous purine metabolite (8, 9), The patterns of neonatal cytokine production appear to be relevant in vivo, in that after birth, during the first week of life, human neonatal peripheral serum levels of TNF remain low (relative to human adult serum) whereas levels of IL-6, a Th2-polarizing cytokine with anti-inflammatory properties, rise.
Multiple studies document that human neonatal monocytes and APCs function suboptimally when tested in vitro with respect to co-stimulatory responses to most stimuli (10). Studies of murine neonatal dendritic cells (DCs) have demonstrated that Agpresentation increases during ontogeny, correlating with increased co-stimulatory molecule expression and increased responses to protein-conjugated, T cell-dependent polysaccharide Ags (11). However, C57BL/6 neonatal mice (1-7 days of age) demonstrated stronger LPS-induced inflammatory cytokine production by splenocytes in the presence of T cells in vitro and after i.p. injection in vivo, ascribed to neonatal quantitative deficiency of CD4+ and CD8+ T-cells (12). Thus, inflammatory responses in neonates appear to be dependent on the species (noting that innate immune genes are hypervariable between species, including between humans and mice (13, 14), experimental model (in vitro verses in vivo), extracellular culture medium (autologous 100% (vol/vol) adenosine-rich neonatal plasma/serum (15) vs. low concentrations of heat-inactivated fetal calf serum that are used in many studies) and particular stimulus studied.
In contrast to neonatal APCs, neonatal CD4+CD25(IL2-Ra)+ T reg cells (Treg) are fully functional and found at high abundance in human fetal lymphoid tissues (16) and newborn cord blood (17, 18). Neonatal Treg cells suppress both T responder cell proliferation and Th1-cytokine (e.g., IFN-γ) production, induced by self-Ags, to limit adaptive immune responses. Treg mediate their effects by both cell contact-dependent and -independent mechanisms, including secretion of IL-10, CD39 and CD73 (ectonucleotidase)-mediated generation of extracellular adenosine, and adenosine A2A receptor-mediated enhancement of cAMP concentrations in target T responder cells (19, 20). Neonatal inhibition of auto-immunity via Treg suppression has clear advantages as neonates first encounter the foreign-Ag rich world, however these effects may be detrimental to neonatal immunity to infection (21) and to neonatal vaccine responses (22, 23).
Innate immune activation enhances adaptive immune responses
Activation of Pattern Recognition Receptors (PRRs) on APCs such as macrophages and DCs enhances antigen-presenting activity and adaptive immune responses via direct and indirect mechanisms (24). PRR signaling is influenced by cross signals mediated via diverse PRR classes, tyrosine kinases, tyrosine phosphatases, ubiquitinating systems, and glucocorticoids, and therefore varies between different cell-types (25). Upon activation, DCs efficiently process and present Ags in the context of MHC, increase production of Th1-polarizing cytokines such as IL-12p70, and up-regulate co-stimulatory molecules (e.g., CD40, CD80, CD86) and chemokine receptors mediating cell migration from the tissues into the draining lymph nodes. Once inside the lymph nodes, DCs interact with naïve T and B cells inducing their differentiation into effector cells, thereby triggering an acquired immune response.
In addition to their direct effect on APCs, PRR agonists also enhance the transition from innate to adaptive immune responses via indirect mechanisms. For example, Tolllike receptor (TLR)-mediated DC activation induces IL-6 that renders T responder cells refractory to inhibition by suppressive Treg (26). Tissue-derived signals may also influence APCs and control the type of effector class generated (27). TLR-mediated activation of DCs enhances lymph node function via triggering DC migration to lymph nodes, expression of vascular endothelial growth factor, increasing high endothelial venule proliferation, remodeling primary feed arterioles, and increasing nodal blood flow and recruitment of rare Ag-specific lymphocytes (28, 29).
The impressive progress in defining the potential roles of PRRs has provided an opportunity to better understand the development of innate immune responses after birth, with potential implications for the optimization of vaccine development. Below, we review the current state of knowledge regarding the developmental expression of key PRRs, highlighting both recent progress and gaps in our knowledge.
Developmental expression of PRRs
Responses to microbial infection are initiated through an innate immune system that features diverse PRRs poised for activation in extracellular and intracellular locations (Figure 1).
Figure 1. Mechanisms of innate immune activation induced by vaccine adjuvants.
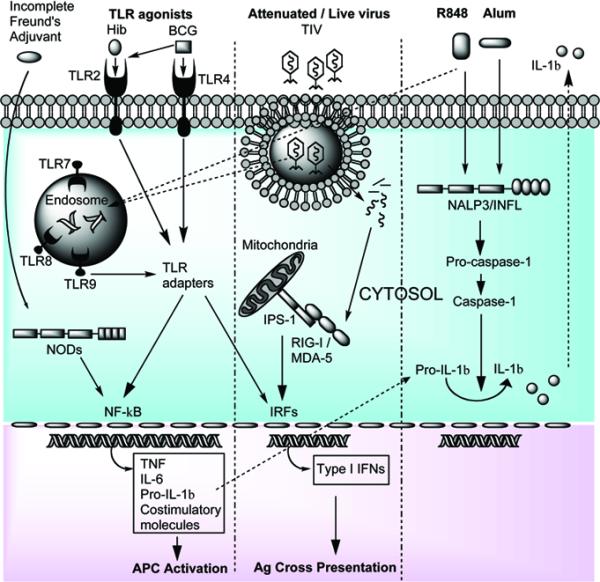
TLR agonists found in multiple neonatal vaccines (Table 1) activate either cell associated or intracellularly located TLRs or NODs. These PRRs then interact with specific adaptor molecules culminating in NF-κB or IRF activation. Viral-derived products (dsRNA or ssRNA) can also activate endosomal TLRs, along with RLRs (such as RIG-I), which induce type-I IFN production. The vaccine adjuvant Alum activates the cytosolic NALP3/inflammasome leading to proIL-1β cleavage into bioactive IL-1β.
TLRs
TLRs are type I transmembrane proteins with an extracellular amino-terminus and an intracellular carboxy-terminus. They are composed of various domains including, extracellular leucine rich repeat (LRR) with one or two cysteine-rich regions and an intracellular toll/interleukin-1 receptor (TIR) domain, named after its homology with the interleukin 1 receptor. Humans express ten TLRs: a) surface expressed TLRs include TLR2 (bacterial lipopeptides), TLR4 (lipopolysaccharide) and TLR5 (flagellin), b) endosomal TLRs include TLR7 and 8 (single stranded RNA, (30, 31)) and TLR9 (unmethylated CpG DNA). Engagement of TLRs activates intracellular signalling cascades via Myeloid differentiation factor 88 (MyD88)-dependent and MyD88-independent pathways (32), including IL-1 receptor-associated kinase-4 (IRAK-4) recruitment, culminating in NF-κB activation and expression of pro-inflammatory cytokines, such as TNF, IL-6, and pro-IL1ß (note that processing to mature IL-1ß requires Nacht Domain Leucine-Rich Repeat and PYD-Containing Protein 3 (NALP3) inflammasome (INFL) action). TLR stimulation can also lead to the activation of several other intracellular signalling pathways such as those involving Jun N-terminal kinase, mitogen activated protein kinase (MAPK) (33, 34), interferon regulatory factor (IRFs), and the FAS-associated death domain (FADD)-induced apoptosis pathway (32).
The importance of the TLR pathway for host defense in newborns and infants is apparent in the clinical consequences of TLR pathway defects. Defects of signaling molecules downstream of TLRs, including IRAK4 and MyD88-deficiency result in selective susceptibility to pyogenic infections (often streptococcal and staphylococcal) during childhood with improvement later in life (35-37).
Cord blood monocytes of full-term human newborns express normal amounts of TLRs, yet upon TLR-mediated stimulation in whole cord blood (i.e., 100% vol/vol autologous, adenosine-rich plasma), agonists of TLRs 1-7 demonstrate a 1-3 log impairment in TNFα production relative to adult peripheral blood monocytes (15). One of the mechanisms accounting for this impairment is that during the hypoxia and stress accompanying the birth process, plasma concentrations of adenosine, an endogenous immunosuppressive purine metabolite, rise. Adenosine can act via A3 adenosine receptors on neonatal mononuclear cells to induce production of cAMP, a key second messenger that inhibits TLR-mediated production of Th1-polarizing cytokines (8, 9). Other studies have demonstrated that LPS-induced responses of newborn mononuclear cells are diminished at birth due to reduced expression of the TLR adaptor molecule MyD88 (38) and by failure of nucleosome remodeling at the IL-12 promoter (39). Impaired TLR agonist-induced production of type I IFNs from human neonatal plasmacytoid DCs (pDCs) and neonatal monocyte-derived DCs (moDC) has also been described (40, 41).
In contrast to agonists of TLRs 1-7, TLR8 agonists, including TLR7/8 agonists, are able to induce robust immune responses by human neonatal APC, comparable with those of healthy adult controls (42). Exposure of human neonatal cord blood-derived monocytes and APCs, including myeloid dendritic cells (mDCs) and moDCs, to TLR8 agonists induces robust (i.e., adult-level) phosphorylation of p38 MAP kinase, NF-κB activation, pro-inflammatory cytokine production (TNF, IL-12), and up-regulation of costimulatory molecules (e.g., CD40).
RLRs
During the infection cycle of some viruses, double stranded (ds) RNA is produced which can be detected in the cell cytosol by retinoic acid inducible gene-like receptors (RLRs), such as retinoic acid inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA-5). These cytoplasmic proteins are composed of amino terminal caspase-recruitment domains (CARDs) and a carboxy-terminal helicase domain (43, 44). RLR activation induces CARD domain interaction with the CARD domain-containing adaptor protein, IFN-β promoter-stimulator (IPS)-1 (also known as mitochondrial antiviral signaling protein (MAVS), virus-induced signaling adaptor (VISA) and CARD adaptor inducing IFN-β (CARDIF)) leading to IRF3 and NF-κB activation. Little is known regarding the developmental expression of RLRs at birth and in the neonate, an important area of future study.
NLRs
Nucleotide oligomerization domain-like receptors (NLRs) detect bacterial components and include members of the nucleotide-binding oligomerization domain (NOD) subfamily as well as NLRs associated with the INFL. The NOD proteins in humans are a family of over 20 cytosolic proteins. Structurally they are composed of: a) a variable N-terminal effector-binding domain, usually consisting of a pyrin domain (PYD) or CARD, cable of regulating homotypic and heterotypic binding; b) a centrally located NOD domain; c) a C-terminal ligand-recognition domain, which can be composed of LRRs. As with the RLRs, little is known regarding the developmental expression of NODs at birth and in the neonate.
The INFL is a cytosolic multi-component protein complex, including caspase-1, which upon activation cleaves pro-IL-1ß to the potent pro-inflammatory cytokine IL-1ß. IL-1ß has a central role in the onset of parturition, particularly in the context of intrauterine infection/inflammation. A recent study, found that: a) caspase-1 concentration in the amniotic fluid increases as a function of gestational age; b) women with spontaneous term labor had a higher median caspase-1 amniotic fluid concentration than women at term without labor, suggesting that the INFL may be activated in spontaneous parturition at term, and c) higher caspase-1 levels were associated with infection/inflammation (45). Human neonatal monocytes, in particular those of preterm newborns, demonstrate impaired IL1-ß production to LPS and to lipoteichoic acids. A search of Pubmed did not yield any publications relating to INFL function at birth.
CLRs
C-type lectins receptors (CLRs) can be produced as secreted soluble proteins, including mannose binding lectin (MBL), lung surfactant protein A (SP-A), or as transmembrane proteins such as selectins, mannose receptor and the DC-specific ICAM-3 grabbing non-intregrin (DC-SIGN). MBL is an acute phase plasma protein whose expression in the liver is up-regulated during inflammation. MBL recognizes a variety of carbohydrate patterns found on infectious microorganisms including, bacteria (e.g. Staphylococcus aureus), fungi (e.g. Saccharomyces cerevisiae), viruses (e.g. HIV), and on altered self-glycoproteins (e.g. aberrant glycosylation of cancer cells). MBL binding to carbohydrate targets triggers activation of MBL-associated serine protease, cleaving complement proteins and promoting opsonisation/membrane attack complex formation (46). MBL can also act directly as an opsonin by binding to receptors which promote phagocytosis (46), and can modulate cytokine production in vitro and in vivo (47) thereby playing important roles in neonatal infection. Plasma MBL concentrations in both premature and full term neonates are lower than those of adults (48), but steadily rise during the first weeks of life, possibly as a consequence of the skew of neonatal cytokine responses towards IL-6, which induces the acute phase response (3, 9). MBL deficiency is associated with an increased risk of bacterial sepsis (49).
From the standpoint of innate immune modulation of vaccine responses, MBL deficiency enhances mouse Ag-specific IgG production following immunization with tetanus toxoid-conjugated Group B streptococcus polysaccharide vaccine or tetanus toxoid alone (50). These data suggest that under certain circumstances MBL can inhibit Ab production. Further characterization of the MBL pathway may inform design of vaccines that minimize such inhibitory MBL effects.
Developmental expression of Antimicrobial proteins and peptides
APPs expressed by leukocytes and epithelial cells are ancient components of innate immune defense that are best known for their ability to kill microorganisms and neutralize their surface components. However, some APPs also demonstrate activity in modulating adaptive immune responses. For example, bactericidal/permeabilityincreasing proten (BPI), a neutrophil-derived primary granule protein with endotoxin binding activity, was recently shown to enhance APC function. Neonates are deficient in BPI expression, raising the possibility that may also impair delivery of naturally shed Gram-negative bacterial LPS outer membrane blebs to APCs. Defensin peptides can enhance production of anti-viral IFNs, that are important to Th1 polarization and crosspresentation (51). Cathelicidin peptides, found in neutrophil secondary granules, enhance adaptive immune responses in vivo. Indeed there is age-dependent maturation in the expression in human neonatal blood plasma of a broad range of APPs, and thus to the extent that these molecules contribute to modulating adaptive immune responses.
The recent progress in defining PRRs and APPs involved in triggering innate immune responses and thereby modulating adaptive immunity provides new opportunities to understand that function of adjuvants in currently administered vaccines, as outlined below.
Engagement of innate immunity by currently approved neonatal and infant vaccines
It is increasingly appreciated that an important determinant of vaccine efficacy is the ability of a given vaccine to activate the innate immune system to enhance APC function and Th1-polarizing adaptive responses (52). Currently, the two vaccines that are regularly given at birth in humans are the bacillus Calmette-Guerin (BCG) vaccine and the hepatitis B (HepB) vaccine (Table 1). BCG is capable of inducing a strong Th1-type immune responses in human neonatal cells in vitro (53) and in vivo (54), which is in part due its ability to activate multiple TLRs expressed by APCs (55). The BCG vaccine demonstrates that under certain conditions, the human neonatal immune system is capable of mounting a protective Th1-type response (56), but the underlying mechanisms of its efficacy at birth is not fully understood. Recent work has indicated that it is very likely that activation of multiple PRRs by BCG plays important roles in its efficacy. The BCG vaccine is a live bacterium and able to activate TLR2 (57, 58) and TLR4 (57, 58) by virtue of its cell wall that consists of peptidoglycan, arabinogalactan, and mycolic acids (59), and TLR9 through its CpG-rich DNA (60). More recently, the possibility that BCG also engages TLR8 has also been raised based upon associations of TLR8 polymorphisms with susceptibility to pulmonary TB, increased susceptibility in males (TLR8 is encoded on the X chromosome), and the ability of BCG to induce macrophage TLR8 expression (61).
Table 1.
Activation of PRRs by approved pediatric vaccines (0-2 years of age)
| Vaccine | Age | Adjuvant | PRR | Ref |
|---|---|---|---|---|
| Hepatitis B (Hep B) vaccine | Birth | Alum | NALP3/INFL | (67, 68) |
| Bacille Calmette-Guerin (BCG) | Birth (not in USA) | CWS† | TLR2 | (57-60) |
| CWS† | TLR4 | (57-59) | ||
| ssRNA | TLR8* | (61) | ||
| CpG DNA | TLR9* | (60) | ||
| NODs, | (97) | |||
| CTLs* | ||||
| Rotavirus vaccine | 2 months | ? | ? | |
| Diphtheria, Tetanus, acellular Pertussis (DTaP) vaccine | 2 months | Alum | NALP3/INFL | (67, 68) |
| Haemophilus influenzae type b conjugate vaccine (Hib) | 6 weeks | Alum | NALP3/INFL | (67, 68) |
| OMPC | TLR2 | (64) | ||
| TLR4* | (63) | |||
| Pneumococcal conjugate vaccine (PCV7) | 6 weeks | Alum | NALP3/INFL | (67, 68) |
| Trivalent inactivated Influenza vaccine (TIV) | 6 months | dsRNA | TLR3* | (98) |
| ssRNA | TLR7 | (30, 99) | ||
| ssRNA | TLR8* | (31) | ||
| dsRNA | RIG-1* | (100) | ||
| Measles, Mumps, Rubella (MMR) vaccine | 12 months | hemagglutinin protein (Measles) | TLR2* | (65) |
| dsRNA (Measles) | MDA-5* | (66) | ||
| Varicella vaccine | 12 months | ? | TLR2* | (101) |
| Hepatitis A (HepA) vaccine | 12 months | Alum | NALP3/INFL | (67, 68) |
| Meningococcal conjugate vaccine (MCV4) | 2 years | bacterial porins | TLR2* | (102) |
PRR activation inferred based on published engagement of PRRs by wild type strains.
Note: The minimum age is given for vaccination (103).
CWS consists of a purified noninfectious material consisting of peptidoglycan, arabinogalactan and mycolic acids.
Abbreviations: Outer membrane protein complex (OMPC), Inflammasome (INFL), Alum (Aluminium hydroxide), Melanoma differentiation-associated gene 5 (MDA-5), C-type lectin receptor (CTLs), Cell wall skeleton (CWS), single stranded (ss).
Hepatitis B virus (HBV) is a global public health threat that has chronically infected over 350 million people worldwide (62). The HepB vaccine is composed of a virus-like particle containing the viral envelope protein hepatitis B surface antigen (HBsAg), prepared using recombinant DNA technology. HepB vaccine is composed of an aluminum-containing ajuvant, but is still incompletely immunogenic as ~10% of vaccinated populations fail to mount immune responses to HBsAg after immunization (Table 1).
Haemophilus influenzae type b (Hib) conjugate vaccines are initially administered at 6 to 8 weeks of age. Hib activates transfected HEK293 cells in a TLR2- and TLR4-dependent manner, likely reflecting expression of bacterial lipopeptides (TLR2) and lipopolysaccharide (TLR4) (63) (64) (Table 1). Indeed, the outer membrane protein complex (OMPC) found within the Hib-OMPC glycoconjugate vaccine is TLR2 and MyD88-dependant, and in the absence of TLR2, the immunogenicity of the Hib-OMPC vaccine is significantly reduced (64).
In addition to TLR-induced vaccine responses (65), activation of RLRs (RIG-I, MDA-5 (66)) has been reported for the wild type measles virus, suggesting that the attenuated strains used for vaccination may also engage this innate immune pathway (Table 1). To date, the US Food and Drug Administration (FDA) have approved a limited number of human vaccine adjuvants, chief among which is aluminium hydroxide, aluminum phosphate (typically referred to as `Alum'). Alum is a commonly used adjuvant present in human and animal vaccines world-wide (HepB, DTaP, Hib, PCV7, HepA; Table 1) and is known for its ability to induce protective Th2-type responses. It was recently demonstrated that the key to Alum's adjuvant activity is its ability to activate the NALP3/INFL (67, 68) (Fig. 1). These conclusions were based on the observations that IL-1β and IL-18 production by macrophages in response to Alum in vitro requires intact INFL signaling. Moreover, mice deficient in NALP3, apoptosis-associated speck-like protein containing a CARD (ASC) or caspase-1 failed to mount a significant Ab response to an Ag administered with Alum, whereas the response to complete Freund's adjuvant remained intact. These data highlight the crucial role of the NALP3/INFL in Alum's adjuvant activity.
MF59 is an oil-in water squalene emulsion and the precise mechanism of its adjuvant effects is still largely unknown (69). Studies using fluorescently-labeled MF59 injected intramuscularly then observed 2 days later indicated partial localization in T-cell areas of the draining lymph node (70). MF59 has also been documented to induce a significant influx of macrophages to the site of infection in mice, which is chemokine receptor 2 (CCR2) dependant (71). Of interest, the oil emulsion incomplete Freund's adjuvant (not licensed in humans due to its toxicity) induces optimal IgG1 and IgG2c in a NOD2-dependant manner (72).
Translational research towards improved adjuvants for newborns and infants
Given that newborns and young infants are susceptible to multiple bacterial and viral pathogens, neonatal immunization is of particular importance because: (a) birth represents a likely point of contact of the newborn with health care providers, (b) early life immunization is associated with a substantially higher rate of vaccination coverage than immunization given at later time-points (2, 73), (c) vaccines that require multiple dosing regimens throughout infancy increase cost and reduce compliance, and (d) strategies involving immunization of the mother pose substantial logistical and medicolegal challenges. There is therefore an unmet medical need to develop new approaches are needed to develop vaccines that would be more effective very early in life or that would require fewer doses to generate durable and protective immune responses.
A novel approach to neonatal vaccination has recently been described which employs an attenuated strain of the intracellular pathogenic bacterium Listeria monocytogenes to deliver Ag to the cytoplasm of APC (74). Vaccinated neonatal mice induced strong CD8+ and CD4+ T cell responses and were protected following wild type challenge. Of note, Listeria monocytogenes is able to activate several PRRs including multiple NODs (IPAF, NALP3) (75) and can induce the expression of RIG-I (76), possibly accounting for its immunostimulatory properties. Therefore this approach could potentially overcome preexisting hurdles of maternal immune response interfering with neonatal vaccine responses (77). This live attenuated vaccine appeared safe in this murine model in that there was no associated mortality and no bacteria were recovered from the spleens or liver of immunized newborn mice 7 days post-vaccination.
Among the TLR agonists, TLR7/8 agonists hold particular promise as potential adjuvants for use in neonatal and infant vaccines as they induce robust production of the Th1-polarizing cytokines TNFα and IL-12 from neonatal (and adult) APCs that substantially exceeds responses induced by agonists of TLR-2, -4, or -7 (alone) (42, 78). Therefore TLR7/8 agonists have potential as novel neonatal vaccine adjuvants, due to their ability to activate both TLR-dependant and independent pathways (NALP3/INFL) mediating Th1-type responses from APC (78). In addition, human Treg express TLR8 and mediate reversal of Treg function upon exposure to TLR8 agonists (79) (Figure 2). Indeed TLR7/8 agonists, such as the synthetic low-molecular weight (< 400 Da) antiviral imidazoquinoline compounds imiquimod and resiquimod (R848), have already been used as immunomodulators for many years against specific viral infections. The U.S. Federal Drug Administration (FDA) approved imiquimod for the treatment of external genital and peri-anal warts caused by certain strains of human papilloma virus (HPV)(80), and it may also have activity against molluscum contagiosum (mulluscipox virus) (81-83). One of the main cellular targets of imiquimod is plasmacytoid DCs (IFN-α producing cells) which express high amounts of TLR7, whose engagement induced IFN-α in a MyD88-dependent manner (80, 84).
Figure 2. TLR8 agonists activate human APCs and reverse human Treg function.
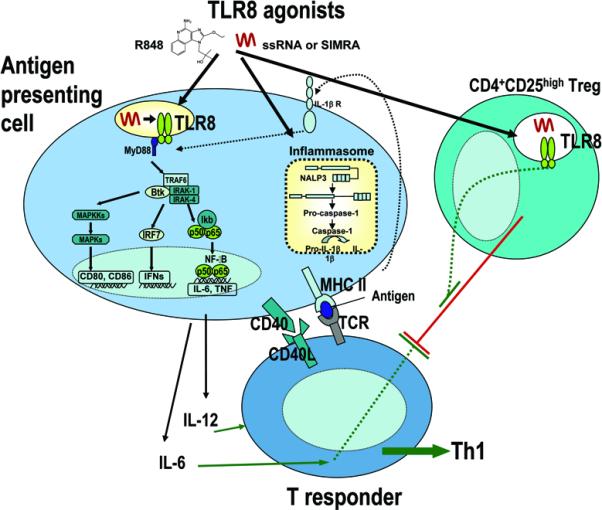
TLR8 agonists, such as R848, ssRNA and stabilized immune modulatory RNA (SIMRA) strongly activate human APCs via TLR8-dependent and TLR8-independent mechanisms including activation of the NALP3 inflammasome inducing IL-1β production. Exposure of human neonatal APCs to TLR8 agonists induces robust phosphorylation of p38 MAP kinase and profound/prolonged disappearance of IkB-κ, resulting in robust induction of protective Th1-type immune responses, including production of IL-12 and up-regulation of the co-stimulatory molecule CD40. TLR8 agonists also reverse suppression mediated by human Treg cells, via both direct action on Treg as well as by induction of APC production of IL-6, a cytokine that renders Tresponder cells refractory to Treg-mediated inhibition.
R848 is an effective adjuvant for HBsAg vaccination in mice, increasing humoral and cellular immune responses. R848 used in combination with the TLR9 agonists CpG ODN further strengthened the immune response and long-lasting HBsAg-specific T cells displaying e■ector memory phenotype were detected in mice (85). R848 or topical application of imiquimod administered with Leishmania Ag induced protective Th1 immune responses in mice, compared with Leishmania Ag alone. In addition, subcutaneous vaccination also induced protective immunity whereas intramuscular vaccination did not (86). Indeed, conjugation of the TLR7/8 agonist to the HIV Gag protein improved the magnitude of Th1 and CD8+ responses in adult Rhesus macaques (87). A combined TLR7/8 agonist compared with a pure TLR8 agonist can also induce greater Th1 responses and IFNα production from pDCs, which express TLR7 but not TLR8. IFNα is a key cytokine within a vaccine adjuvant setting, inducing Th1 differentiation (80, 88), induction of cytotoxic T lymphocytes, enhancement of cross presentation and of primary antibody responses and DC activation (89). Novel TLR7 and TLR8 agonists referred to as stabilized immune modulatory RNA (SIMRA) compounds have recently developed and have distinct pharmacodynamic characteristics (90). SIMRA compounds demonstrate greater stability in human sera compared with linear RNA, which is rapidly degraded by ubiquitous RNases. In addition, SIMRA compounds and are able to activate TLR7 or TLR8 in HEK293 cells without the need for lipid carriers.
TLR9 agonists are also undergoing biopharmaceutical development for multiple indications, including as vaccine adjuvants for HBV by linking an immunostimulatory DNA sequence to the recombinant HBsAg. This vaccine formulation, which has currently completed phase III trials, may help drive Th1-type responses and reduce Th2 responses (91).
A murine study demonstrated that although the IPS-1 signaling pathway appears to be important for initial type I IFN responses, the TLR7/MyD88 pathway is needed for induction of protective immune responses to influenza A infection. Inactivated influenza virus vaccine failed to confer protection against lethal challenge with live influenza virus in TLR7- and MyD88-deficient mice. Thus protective adaptive immune responses to live attenuated influenza A virus strains are likely dependent on the TLR7-MyD88 pathway (92).
Priorities For Future Studies
In summary, infectious diseases account for over > 2 million deaths per year in newborns and infants less than 6 months of age. Early life vaccination offers several advantages, particularly for developing countries in which birth maybe the only point of contact with the healthcare system. There is thus an unmet medical need for improved neonatal vaccine adjuvants, which are able to improve the level, speed and longevity of protection, particularly by inducing Th1-polarizing cell-mediated immunity. In this context, neonatal vaccination is a feasible approach that is likely to be expanded in future years, an effort that will require the development of new adjuvants capable activating specific PRRs. The recent expansion in knowledge of PRRs in the neonate as described above provides new opportunities for developing novel delivery systems (e.g., intracellular/cytosolic) and/or adjuvants. As always, safety considerations will be paramount, but the possibility of vaccinating this vulnerable population provides a strong motivation to pursue effective vaccines for neonates and infants. Although neonatal vaccination offers many advantages safety concerns will be paramount and have recently been reviewed (93). There are concerns that neonatal vaccination could trigger autoimmunity through molecular mimicry or antigen immune system polarization (94, 95), however self-reactive neonatal T and B cells are eliminated by peripheral tolerance and there is substantial evidence that multiple pediatric vaccines, including BCG, are not linked to allergy or autoimmunity (96). Nevertheless, as with any new drugs, all novel adjuvant/Ag combinations, must undergo rigorous safety analysis. The rapidly expanding menu of antigen-adjuvant combinations will require federal authorities to update and enhance the pathways to establishing vaccine safety and efficacy.
Acknowledgments
We thank Drs. Michael Wessels and Raif Geha for mentorship and Dr. Richard Malley for helpful conversations.
Financial Support: This study was funded in part by AHA postdoctoral fellowship (VJP) and NIH R01 grant AI067353, the Patterson Trust, reagent support from 3M Pharmaceuticals, and a sponsorship by Idera Pharmaceuticals.
Abbreviations
- APC
antigen-presenting cell
- BCG
Bacille Calmette-Guerin
- MyD88
Myeloid differentiation factor 88
- NALP3
Nacht Domain Leucine-Rich Repeat and PYD-Containing Protein 3
- RLRs
retinoic acid inducible gene-like receptors
- TLR
Toll-like receptor
- Treg
T-regulatory cell
References
- 1.W.H.O. The World Health Report. World Health Organization; Geneva: 2005. [Google Scholar]
- 2.Siegrist CA. Immunologic Requirements for Vaccines to be used in Early Life. In: Bloom B, Lambert PH, editors. The Vaccine Book. Academic Press; Boston: 2003. pp. 73–83. [Google Scholar]
- 3.Angelone DF, Wessels MR, Coughlin M, Suter EE, Valentini P, Kalish LA, Levy O. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatr Res. 2006;60:205–209. doi: 10.1203/01.pdr.0000228319.10481.ea. [DOI] [PubMed] [Google Scholar]
- 4.Kallewaard NL, McKinney BA, Gu Y, Chen A, Prasad BV, Crowe JE., Jr Functional maturation of the human antibody response to rotavirus. J Immunol. 2008;180:3980–3989. doi: 10.4049/jimmunol.180.6.3980. [DOI] [PubMed] [Google Scholar]
- 5.Morein B, Blomqvist G, Hu K. Immune responsiveness in the neonatal period. J Comp Pathol. 2007;137:S27–S31. doi: 10.1016/j.jcpa.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Jones CA, Holloway JA, Warner JO. Phenotype of fetal monocytes and B lymphocytes during the third trimester of pregnancy. J Reprod Immunol. 2002;56:45–60. doi: 10.1016/s0165-0378(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 7.Saito S. Cytokine network at the feto-maternal interface. J Reprod Immunol. 2000;47:87–103. doi: 10.1016/s0165-0378(00)00060-7. [DOI] [PubMed] [Google Scholar]
- 8.Levy O, Coughlin M, Cronstein B, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006;177:1956–1966. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy O. Innate immunity of the newborn: basic mechansims and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 10.Langrish CL, Buddle JC, Thrasher AJ, Goldblatt D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin Exp Immunol. 2002;128:118–123. doi: 10.1046/j.1365-2249.2002.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muthukkumar S, Goldstein J, Stein KE. The ability of B cells and dendritic cells to present antigen increases during ontogeny. J Immunol. 2000;165:4803–4813. doi: 10.4049/jimmunol.165.9.4803. [DOI] [PubMed] [Google Scholar]
- 12.Zhao J, Kim KD, Yang X, Auh S, Fu YX, Tang H. Hyper innate responses in neonates lead to increased morbidity and mortality after infection. Proc Natl Acad Sci USA. 2008;105:7528–7533. doi: 10.1073/pnas.0800152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, Hood LE, Aderem A. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci USA. 2005;102:9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Philbin VJ, Iqbal M, Boyd Y, Goodchild MJ, Beal RK, Bumstead N, Young J, Smith AL. Identification and characterization of a functional, alternatively spliced Toll-like receptor 7 (TLR7) and genomic disruption of TLR8 in chickens. Immunology. 2005;114:507–521. doi: 10.1111/j.1365-2567.2005.02125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004;173:4627–4634. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 16.Michaelsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. J Immunol. 2006;176:5741–5748. doi: 10.4049/jimmunol.176.10.5741. [DOI] [PubMed] [Google Scholar]
- 17.Godfrey WR, Spoden DJ, Ge YG, Baker SR, Liu B, Levine BL, June CH, Blazar BR, Porter SB. Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 2005;105:750–758. doi: 10.1182/blood-2004-06-2467. [DOI] [PubMed] [Google Scholar]
- 18.Wing K, Larsson P, Sandstrom K, Lundin SB, Suri-Payer E, Rudin A. CD4+ CD25+ FOXP3+ regulatory T cells from human thymus and cord blood suppress antigen-specific T cell responses. Immunology. 2005;115:516–525. doi: 10.1111/j.1365-2567.2005.02186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodor J, Fehervari Z, Diamond B, Sakaguchi S. Regulatory T cell-mediated suppression: potential role of ICER. J Leukoc Biol. 2007;81:161–167. doi: 10.1189/jlb.0706474. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez MA, Puttur FK, Wang YM, Howden W, Alexander SI, Jones CA. T regulatory cells contribute to the attenuated primary CD8+ and CD4+ T cell responses to herpes simplex virus type 2 in neonatal mice. J Immunol. 2008;180:1556–1564. doi: 10.4049/jimmunol.180.3.1556. [DOI] [PubMed] [Google Scholar]
- 22.Toka FN, Suvas S, Rouse BT. CD4+ CD25+ T cells regulate vaccine-generated primary and memory CD8+ T-cell responses against herpes simplex virus type 1. J Virol. 2004;78:13082–13089. doi: 10.1128/JVI.78.23.13082-13089.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stober CB, Lange UG, Roberts MT, Alcami A, Blackwell JM. IL-10 from regulatory T cells determines vaccine efficacy in murine Leishmania major infection. J Immunol. 2005;175:2517–2524. doi: 10.4049/jimmunol.175.4.2517. [DOI] [PubMed] [Google Scholar]
- 24.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 25.O'Neill LA. When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity. 2008;29:12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 27.Matzinger P. Friendly and dangerous signals: is the tissue in control? Nat Immunol. 2007;8:11–13. doi: 10.1038/ni0107-11. [DOI] [PubMed] [Google Scholar]
- 28.Soderberg KA, Payne GW, Sato A, Medzhitov R, Segal SS, Iwasaki A. Innate control of adaptive immunity via remodeling of lymph node feed arteriole. Proc Natl Acad Sci USA. 2005;102:16315–16320. doi: 10.1073/pnas.0506190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webster B, Ekland EH, Agle LM, Chyou S, Ruggieri R, Lu TT. Regulation of lymph node vascular growth by dendritic cells. J Exp Med. 2006;203:1903–1913. doi: 10.1084/jem.20052272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 31.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 32.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 33.Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, Tsichlis PN. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–1083. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 34.Huang Q, Yang J, Lin Y, Walker C, Cheng J, Liu ZG, Su B. Differential regulation of interleukin 1 receptor and Toll-like receptor signaling by MEKK3. Nat Immunol. 2004;5:98–103. doi: 10.1038/ni1014. [DOI] [PubMed] [Google Scholar]
- 35.von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, Chrabieh M, Mustapha IB, Ghandil P, Camcioglu Y, Vasconcelos J, Sirvent N, Guedes M, Vitor AB, Herrero-Mata MJ, Arostegui JI, Rodrigo C, Alsina L, Ruiz-Ortiz E, Juan M, Fortuny C, Yague J, Anton J, Pascal M, Chang HH, Janniere L, Rose Y, Garty BZ, Chapel H, Issekutz A, Marodi L, Rodriguez-Gallego C, Banchereau J, Abel L, Li X, Chaussabel D, Puel A, Casanova JL. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ku CL, von Bernuth H, Picard C, Zhang SY, Chang HH, Yang K, Chrabieh M, Issekutz AC, Cunningham CK, Gallin J, Holland SM, Roifman C, Ehl S, Smart J, Tang M, Barrat FJ, Levy O, McDonald D, Day-Good NK, Miller R, Takada H, Hara T, Al-Hajjar S, Al-Ghonaium A, Speert D, Sanlaville D, Li X, Geissmann F, Vivier E, Marodi L, Garty BZ, Chapel H, Rodriguez-Gallego C, Bossuyt X, Abel L, Puel A, Casanova JL. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J Exp Med. 2007;204:2407–2422. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medvedev AE, Lentschat A, Kuhns DB, Blanco JC, Salkowski C, Zhang S, Arditi M, Gallin JI, Vogel SN. Distinct mutations in IRAK-4 confer hyporesponsiveness to lipopolysaccharide and interleukin-1 in a patient with recurrent bacterial infections. J Exp Med. 2003;198:521–531. doi: 10.1084/jem.20030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan SR, Qing G, Byers DM, Stadnyk AW, Al-Hertani W, Bortolussi R. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect Immun. 2004;72:1223–1229. doi: 10.1128/IAI.72.3.1223-1229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goriely S, Van Lint C, Dadkhah R, Libin M, De Wit D, Demonte D, Willems F, Goldman M. A defect in nucleosome remodeling prevents IL-12(p35) gene transcription in neonatal dendritic cells. J Exp Med. 2004;199:1011–1016. doi: 10.1084/jem.20031272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Wit D, Olislagers V, Goriely S, Vermeulen F, Wagner H, Goldman M, Willems F. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood. 2004;103:1030–1032. doi: 10.1182/blood-2003-04-1216. [DOI] [PubMed] [Google Scholar]
- 41.Aksoy E, Albarani V, Nguyen M, Laes JF, Ruelle JL, De Wit D, Willems F, Goldman M, Goriely S. Interferon regulatory factor 3-dependent responses to lipopolysaccharide are selectively blunted in cord blood cells. Blood. 2007;109:2887–2893. doi: 10.1182/blood-2006-06-027862. [DOI] [PubMed] [Google Scholar]
- 42.Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006;108:1284–1290. doi: 10.1182/blood-2005-12-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 44.Andrejeva J, Childs KS, Young DF, Carlos TS, Stock N, Goodbourn S, Randall RE. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci USA. 2004;101:17264–17269. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, Kusanovic JP, Mittal P, Mazaki-Tovi S, Kim CJ, Kim JS, Edwin S, Nhan-Chang CL, Hamill N, Friel L, Than NG, Mazor M, Yoon BH, Hassan SS. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21:605–616. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gadjeva M, Takahashi K, Thiel S. Mannan-binding lectin--a soluble pattern recognition molecule. Mol Immunol. 2004;41:113–121. doi: 10.1016/j.molimm.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 47.Jack DL, Read RC, Tenner AJ, Frosch M, Turner MW, Klein NJ. Mannose-binding lectin regulates the inflammatory response of human professional phagocytes to Neisseria meningitidis serogroup B. J Infect Dis. 2001;184:1152–1162. doi: 10.1086/323803. [DOI] [PubMed] [Google Scholar]
- 48.Oudshoorn AM, van den Dungen FA, Bach KP, Koomen I, Fetter WP, Catsburg A, Savelkoul PH, van Elburg RM. Mannose-binding lectin in term newborns and their mothers: genotypic and phenotypic relationship. Hum Immunol. 2008;69:344–348. doi: 10.1016/j.humimm.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 49.Dzwonek AB, Neth OW, Thiebaut R, Gulczynska E, Chilton M, Hellwig T, Bajaj-Elliott M, Hawdon J, Klein NJ. The role of mannose-binding lectin in susceptibility to infection in preterm neonates. Pediatr Res. 2008;63:680–685. doi: 10.1203/PDR.0b013e31816fdbff. [DOI] [PubMed] [Google Scholar]
- 50.Guttormsen HK, Stuart LM, Shi L, Carroll MC, Chen J, Kasper DL, Ezekowitz RA, Takahashi K. Deficiency of mannose-binding lectin greatly increases antibody response in a mouse model of vaccination. Clin Immunol. 2008 doi: 10.1016/j.clim.2008.09.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oppenheim JJ, Biragyn A, Kwak LW, Yang D. Roles of antimicrobial peptides such as defensins in innate and adaptive immunity. Ann Rheum Dis. 2003;62:ii17–ii21. doi: 10.1136/ard.62.suppl_2.ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kornbluth RS, Stone GW. Immunostimulatory combinations: designing the next generation of vaccine adjuvants. J Leukoc Biol. 2006;80:1084–1102. doi: 10.1189/jlb.0306147. [DOI] [PubMed] [Google Scholar]
- 53.Vekemans J, Amedei A, Ota MO, D'Elios MM, Goetghebuer T, Ismaili J, Newport MJ, Del Prete G, Goldman M, McAdam KP, Marchant A. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur J Immunol. 2001;31:1531–1535. doi: 10.1002/1521-4141(200105)31:5<1531::AID-IMMU1531>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 54.Marchant A, Goetghebuer T, Ota MO, Wolfe I, Ceesay SJ, De Groote D, Corrah T, Bennett S, Wheeler J, Huygen K, Aaby P, McAdam KP, Newport MJ. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J Immunol. 1999;163:2249–2255. [PubMed] [Google Scholar]
- 55.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 2004;6:1382–1387. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 56.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 57.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–3927. [PubMed] [Google Scholar]
- 58.Uehori J, Matsumoto M, Tsuji S, Akazawa T, Takeuchi O, Akira S, Kawata T, Azuma I, Toyoshima K, Seya T. Simultaneous blocking of human Toll-like receptors 2 and 4 suppresses myeloid dendritic cell activation induced by Mycobacterium bovis bacillus Calmette-Guerin peptidoglycan. Infect Immun. 2003;71:4238–4249. doi: 10.1128/IAI.71.8.4238-4249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsuji S, Matsumoto M, Takeuchi O, Akira S, Azuma I, Hayashi A, Toyoshima K, Seya T. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: involvement of toll-like receptors. Infect Immun. 2000;68:6883–6890. doi: 10.1128/iai.68.12.6883-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med. 2005;202:1715–1724. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davila S, Hibberd ML, Hari Dass R, Wong HE, Sahiratmadja E, Bonnard C, Alisjahbana B, Szeszko JS, Balabanova Y, Drobniewski F, van Crevel R, van de Vosse E, Nejentsev S, Ottenhoff TH, Seielstad M. Genetic association and expression studies indicate a role of toll-like receptor 8 in pulmonary tuberculosis. PLoS Genet. 2008;4:e1000218. doi: 10.1371/journal.pgen.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams R. Global challenges in liver disease. Hepatology. 2006;44:521–526. doi: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- 63.Mogensen TH, Paludan SR, Kilian M, Ostergaard L. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J Leukoc Biol. 2006;80:267–277. doi: 10.1189/jlb.1105626. [DOI] [PubMed] [Google Scholar]
- 64.Latz E, Franko J, Golenbock DT, Schreiber JR. Haemophilus influenzae type bouter membrane protein complex glycoconjugate vaccine induces cytokine production by engaging human toll-like receptor 2 (TLR2) and requires the presence of TLR2 for optimal immunogenicity. J Immunol. 2004;172:2431–2438. doi: 10.4049/jimmunol.172.4.2431. [DOI] [PubMed] [Google Scholar]
- 65.Bieback K, Lien E, Klagge IM, Avota E, Schneider-Schaulies J, Duprex WP, Wagner H, Kirschning CJ, Ter Meulen V, Schneider-Schaulies S. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J Virol. 2002;76:8729–8736. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berghall H, Siren J, Sarkar D, Julkunen I, Fisher PB, Vainionpaa R, Matikainen S. The interferon-inducible RNA helicase, mda-5, is involved in measles virus-induced expression of antiviral cytokines. Microbes Infect. 2006;8:2138–2144. doi: 10.1016/j.micinf.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 67.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1127. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 69.Ott G, Barchfeld GL, Chernoff D, Radhakrishnan R, van Hoogevest P, Van Nest G. MF59. Design and evaluation of a safe and potent adjuvant for human vaccines. Pharm Biotechnol. 1995;6:277–296. doi: 10.1007/978-1-4615-1823-5_10. [DOI] [PubMed] [Google Scholar]
- 70.Schultze V, D'Agosto V, Wack A, Novicki D, Zorn J, Hennig R. Safety of MF59 adjuvant. Vaccine. 2008;26:3209–3222. doi: 10.1016/j.vaccine.2008.03.093. [DOI] [PubMed] [Google Scholar]
- 71.Dupuis M, Denis-Mize K, LaBarbara A, Peters W, Charo IF, McDonald DM, Ott G. Immunization with the adjuvant MF59 induces macrophage trafficking and apoptosis. Eur J Immunol. 2001;31:2910–2918. doi: 10.1002/1521-4141(2001010)31:10<2910::aid-immu2910>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 72.Moreira LO, Smith AM, Defreitas AA, Qualls JE, El Kasmi KC, Murray PJ. Modulation of adaptive immunity by different adjuvant-antigen combinations in mice lacking Nod2. Vaccine. 2008;26:5808–5813. doi: 10.1016/j.vaccine.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lambert PH. Vaccines for the world: major challenges for the future. Southeast Asian J Trop Med Public Health. 1997;28:122–126. [PubMed] [Google Scholar]
- 74.Kollmann TR, Reikie B, Blimkie D, Way SS, Hajjar AM, Arispe K, Shaulov A, Wilson CB. Induction of protective immunity to Listeria monocytogenes in neonates. J Immunol. 2007;178:3695–3701. doi: 10.4049/jimmunol.178.6.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Warren SE, Mao DP, Rodriguez AE, Miao EA, Aderem A. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J Immunol. 2008;180:7558–7564. doi: 10.4049/jimmunol.180.11.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Imaizumi T, Sashinami H, Mori F, Matsumiya T, Yoshida H, Nakane A, Wakabayashi K, Oyama C, Satoh K. Listeria monocytogenes induces the expression of retinoic acid-inducible gene-I. Microbiol Immunol. 2006;50:811–815. doi: 10.1111/j.1348-0421.2006.tb03857.x. [DOI] [PubMed] [Google Scholar]
- 77.Starks H, Bruhn KW, Shen H, Barry RA, Dubensky TW, Brockstedt D, Hinrichs DJ, Higgins DE, Miller JF, Giedlin M, Bouwer HG. Listeria monocytogenes as a vaccine vector: virulence attenuation or existing antivector immunity does not diminish therapeutic efficacy. J Immunol. 2004;173:420–427. doi: 10.4049/jimmunol.173.1.420. [DOI] [PubMed] [Google Scholar]
- 78.Philbin VJ, Levy O. Immunostimulatory activity of Toll-like receptor 8 agonists towards human leucocytes: basic mechanisms and translational opportunities. Biochem Soc Trans. 2007;35:1485–1491. doi: 10.1042/BST0351485. [DOI] [PubMed] [Google Scholar]
- 79.Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 80.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [comment] [DOI] [PubMed] [Google Scholar]
- 81.Bayerl C, Feller G, Goerdt S. Experience in treating molluscum contagiosum in children with imiquimod 5% cream. Br J Dermatol. 2003;149:25–29. doi: 10.1046/j.0366-077x.2003.05631.x. [DOI] [PubMed] [Google Scholar]
- 82.Hengge UR, Cusini M. Topical immunomodulators for the treatment of external genital warts, cutaneous warts and molluscum contagiosum. Br J Dermatol. 2003;149:15–19. doi: 10.1046/j.0366-077x.2003.05623.x. [DOI] [PubMed] [Google Scholar]
- 83.Miller RL, Meng TC, Tomai MA. The antiviral activity of Toll-like receptor 7 and 7/8 agonists. Drug News Perspect. 2008;21:69–87. doi: 10.1358/dnp.2008.21.2.1188193. [DOI] [PubMed] [Google Scholar]
- 84.Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, Lipford G, Bauer S. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [comment] [DOI] [PubMed] [Google Scholar]
- 85.Ma R, Du JL, Huang J, Wu CY. Additive effects of CpG ODN and R-848 as adjuvants on augmenting immune responses to HBsAg vaccination. Biochem Biophys Res Commun. 2007;361:537–542. doi: 10.1016/j.bbrc.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 86.Zhang WW, Matlashewski G. Immunization with a Toll-like receptor 7 and/or 8 agonist vaccine adjuvant increases protective immunity against Leishmania major in BALB/c mice. Infect Immun. 2008;76:3777–3783. doi: 10.1128/IAI.01527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci USA. 2005;102:15190–15194. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gibson SJ, Lindh JM, Riter TR, Gleason RM, Rogers LM, Fuller AE, Oesterich JL, Gorden KB, Qiu X, McKane SW, Noelle RJ, Miller RL, Kedl RM, Fitzgerald-Bocarsly P, Tomai MA, Vasilakos JP. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74–86. doi: 10.1016/s0008-8749(02)00517-8. [DOI] [PubMed] [Google Scholar]
- 89.Bracci L, La Sorsa V, Belardelli F, Proietti E. Type I interferons as vaccine adjuvants against infectious diseases and cancer. Expert Rev Vaccines. 2008;7:373–381. doi: 10.1586/14760584.7.3.373. [DOI] [PubMed] [Google Scholar]
- 90.Lan T, Kandimalla ER, Yu D, Bhagat L, Li Y, Wang D, Zhu F, Tang JX, Putta MR, Cong Y, Trombino AF, Sullivan T, Agrawal S. Stabilized immune modulatory RNA compounds as agonists of Toll-like receptors 7 and 8. Proc Natl Acad Sci USA. 2007;104:13750–13755. doi: 10.1073/pnas.0706059104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sung JJ, Lik-Yuen H. HBV-ISS (Dynavax) Curr Opin Mol Ther. 2006;8:150–155. [PubMed] [Google Scholar]
- 92.Koyama S, Ishii KJ, Kumar H, Tanimoto T, Coban C, Uematsu S, Kawai T, Akira S. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol. 2007;179:4711–4720. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- 93.Demirjian A, Levy O. Safety and efficacy of neonatal vaccination. Eur J Immunol. 2008;39:36–46. doi: 10.1002/eji.200838620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Offit PA. Multiple Vaccines and the Immune System. In: Plotkin SA, Orenstein WA, editors. Vaccines. Saunders; Philadelphia: 2004. pp. 1583–1589. [Google Scholar]
- 95.Goriely S, Goldman M. From tolerance to autoimmunity: is there a risk in early life vaccination? J Comp Pathol. 2007;137:S57–S61. doi: 10.1016/j.jcpa.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 96.Gruber C, Nilsson L, Bjorksten B. Do early childhood immunizations influence the development of atopy and do they cause allergic reactions? Pediatr Allergy Immunol. 2001;12:296–311. doi: 10.1034/j.1399-3038.2001.1r046.x. [DOI] [PubMed] [Google Scholar]
- 97.Jo EK. Mycobacterial interaction with innate receptors: TLRs, C-type lectins, and NLRs. Curr Opin Infect Dis. 2008;21:279–286. doi: 10.1097/QCO.0b013e3282f88b5d. [DOI] [PubMed] [Google Scholar]
- 98.Le Goffic R, Pothlichet J, Vitour D, Fujita T, Meurs E, Chignard M, Si-Tahar M. Cutting Edge: Influenza A virus activates TLR3-dependent inflammatory and RIG-Idependent antiviral responses in human lung epithelial cells. J Immunol. 2007;178:3368–3372. doi: 10.4049/jimmunol.178.6.3368. [DOI] [PubMed] [Google Scholar]
- 99.Geeraedts F, Goutagny N, Hornung V, Severa M, de Haan A, Pool J, Wilschut J, Fitzgerald KA, Huckriede A. Superior immunogenicity of inactivated whole virus H5N1 influenza vaccine is primarily controlled by Toll-like receptor signalling. PLoS Pathog. 2008;4:e1000138. doi: 10.1371/journal.ppat.1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matikainen S, Siren J, Tissari J, Veckman V, Pirhonen J, Severa M, Sun Q, Lin R, Meri S, Uze G, Hiscott J, Julkunen I. Tumor necrosis factor alpha enhances influenza A virus-induced expression of antiviral cytokines by activating RIG-I gene expression. J Virol. 2006;80:3515–3522. doi: 10.1128/JVI.80.7.3515-3522.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang JP, Kurt-Jones EA, Shin OS, Manchak MD, Levin MJ, Finberg RW. Varicella-zoster virus activates inflammatory cytokines in human monocytes and macrophages via Toll-like receptor 2. J Virol. 2005;79:12658–12666. doi: 10.1128/JVI.79.20.12658-12666.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Massari P, Henneke P, Ho Y, Latz E, Golenbock DT, Wetzler LM. Cutting edge: Immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J Immunol. 2002;168:1533–1537. doi: 10.4049/jimmunol.168.4.1533. [DOI] [PubMed] [Google Scholar]
- 103.American Academy of Pediatrics Committee on Infectious Diseases Recommended childhood and adolescent immunization schedules--United States, 2009. Pediatrics. 2009;123:189–190. doi: 10.1542/peds.2008-3306. [DOI] [PubMed] [Google Scholar]


