Abstract
Objective
To determine the relative efficacy of peer support and pager messaging strategies versus usual care to improve medication adherence and clinical outcomes among HIV-positive outpatients initiating or switching to a new highly active antiretroviral therapy regimen.
Design
A 2 × 2 factorial randomized controlled trial of a 3-month intervention with computer-assisted self-interviews and blood draws administered at baseline, 3, 6, and 9 months.
Methods
HIV-positive patients at a public HIV specialty clinic in Seattle, WA (N= 224) were randomly assigned to peer support, pager messaging, both strategies, or usual care. The main outcomes were adherence according to self-report and electronic drug monitoring, CD4 count, and HIV-1 RNA viral load.
Results
Intent-to-treat analyses suggested the peer intervention was associated with greater self-reported adherence at immediate post-intervention. However, these effects were not maintained at follow-up assessment; nor were there significant differences in biological outcomes. The pager intervention, on the other hand, was not associated with greater adherence but did appear to have effects on biological outcomes at post-intervention that were sustained at follow-up.
Conclusions
Analyses indicate the potential efficacy of peer support but not pager messaging in promoting short-term antiretroviral adherence. Strategies to maintain adherence over time still are needed.
Keywords: HIV/AIDS, HAART, adherence, peers, social support, pager
Introduction
Widespread adoption of highly active antiretroviral therapy (HAART) has significantly extended the lives of persons living with HIV/AIDS,1, 2 with one report estimating the potent therapies have saved at least 3.0 million years of life in the United States (USA) alone3. Consistently strict adherence remains key to successful viral suppression.4 However, in both resource-rich and -constrained settings, the majority of patients on HAART struggle to maintain optimal levels of adherence.5, 6
Two meta-analyses indicated that various behavioral strategies can be efficacious in promoting adherence as well as improving clinical outcomes.7, 8 Successful behavioral strategies include couples-based counseling9; modified directly observed therapy10; and cognitive-behavioral, problem-solving, and motivational interviewing techniques11. Research on peer support and electronic reminders12 is more limited, with mixed findings. However, both strategies are potentially cost effective, culturally adaptable, and easily translatable and, therefore, merit further investigation.
The present study builds upon preliminary work of a peer support intervention13 that indicated non-significant between-group findings but a dose-response effect among participants within the peer support arm. The current study supplemented the peer support program with messaging from a programmable pager. Moreover, this follow-up study involved a larger and more restricted sample, a lengthier follow-up assessment period, and monetary incentives to encourage greater engagement in the peer support intervention.
Methods
Procedures
Data were collected from 3/30/2003 – 5/30/2007 at the adult HIV primary care outpatient clinic at Harborview Medical Center, a public institution affiliated with University of Washington in Seattle, Washington, USA. Advertisements for the study were posted in the clinic waiting room, and all providers were informed of the study. Referrals came from clinic providers, the patients themselves, and a nurse at the clinic dedicated to soliciting research participants. The nurse approached all potentially eligible patients at each clinic session.
Eligible participants were (a) at least 18 years of age, (b) proficient in English, (c) living within the service area of the pager, and (e) initiating or changing at least two medications of a HAART regimen. Some of the changes were prescribed because of poor response, but in other cases new formulations were prescribed in order to reduce pill burden and/or side effects. Individuals who were cognitively impaired, actively psychotic, or had a known history of harming others were excluded. Study staff provided detailed information about the scope of the trial to all eligible patients referred to them and scheduled a baseline appointment to obtain written informed consent and enroll participants. The baseline appointment was scheduled to coincide as closely as possible with the day patients were to initiate their HAART regimen.
At the baseline appointment, participants first completed an approximately 1-hour computer-assisted self-interview assessing adherence as well as other psychosocial and attitudinal variables (i.e., knowledge about treatment, adherence self-efficacy, side effects, substance use, sexual risk behaviors, social support, coping, negative affective states, and quality of life). Next, they were randomly assigned to one of the four study arms: peer support, pager, both peer and pager, or usual care. Allocation concealment involved the use of sequentially numbered, opaque, sealed envelopes containing the study arm assignment, which the study staff opened at the moment of randomization. An external statistician had used a computerized random number generator to select random permuted blocks of four. Due to the nature of the intervention, participants, study staff, and data analysts could not be completely blinded to study arm assignment.
Electronic drug monitors (EDM; i.e., the Medication Event Monitoring System or MEMS®; http://www.aardex.ch) were provided to all participants for the duration of the trial. EDM technology consists of a plastic pill vial and modified cap containing a microprocessor capable of recording the precise date and time of each vial opening as a presumptive dose. Each participant was given one EDM to use with the most frequently dosed antiretroviral.
All participants continued to receive medical care at the clinic and were asked to return for assessments at 2 weeks (for a brief interview) and 3, 6, and 9 months (for full follow-up assessments). At each full assessment, EDM data were uploaded and blood was drawn to determine HIV-1 RNA viral load (VL) and CD4 lymphocyte count. Participants were reimbursed $60 for the baseline interview, $20 for the 2-week interview, and $35 for the 3-, 6-, and 9-month interviews, with a $25 bonus if they completed all five assessments and returned the EDM. Tracking efforts included unscripted reminder phone calls 1 week and 1 day before each scheduled interview appointment and mailed correspondence in the face of non-response to phone calls.
Peer Support Arm
Clinic patients who were HIV-positive and currently on HAART served as “peers” who provided medication-related social support in the peer support arm. Medical providers in the clinic assisted study staff in identifying appropriate peers from among current patients who reported consistently high levels of adherence, attended clinic appointments regularly, were socially skilled, and were able to participate in an initial training and commit to ongoing supervision. Research staff (i.e., the principal investigator, psychology doctoral candidates, and clinical staff including a pharmacist, medical provider, nutritionist, and case manager) provided approximately 15 hours of training to a total of 17 peers during the course of the study. During the trainings, peers learned how to assess for negative affective states and other barriers to adherence and to sensitively provide emotional, informational, and affirmational social support. Other topics covered in the training included an overview of HIV and HAART, how to maintain appropriate limits on the peer relationship, overcoming potential barriers to the acceptance of support, harm reduction approaches to substance use, appropriate referrals for medical inquiries, and strategies for working with diverse participants. Peers received $25 – $40 twice monthly as an incentive for their involvement based on the number of participants (1 – 3) to whom they were assigned as peers. On average, peers remained actively involved for approximately 1 year.
The 3-month peer support intervention consisted of two parts: (a) six twice-monthly one-hour gatherings (i.e., “peer meetings”) held at the clinic, consisting of all peers and actively enrolled participants in the peer support arm and (b) weekly phone calls from peers to participants. At the time of enrollment, participants were assigned to individual peers by study staff based on peer availability and presumed compatibility (i.e., whenever possible, an effort was made to match peers to participants based on race/ethnicity, sex, and sexual orientation). In the group setting, participants had the opportunity to interact face-to-face with their assigned peer as well as meet the other peers and participants, with the goal of benefiting from the discussion of the shared experiences of the group. The meetings were designed to identify barriers to HAART adherence and generate problem-solving strategies to overcome them. Other emerging themes included life issues that impact adherence, including HIV status disclosure, dating, substance use, and struggles with mental health issues. One of several research staff members (each with graduate training in psychology) coordinated the groups by making reminder phone calls, preparing the meeting room, and providing refreshments. With assistance from the peers, they facilitated the meetings by refocusing the discussion on adherence-related topics when appropriate. Otherwise, they refrained from interfering with the group process and the exchange of support among peers and participants, resulting in predominantly peer-led groups. Participants received a $15 incentive for attending each of the six sessions. All were welcomed to continue attending thereafter but were provided neither reminder phone calls nor monetary incentives for additional meetings.
Between group meetings, peers were instructed to call each of their study participants weekly to provide more in-depth one-on-one attention and feedback. Phone calls also were better suited for participants with confidentiality concerns and those who had difficulty traveling to the clinic or had scheduling conflicts with the set meeting times. Peers were instructed not to initiate contact with participants following the 3-month intervention period, but they were allowed to respond to requests for contact from the participants at their own discretion.
In order to preserve and monitor intervention fidelity, peers were evaluated at the end of the training to ensure they acquired competency in their respective tasks. Peers also received ongoing supervision from the group facilitator after each group meeting and at twice-monthly telephone check-ins. Additionally, they completed a one-page log for each telephone and face-to-face contact with a participant. These logs were reviewed with project staff during supervision.
Pager Messaging Arm
The 3-month pager intervention consisted of a customized pager system that combined the communicating abilities of the World Wide Web and two-way pagers. A computer program allowed for the timing of all text messages to be specified up front and then sent automatically without any further intervention from study staff. At randomization, the study coordinator distributed a two-way pager and customized a message schedule to the participant’s daily medication regimen, which was confirmed by the clinic pharmacist. In addition to dose reminders (which included medication names familiar to the patient and number of pills to be taken), three other types of text messages were sent: (1) educational (referring to side effects and their management, medication benefits, understanding laboratory values, the importance of adherence, drug interactions, proper medication storage, resistance, and self-advocacy); (2) entertainment (jokes or thoughts for the day), and (3) adherence assessments. There was some flexibility in the frequency and content of messages to accommodate participants’ differing needs and schedules, but there was a minimum of three pager messages sent daily to participants for the first two months. Pages gradually tapered in the last month of the 3-month intervention to avoid a rebound in nonadherence. A confirmation return page was requested for every message sent. Project staff periodically reviewed the return page log and if no responses were received, the participant could be called to determine if they were having any problems with the pager.
Between the first contact and the end of the 3-month intervention, the participants were asked to wear the pager at all waking moments. Participants had to use the study pagers and could not substitute with their own pager or cell phone. They were instructed not to use the devices for personal use. Participants could select from a variety of auditory alarms or a vibrate-only option if desired. If the pager was lost or failed it was promptly replaced at no cost to the subject.
Usual Care
Before initiation of HAART, all clinic patients who are naïve patients or off HAART for more than six months are required to complete the HAART Protocol, a clinic-based program designed to provide education regarding HAART and adherence as well as to identify and correct adherence barriers prior to HAART initiation. The HAART Protocol involves a series of three separate appointments with a pharmacist, nutritionist, and case manager after an initial meeting with a provider and before a final meeting when the provider decides whether to prescribe HAART. During these preliminary interviews, patients are given social and mental health referrals as appropriate, a daily medication schedule, and information on medication side effects and techniques to help manage them. All participants in each of the study’s four arms participated in the HAART Protocol if they had not received it in the last six months. There were no time or attention control activities.
Outcome Measures
The primary outcome measures were self-reported and EDM-based medication adherence at 3 months (immediate post-intervention) and at 6 and 9 months (post intervention follow-ups). The secondary outcomes were the clinical laboratory biomarkers of CD4 lymphocyte count and VL at 3, 6, and 9 months.
Self-reported medication adherence was assessed with one item from the Simplified Medication Adherence Questionnaire (SMAQ)14 assessing the number of doses missed during the previous week (none of the time, 1–2 times, 3–5 times, or 6–10 times). A dichotomous variable for past-week 100% adherence was created with participants classified as perfectly adherent if they missed zero doses and non-adherent if they missed 1 or more doses.10, 15 We computed a continuous EDM adherence variable, defined as the number of bottle openings recorded by the MEMS cap during the 7 days before each assessment date divided by the number of prescribed doses. The EDM adherence outcome was analyzed as a continuous variable and as dichotomous variables with cut-offs at 80, 85, 90, 95, and 100% at each assessment point and across all three follow-up assessments.
Study staff abstracted VL and CD4 lymphocyte counts from patient medical records if they were available within 30 days of the baseline, 3, 6, and 9-month interviews; otherwise, blood draws for VL and CD4 counts were conducted the day of the interview. As VL data were not normally distributed, we conducted a log transformation and used the transformed values in all further analyses. Clinical outcomes were analyzed as continuous variables and as dichotomous variables with cut-offs for VL of undetectable, 1,000, and 10,000 and for CD4 of 350 at each assessment point and across all three follow-up assessments.
Power Analyses
Initial power analyses were based on a dichotomous measure of adherence as the outcome and .80 power to detect a 15–20% difference in the percentage of 100% adherent patients. An analysis “at the margins” was planned whereby all patients treated with one intervention are compared to all patients not treated with the intervention. For example, in evaluating the peer support intervention, all participants in the peer support arm and those in the peer support and pager arm would be compared with those in the pager only and usual care arms. This method maximizes the statistical power for detecting intervention effects when analyzing a factorial trial.16 These power calculations indicated that 120 patients were required to receive each intervention, resulting in a total sample of 240.
Statistical Methods
All participants who were randomly assigned were included in the analyses (i.e., an intent-to-treat approach), with adjustments made only in the case of two participants who were dropped from the study when they were determined during the baseline interview to be ineligible. Missing data were set equal to zero for the primary adherence outcomes and the detection limits for the secondary biomarker outcomes.
To evaluate efficacy, repeated-measures analyses were conducted using generalized estimating equations (GEE).17 In these analyses, each outcome was regressed on pager arm, peer support arm, pager × peer support arm, time (assessment point), all arm × time interactions, and previous experience with HAART as a control variable (i.e., HAART naïve or not). Time was partitioned into contrasts of 2-week assessment point (or, in the case of the biomedical outcomes, baseline) against each follow-up (i.e., 2 weeks vs. 3, 6, and 9 months). The statistical tests of the intervention effects were the peer support × time and pager support × time interactions. A logit link function was utilized for the binary outcomes and Gaussian link function for continuous outcomes. An unstructured working correlation structure was specified to account for correlated outcome data across assessments.
To assess whether an analysis “at the margins” was appropriate, the full factorial models were compared to models that excluded any interaction effects between the pager and peer support interventions. Wald tests were performed to evaluate whether the pager × peer support and pager × peer support × time terms were collectively significant for each primary and secondary outcome. Analyses conducted at the margins were comparable to the full factorial models for 3 out of the 4 outcomes, so results from the analyses “at the margins” are reported. All trend-level findings at p < .12 are reported for interpretive purposes.
Additional post hoc analyses evaluated whether dichotomous versions of the primary (EDM adherence) and secondary (VL, CD4 count) outcomes would be associated with the interventions. In the first set of analyses, we substituted dichotomous version of EDM adherence, VL, and CD4 count in the GEE analyses. In the second set of analyses, we used a stricter dichotomous outcome that required meeting the dichotomous outcome across all follow-up assessments (e.g., EDM adherence > 80% at 3, 6, and 9 months). In these analyses, the strict dichotomous outcome for (i.e., meeting the cutoff at all follow-up assessments), was regressed on peer support, pager support, and previous experience with HAART and baseline levels as control variables.
In further post hoc analyses, we examined whether the number of mandatory peer support meetings attended or pager response rate was associated with intervention effectiveness among those in the experimental arms. In these quasi dose response analyses, each outcome was regressed on dose, time (assessment point), dose × time, and previous experience with HAART and baseline levels as a control variable. The statistical test of the dose response effect for each outcome was the dose × time interaction.
Results
Flow of participants
As seen in the Figure, of the 909 patients screened for eligibility, 308 (34%) did not meet study eligibility criteria, mainly because they were not currently prescribed HAART, not switching at least two medications, or were severely psychologically or cognitively impaired. A minority (232; 26%) of eligible patients approached declined to participate. The primary reasons cited for refusal were lacking interest, being too busy, having transportation difficulties that made it difficult to accommodate the additional study-related clinic visits, and feeling uncomfortable in groups. Other participants (143; 16%) were excluded because they died or could not be re-contacted prior to enrollment. Across the four assessment points, 79% of participants had complete data, 13% missed a single assessment, and 9% missed two or more assessments. No significant differences were found in retention by study arm.
Figure 1.
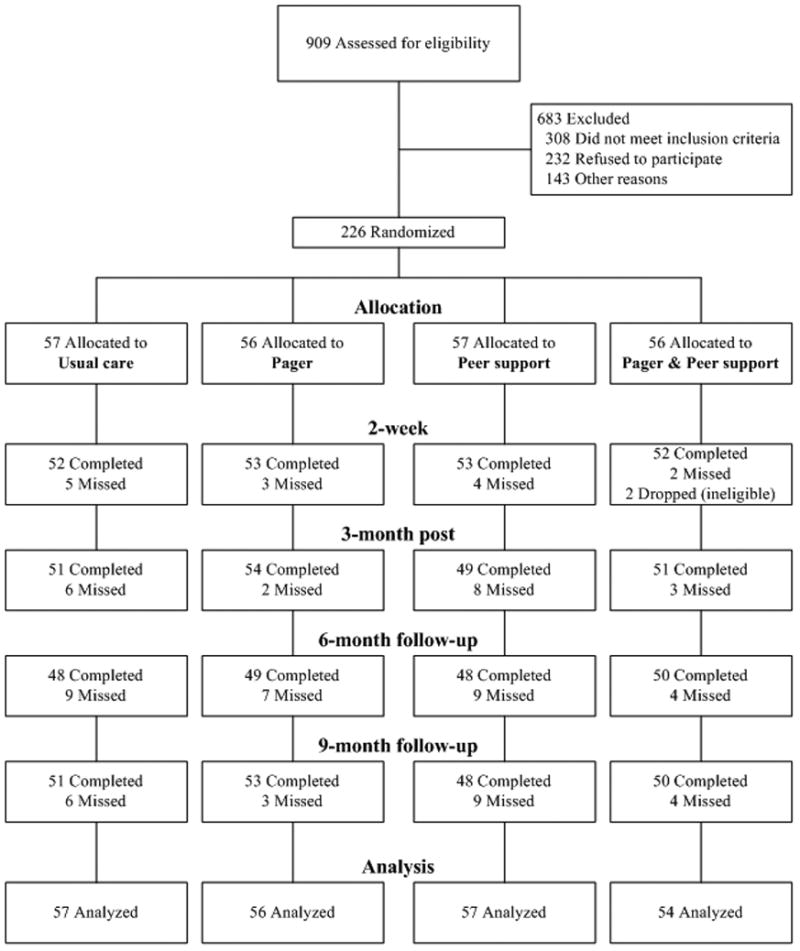
Flow chart of participants
Description of Participants
The final analytic sample of 224 patients ranged in age from 19 to 60 years (M/SD = 40.0/8.2) at baseline and were mostly (76%) male. On average, participants had been diagnosed with HIV for 9 years; 62% were HAART naïve at baseline and 38% were switching or restarting an ART regimen. The sample was 30% African American, 11% Hispanic, 47% White, and 12% of other or mixed racial heritage. The sample was fairly well educated (71% had a high school degree or GED and 9% had a college degree) and 81% were employed full- or part-time). Nearly half (44%) reported injection drug use at some point in the past.
With respect to baseline medication, 59% of participants were on a once daily regimen and 41% were on a twice daily regimen. Participants were prescribed between 2 and 5 antiretroviral products, either individual or combination tablets (M/SD = 3.0/0.8), with 64% on a protease inhibitor (PI) based regimen, 32% on a non-nucleoside reverse transcriptase inhibitor (NNRTI) based regimen, 4% on both PI(s) and a NNRTI, and 1% using a fusion inhibitor.
Baseline comparison of participants in the four arms demonstrated that randomization was successful. No important differences were found on any socio-demographic, regimen, or outcome variable except that participants in both the pager (M/SD = 7.7/6.9, t(111)= 1.99, p= .05) and peer support arms (M/SD = 7.0/5.6, t(112) = 2.77, p = .01) received their HIV diagnosis fewer years ago than those receiving standard of care (M/SD = 10.3/6.9).
Intervention Participation
Among the 111 participants randomized to the two arms including the peer intervention, attendance at the twice-monthly meetings ranged from 0 to all 6 meetings (M/SD = 2.8/2.2); 54% attended 3 or more of the 6 required meetings. An additional 36 participants attended from 1 to 12 of the additional voluntary meetings as well (M/SD = 1.5/1.9).
Among the 110 participants randomized to the two arms including the pager intervention, the average number of pages received ranged from 0 to 343 (M/SD = 157.0/71.0). The average response rate was 41% (SD = 31.1%) as measured by the proportion of messages that participants acknowledged receipt out of the total received. A total of 8 participants in the pager arm did not use the pager at all.18
Missing Data
To assess for differences between participants with complete self-report data (79%), those who missed a single assessment (13%), and those who missed two or more assessments (9%), χ2 tests and one-way ANOVAs were conducted on categorical and continuous socio-demographic characteristics, respectively. No significant differences were found among these three groups. Missing EDM adherence data were tabulated separately as this data could be available even if a self-report assessment was missing. For EDM adherence, 88% of participants had complete data, 5% were missing a single assessment, and 8% were missing two or more assessments. No significant differences were found among these three groups.
Adherence Levels
Self-reported and EDM adherence data at 2 weeks and 3, 6, and 9 months are presented in the Table. Note these are the findings for each of the experimental arms, but in the analyses of intervention effects, these groups were combined “at the margins” as described above. Overall, average adherence was low at initiation and slowly declined over time with 70% of participants at 2 weeks, 58% at 3 months, 51% at 6 months, and 49% at 9 months self-reporting 100% adherence in the past seven days. Descriptive data from the “at the margins” analyses indicated that among those in the two peer intervention arms, the percentage self-reporting 100% adherence in the past seven days at 2 weeks and 3, 6, and 9 months was 67%, 63%, 49%, and 47%, compared with 73%, 52%, 53%, and 51% for those not receiving the peer intervention. Among those in the two pager intervention arms, the percentage self-reporting 100% adherence in the past seven days at 2 weeks and 3, 6, and 9 months was 75%, 64%, 49%, and 55%, compared with 64%, 52%, 53%, and 44% for those not receiving the pager intervention.
With respect to EDM data, the mean percentage of doses taken in the past seven days was 63% at 2 weeks, 44% at 3 months, 38% at 6 months, and 33% at 9 months. Among those in the two peer intervention arms, the percentage of doses taken in the past seven days at 2 weeks, 3, 6, and 9 months was 62%, 49%, 36%, and 32%, compared with 63%, 40%, 39%, and 33% for those not receiving the intervention. Among those in the pager intervention arms, the percentage of doses taken in the past seven days at 2 weeks, 3, 6, and 9 months was 63%, 46%, 36%, and 34%, compared with 62%, 43%, 39%, and 31% for those not receiving the intervention.
Evaluation of Intervention Effects
Peer intervention
With respect to the dichotomous self-report 100% adherence outcome, GEE at-the-margins analyses revealed a significant peer support × time (2 weeks vs. 3 months) interaction indicating a two-fold increase in the odds of 100% adherence for those receiving versus not receiving peer support at post-intervention (OR = 2.10, SE = 0.69, 95% CI = 1.10 – 4.01, p = .02). This effect was partially supported by findings from the EDM dose adherence continuous outcome measure, for which there was a trend toward a peer support × time (2 weeks vs. 3 months) interaction suggesting that peer support (versus no peer support) was associated with 9% higher adherence at post-intervention (Est = 8.88, SE = 5.60, 95% CI = −2.09 – 19.85, p = 0.11). The findings for the efficacy of peer support at post-intervention did not persist at the 6-and 9-month follow-ups for either self-reported (p’s > .77) or EDM adherence (p’s > .74); nor were there any peer intervention effects for the secondary outcomes of VL (p’s > .70) or CD4 (p’s > .66).
Pager intervention
According to the self-report measure, pager support did not predict improved odds of 100% adherence at 3 or 9 months (p’s > .74). However, pager support was associated with a marginally significant decrease in 100% adherence at 6 months (OR = 0.50, SE = 0.18, 95% CI = 0.24 – 1.03, p = .06). Analyses with the EDM dose adherence continuous outcome measure also indicated no effect for pager support at 3, 6, or 9 months (p’s > .54). There were no intervention effects (i.e., pager support × time interactions) at any time point for either of the secondary outcomes of log10 VL (p’s > .17) and CD4 count (p’s > .12) as continuous variables.
Post Hoc Analyses
Peer intervention
Analyses of the primary EDM adherence and secondary VL and CD4 outcomes as dichotomous variables revealed no intervention effects for peer support at each time point (p’s > .19) or when the outcome was evaluated more strictly across time points (p’s > .50).
Dose response analyses indicated that attendance at the peer meetings did not predict differences in either the self-report or EDM adherence outcomes (p’s > .50). However, associations emerged between attendance and the secondary clinical outcomes. Greater attendance had a marginal association with reduced log10 VL at 3 months (Est = −0.13, SE = 0.08, 95% CI = −0.28 – 0.02, p= 0.08) and 6 months (Est = −0.17, SE = .09, 95% CI = −0.34 – 0.01, p = .06). At 9-month assessment, greater attendance predicted significant reductions in log10 VL (Est = −0.22, SE = 0.08, 95% CI = −0.38 – −0.06, p = .01). Attendance had a marginal association with elevated CD4 count at 3 months (Est = 12.35, SE = 6.67, 95% CI = −0.72 – 25.42, p = .06).
Pager intervention
Analyses of the clinical outcomes revealed effects for pager support when (1) CD4 count was analyzed at each time point using a 350 cells/mm3 cutoff and (2) viral load was evaluated using the strict criteria of remaining below 1000 copies/mL across all 3 follow-up time points, controlling for baseline. With respect to the dichotomous CD4 measure, there was a two-fold greater odds (OR = 2.20, SE = 0.78, 95% CI = 1.10 – 4.42, p = .03) of possessing a CD4 count above 350 cells/mm3 for those receiving versus not receiving pager support at post-intervention. At 9-month follow-up, pager participants were marginally more likely to have CD4 count above 350 cells/mm3 (OR = 1.94, SE = 0.74, 95% CI = 0.92 – 4.08, p = .08). With respect to VL as a dichotomous measure, pager participants had a nearly two-fold greater odds of reporting VL less than 1000 copies/mL at all 3 follow-ups (OR = 1.78, SE = 0.50, 95% CI = 1.03 – 3.09, p = .04) than those not receiving a pager.
Dose response analyses indicated that increased percentage of pager response was associated with lower EDM adherence at 6 months (Est = −0.30, SE = 0.13, 95% CI = −.34 – .01, p = .06). Greater pager response predicted significant reductions in log10 VL at 3 months (Est = −0.01, SE = 0.01, 95% CI = −0.024 – −0.004, p= 0.01) and 9-months (Est = −0.01, SE = .01, 95% CI = −0.025 – 0.003, p = .01). Additionally, pager response predicted significant increases in CD4 count at 3 months (Est = 1.84, SE = 0.39, 95% CI = 1.08 – 2.60, p < .001), 6 months (Est = 1.15, SE = 0.53, 95% CI = 0.11 – 2.20, p = .03), and 9-months (Est = 2.38, SE = 0.52, 95% CI = 1.37 – 3.39, p < .001).
Discussion
In an effort to address the widespread problem of non-adherence to antiretroviral medication, we developed peer and pager support interventions and evaluated them among a sample of 224 men and women on HAART in a publicly funded clinic in Seattle, Washington. Findings from intent-to-treat, missing equals failure analyses indicated a two-fold greater odds of self-reported 100% medication adherence at immediate post-intervention among individuals receiving peer support versus not receiving peer support. EDM data partially supported this finding. However, neither effect was maintained at the follow-ups and there was no effect of the peer intervention on the secondary outcomes of CD4 count or VL at any time point. Post hoc analyses indicated that, among participants receiving the peer intervention, increasing attendance at the peer meetings was associated with some clinical benefit (but not improved adherence).
These findings suggest that receiving information, emotional, and affirmation support from peers might promote adherence, but that effect did not persist when the support was discontinued. Ongoing social support, perhaps from individuals more integrated into the participants’ lives, may hold greater promise in promoting and sustaining long-term adherence. Indeed, Remien et al.9 demonstrated that incorporating a partner in a 4-week couples-based intervention led to increases in adherence over usual care. It is likely that the more intensive and structured intervention in that study, delivered by a highly skilled counselor, contributed as well to its efficacy. The lack of peer support’s impact on clinical outcomes, despite associated improvements in adherence, may be due to its small and short-lived effects.
In contrast, the pager messaging intervention was associated with some clinical improvement, which partially persisted over time, even though it did not appear to be successful in promoting adherence at any time point. Dose response post hoc analyses indicated that, among pager participants, greater response to pagers was associated with better clinical outcomes. Although the pager message could serve as a reminder to take a dose at the scheduled time of the dose, this assistance alone apparently was not enough to improve adherence. Adherence involves a complex set of behaviors that includes maintaining a supply of medication that is available at the prescribed dosing time and being in a situation at that time in which medication ingestion is feasible. The success of the pager as well depends on the participant’s maintaining the pager in working order (with operating batteries) and carrying it at all times. Moreover, the pagers were not used in this study to link participants to study personnel; they functioned as high technology reminder systems. It is possible that more individualized pages from a known study staff member might solidify a personal connection and be more effective in promoting long-term adherence. Any of these factors may explain the inconsistent findings in the literature regarding the efficacy of reminder devices to promote adherence.12, 19
How do we explain the post hoc findings of an association between both peer meeting attendance and pager messaging on clinical outcomes in the absence of improvements in adherence? One possibility lies in the difficulty of reliably and validly capturing adherence behavior. Although adherence is generally associated with VL,15 it is possible that our operationalization of adherence did not capture improvements in adherence that led to better clinical outcomes. For instance, perhaps a more finely tuned assessment of the timing of medication taking or the extent of drug holidays may have captured the benefits of the pager intervention that led to improved VL and CD4 count.
One caveat involves the trend for worsened adherence at 6 months among participants in the pager arms versus others. Although the pagers were tapered to reduce an iatrogenic rebound effect, these data suggest the need for greater attention to compensatory strategies when such alarms are removed. Alternatively, alarm interventions might be viewed as continuously needed, at least until the participants themselves opt to discontinue their use. Note this strategy was used successfully in the peer intervention, where participants were allowed to continue attending group meetings after the 3-month period at their own discretion.
One possible explanation for the lack of stronger intervention effects may be the intensive usual care provided by the clinic. Prior to initiating their HAART regimens, all HAART naïve patients or those off medications for more than 6 months are required to undergo a series of appointments with various staff at the clinic to identify and address potential barriers to adherence. There is some evidence that this approach itself might lead to improvements in adherence,20 setting a high standard that the intervention arm could not surpass. Relatedly, post-intervention interviews indicated that some participants in the usual care arm reported benefitting from the sustained contact with study staff and considered their EDM device to be a potent visual reminder to take their medications.
Another possibility is that not all participants got a full “dose” of either intervention, because they failed to attend peer support group meetings, return the phone calls of their peers, or consistently use their pagers. It should be noted that peer support participants who attended more group meetings as well as pager participant who responded more consistently to their pagers had improved clinical outcomes. This suggests that the peer support intervention was less effective when participants missed the group meetings and pager messaging requires active participation. However, to be successful, any adherence intervention must limit the burden to patients who are often already overwhelmed with caring for their HIV disease and other life responsibilities.
The findings are consistent with recent reviews of the field. In two meta-analyses, behavioral interventions to improve HAART adherence have been shown to have positive though minor effects on adherence and clinical outcomes that diminish over time.7, 8 Perhaps interventions need to be more highly tailored and, like adherence behavior itself, dynamic over time. Perhaps offering individuals a choice of intervention strategies would be more beneficial as well.
There were some limitations to the present study. Our eligibility criteria were relatively broad. For example, we did not exclude participants who were already resistant to medications, which would make it difficult to demonstrate effects on VL even among participants who were adherent to their medications. Also, we did not exclude individuals whose adherence was already satisfactory; these participants may have been less motivated to fully engage in the interventions and would affect our power to find a significant intervention effect. Indeed, in their meta-analysis, Amico and colleagues noted that interventions targeting participants with known or anticipated problems with ART adherence demonstrated larger effects on adherence than interventions that did not.7 With respect to methodology, adherence assessment strategy appeared to influence our estimates of intervention effects, with self-reported data indicating a larger effect size than did EDM data for the peer intervention. It is possible that participants in the intervention arm were subject to a “social desirability” bias and, after receiving an intervention obviously designed to enhance their adherence, would be more likely to report improved adherence than participants in the usual care arm. Given this possibility, future studies would do well to employ triangulation approaches to adherence, as we did, using multiple methods and avoiding reliance solely on self-report. Also, our post hoc analyses suggesting improvements in clinical outcomes for pager participants suggests that creating more stringent outcome measures (i.e., the achievement of a pre-determined cut-off at each of successive assessment points) may capture small effects. This would be especially helpful in the presence of ceiling effects, which are common in self-reported adherence.
The findings can likely generalize to other outpatient HIV clinic populations in the U.S., although our participants included fewer ethnic minorities and were more highly educated than most HIV-positive individuals in the USA. In international settings, peers may be even more influential given the greater stigma of HIV that inhibits disclosure. For example, one study in Mozambique utilizing peer-delivered modified directly observed therapy led to significantly higher adherence.10 Also, almost one third of eligible patient declined to participate in our intervention, suggesting that the findings may be generalizable to a subgroup of patients who are open to disclosing their HIV infection and receptive to social support. Perhaps targeting such individuals would result in greater adherence to the intervention and more positive effects.
Optimal intervention designs might involve conducting needs assessments at baseline and offering a range of potentially appealing and useful strategies to enhance adherence. Maintaining optimal adherence requires a set of complex and dynamic behaviors that may necessitate interventions of equal sophistication with personnel trained and dedicated to providing them on an ongoing basis.
Table 1.
Data on Adherence and Clinical Outcomes of Peer and Page Support Interventions by Arm at all Assessment Points
| All participants | Usual care participants | Pager Arm participants | Peer Arm participants | Pager and Peer Arm participants | |
|---|---|---|---|---|---|
| M(SD) N = 224 | M(SD) n = 57 | M(SD) n = 56 | M(SD) n = 57 | M(SD) n = 54 | |
| Dose Adherence | |||||
| Self-Report 100% (1-week) | |||||
| 2 weeks | 69.6 | 64.9 | 80.4 | 63.2 | 70.4 |
| 3 Months | 57.6 | 47.4 | 57.1 | 56.1 | 70.4 |
| 6 Months | 50.9 | 63.2 | 42.9 | 42.1 | 55.6 |
| 9 Months | 49.1 | 43.9 | 58.9 | 43.9 | 50.0 |
| EDM % (1-week) | |||||
| 2 weeks | 62.7 (41.2) | 63.0 (41.5) | 63.0 (41.9) | 61.7 (41.2) | 63.2 (41.1) |
| 3 Months | 44.4 (42.8) | 38.7 (43.0) | 41.8 (42.6) | 47.0 (44.6) | 50.1 (41.2) |
| 6 Months | 37.7 (42.8) | 41.0 (44.0) | 36.9 (42.6) | 37.2 (44.5) | 35.4 (40.9) |
| 9 Months | 32.5 (41.5) | 29.1 (39.7) | 36.5 (44.2) | 32.3 (42.5) | 32.1 (40.0) |
| HIV-1 RNA viral load (Log10 copies/ml) | |||||
| Baseline | 4.4 (1.3) | 4.3 (1.2) | 4.5 (1.2) | 4.6 (1.2) | 4.3 (1.4) |
| 3 Months | 3.1 (1.7) | 3.2 (1.7) | 3.1 (1.8) | 3.3 (1.8) | 2.8 (1.6) |
| 6 Months | 3.3 (1.9) | 3.0 (1.7) | 3.4 (1.9) | 3.8 (2.0) | 3.0 (1.7) |
| 9 Months | 3.4 (1.9) | 3.5 (1.9) | 3.2 (1.8) | 3.5 (2.0) | 3.1 (1.8) |
| Across 3 follow-upsa | 39.3% | 31.6% | 42.9% | 33.3% | 50.0% |
| CD4 count (cells/mm3) | |||||
| Baseline | 204.5 (166.8) | 198.5 (160.4) | 194.3 (149.8) | 229.2 (153.1) | 195.4 (202.3) |
| 3 Months | 246.3 (215.9) | 232.5 (204.2) | 256.2 (223.2) | 246.6 (209.4) | 250.3 (231.7) |
| 6 Months | 248.4 (218.2) | 255.6 (222.8) | 235.3 (214.2) | 259.6 (238.8) | 242.4 (199.1) |
| 9 Months | 259.0 (234.9) | 243.5 (216.7) | 254.6 (206.6) | 280.0 (258.2) | 257.7 (258.9) |
| Across 3 follow-upsb | 17.0% | 12.3% | 19.6% | 21.1% | 14.8% |
Percentage of participants with HIV-1 RNA viral load < 1000 copies/ml at all three follow-up assessments
Percentage with CD4 count > 350 cells/mm3 at all three follow-up assessment
Acknowledgments
We thank clinic staff Carol Glenn, Robert Harrington, and Bobby Santucci; all other research assistants and staff; and all participants – especially the trained peers.
Sources of support
This research was supported by a National Institute of Mental Health Grant (R01 MH58986) to Jane M. Simoni. This publication resulted, in part, from research supported by the University of Washington Center for AIDS Research (CFAR), a NIH-funded program (P30 AI 27757).
Footnotes
Meetings at which preliminary findings were presented
2nd International Conference on HIV Treatment Adherence, Jersey City, NJ, March 2007
4th International Conference on HIV Treatment Adherence, Miami, FL, April 2009
References
- 1.Crum NF, Riffenburgh RH, Wegner S, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006 Feb;41(2):194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 2.Jones J, Taylor B, Wilkin TJ, Hammer SM. Advances in antiretroviral therapy. Top HIV Med. 2007 Apr–May;15(2):48–82. [PubMed] [Google Scholar]
- 3.Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006 Jul;194(1):11–19. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett J. Addressing the challenges of adherence. J Acquir Immune Defic Syndr. 2002 Feb;29 (Suppl 1):S2–10. doi: 10.1097/00126334-200202011-00002. [DOI] [PubMed] [Google Scholar]
- 5.Mills EJ, Nachega JB, Bangsberg DR, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006 Nov;3(11):e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nieuwkerk PT, Sprangers MA, Burger DM, et al. Limited patient adherence to highly active antiretroviral therapy for HIV-1 infection in an observational cohort study. Arch Intern Med. 2001 Sep;161(16):1962–1968. doi: 10.1001/archinte.161.16.1962. [DOI] [PubMed] [Google Scholar]
- 7.Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: a research synthesis of trials, 1996 to 2004. J Acquir Immune Defic Syndr. 2006 Mar;41(3):285–297. doi: 10.1097/01.qai.0000197870.99196.ea. [DOI] [PubMed] [Google Scholar]
- 8.Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr. 2006 Dec;43(Suppl 1):S23–35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remien RH, Stirratt MJ, Dolezal C, et al. Couple-focused support to improve HIV medication adherence: a randomized controlled trial. AIDS. 2005 May;19(8):807–814. doi: 10.1097/01.aids.0000168975.44219.45. [DOI] [PubMed] [Google Scholar]
- 10.Pearson CR, Micek MA, Simoni JM, et al. Randomized control trial of peer-delivered, modified directly observed therapy for HAART in Mozambique. J Acquir Immune Defic Syndr. 2007 Oct;46(2):238–244. doi: 10.1097/QAI.0b013e318153f7ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Safren SA, Otto MW, Worth JL, et al. Two strategies to increase adherence to HIV antiretroviral medication: life-steps and medication monitoring. Behav Res Ther. 2001 Oct;39(10):1151–1162. doi: 10.1016/s0005-7967(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 12.Wise J, Operario D. Use of electronic reminder devices to improve adherence to antiretroviral therapy: a systematic review. AIDS Patient Care STDS. 2008 Jun;22(6):495–504. doi: 10.1089/apc.2007.0180. [DOI] [PubMed] [Google Scholar]
- 13.Simoni JM, Pantalone DW, Plummer MD, Huang B. A randomized controlled trial of a peer support intervention targeting antiretroviral medication adherence and depressive symptomatology in HIV-positive men and women. Health Psychol. 2007 Jul;26(4):488–495. doi: 10.1037/0278-6133.26.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knobel H, Alonso J, Casado JL, et al. Validation of a simplified medication adherence questionnaire in a large cohort of HIV-infected patients: the GEEMA Study. AIDS. 2002 Mar;16(4):605–613. doi: 10.1097/00002030-200203080-00012. [DOI] [PubMed] [Google Scholar]
- 15.Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS Behav. 2006 May;10(3):227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAlister FA, Straus SE, Sackett DL, Altman DG. Analysis and reporting of factorial trials: a systematic review. JAMA. 2003 May;289(19):2545–2553. doi: 10.1001/jama.289.19.2545. [DOI] [PubMed] [Google Scholar]
- 17.Liang K, Zeger SL. Longitudinal Data Analysis Using Generalized Linear Models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 18.Harris LT, Lehavot K, Simoni JM, Huh D, Andrasik MP, Dunbar PJ. Two-way text messaging for health behavior change: How can we engage the user? doi: 10.1089/tmj.2010.0050. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Operario D, Smith CD, Kegeles S. Social and psychological context for HIV risk in non-gay-identified African American men who have sex with men. AIDS Educ Prev. 2008 Aug;20(4):347–359. doi: 10.1521/aeap.2008.20.4.347. [DOI] [PubMed] [Google Scholar]
- 20.Frick P, Tapia K, Grant P, Novotny M, Kerzee J. The effect of a multidisciplinary program on HAART adherence. AIDS Patient Care STDs. 2006 July;20(7):511–524. doi: 10.1089/apc.2006.20.511. [DOI] [PubMed] [Google Scholar]


