Abstract
Mechanisms for identifying appropriate mating partners are required for any species to survive. In many types of animals, males employ multiple sensory modalities to initially search for females and to subsequently determine if they are fit and/or receptive. In this paper we will detail the multiple types of sensory information that are used to initiate and drive courtship in Drosophila melanogaster and discuss the importance of context in the interpretation of chemosensory cues. We find that food-related olfactory cues increase the salience of the aversive pheromone cis-vaccenyl acetate.
Keywords: Drosophila, olfaction, gustation, hearing, cis-vaccenyl acetate, pheromone
In Drosophila melanogaster, males respond to females by performing a stereotyped set of behaviors collectively known as “courtship.” This behavior is quickly recognizable to the observer: the male orients toward the female, he taps her with his foreleg, he sings her a courtship song by vibrating his wings, he licks her genitals, and then he attempts to copulate.1,2 If the female is receptive, this display causes her to slow her locomotion, orient toward the male, present her abdomen, and eventually to spread her wings and genital plates to allow intromission.3 Both the male and the female are integrating visual, auditory, mechanosensory, and chemosensory signals that will allow them to make a decision as to the suitability of their mate.
From the male point of view, each of the stages of active courtship is an opportunity to obtain information about the mating status of the female he is courting and, if that information is appropriate, to increase the enthusiasm with which he courts. Orienting toward the female puts him in close proximity, which is important for sensing pheromonal olfactory cues with low volatility.4 Tapping her abdomen with his foreleg allows him to taste nonvolatile cuticular compounds with gustatory receptors located in chemosensory bristles of the leg.5 Hearing courtship song stimulates both the female's receptivity6 and the male's courtship drive.7 Licking her genitalia allows him to taste a potentially different set of nonvolatile compounds.
All of these stimuli are presented over small distances, that is, they are only sensed after the male has made the decision to court and is close to the female. The decision to court is made with a separate set of cues that act over longer distances and is called “initiation.” Initiation can be investigated by measuring the time between when the male is put into the courtship chamber with a female and the first active courtship event—usually orientation. The latency to first courtship is highly dependent on the conditions under which it is measured8; it takes a male longer to find a female in a large chamber. Not surprisingly, vision plays a big role in female localization in large arenas. Turning off the lights or using males with decreased visual acuity increases initiation latency.9 Olfaction also appears to have a role, indicating that some pheromonal cues can act over distance.10 Recently we have also shown a role for hearing in initiation.11 Noise made by the female fly in the chamber can decrease latency. Unlike the chemosensory cues used in courtship, which are exquisitely species-specific and information-rich, auditory cues are completely nonspecific. Even white noise can decrease initiation latency, suggesting that noise is acting to enhance some attentional process that either stimulates the male to search or increases his sensitivity to female-specific cues that are presented to other sensory systems.
Initiation of courtship also appears to be a stage at which courtship can be modulated by learning.10 Given that subsequent close-quarters, active courtship results in a rapid and strong increase in the vigor of the behavior, the initial decision to court is a good stage at which to regulate this behavior. Courtship conditioning,12 the ability of a male to decrease his courtship of classes of females that have been previously found to be unreceptive, has been shown to cause large increases in courtship latency, but relatively smaller changes in the intensity of courtship (as measured by bout length) once it has been started.10 This behavior has been shown to be a form of associative learning that depends on the male sensing both a stimulatory pheromone and some aversive stimulus.
In our lab we have found two types of courtship conditioning that have different aversive cues and result in different behavioral outcomes. When a mated female is used as the trainer in the “classical” paradigm, the aversive stimulus is cis-vaccenyl acetate (cVA), a lipid transferred from males to females during mating.13 The presence of cVA is a “generalizer” acting to make the male suppress courtship toward all females: mature virgins, mated females, and immature virgins (which are known to have a cuticular hydrocarbon profile distinct from mature females). This implies that the conditioned stimulus is a volatile chemosensory cue common to all types of females. When virgin females are used as trainers, the conditioned stimulus is an age-specific volatile pheromone and the unconditioned stimulus is the failure to copulate, which is apparently aversive to the male.10 Unsuccessful courtship of a virgin results in males that avoid only females of the same age as the trainer. This paradigm has been termed “trainer type-specific” courtship conditioning.
To study the associative nature of classical courtship conditioning we have used training protocols that artificially “recreate” the mated female trainer: pairing a virgin female with synthetic cVA. This allows us to manipulate conditions more precisely and probe the role of the aversive pheromone cVA. cVA is a well-studied lipid. It is made only in males,14 and is present in ejaculate. It is sensed by two different olfactory receptors, Or67d and Or65a.15 Females acquire it during mating, and retain it in their vaginal tracts where it is released during ovipositor extrusion and egg-laying. The biological role of cVA is diverse. Early studies suggested that it was an attractant, and that cVA found on eggs laid at food sites caused aggregation of flies.16 Subsequently, cVA has been shown to be attractive in a Y-maze paradigm only in the context of propionic acid, a volatile found in fermenting organics.17 This attractive role of cVA requires Or67d.17,18 The repellent role of cVA in courtship learning is observed only in the presence of pheromone and courtship10 and has been shown by different groups to be mediated by either Or65a13 or Or67d.19 The diversity of responses to this lipid suggests that its hedonic valence may be context dependent: in the presence of food it is an attractant, in the presence of female pheromone it is aversive.
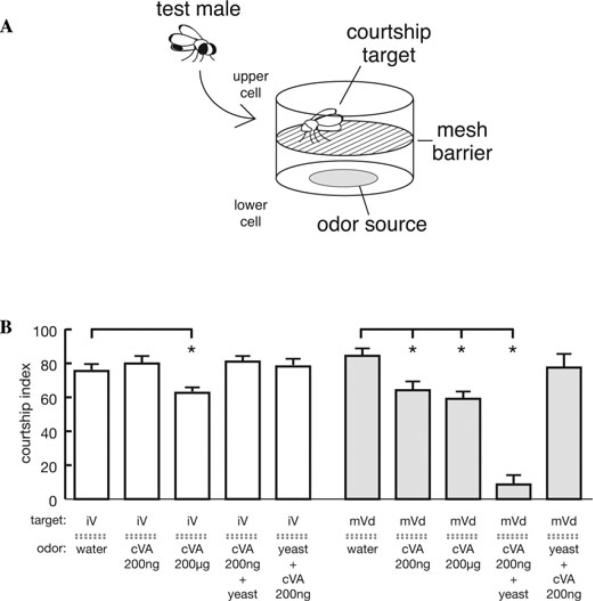
To look at these context odors, we examined the effect of cVA on initial courtship (first 10 min of exposure to a female) in the presence of different female pheromones and food odors. Figure 1A shows the behavioral apparatus. Flies were placed in the upper chamber, while odors were placed in the lower chamber. A mesh floor allowed flies to be exposed to volatile components of the compounds, but prevents direct contact with the substances, which could give gustatory information. Two types of odor presentation were done. In the first, cVA was added to the filter, which was dried, then covered in yeast paste. The yeast paste acts as a barrier to prevent cVA from volatilizing, and is functionally like presenting the yeast odor alone. The second presentation was to add the yeast paste to the filter first, then layer cVA on top. In this configuration, both yeast and cVA can volatilize and be sensed by the flies in the top of the chamber.
Figure 1.
The courtship response to cVA is modulated by both the age of the female and the presence of food odors from yeast, the fly's main food source. (A) Diagram of the behavior chamber. The male and the female are videotaped in an 8 mm diameter × 3 mm high chamber with a mesh floor. Odors are presented in a chamber of the same size below the courting pair so that they have no direct contact with odorants. (B) Courtship in the presence of pheromone and food odor (10% v/v yeast paste). Courtship index (% of a 10-min observation period spent in active courtship, measured as described8) was assessed for 4–5 day old Canton-S wild-type males presented with either a 0 day old immature virgin (iV; white bars) or a 4–5 day old decapitated mature virgin (mVd; gray bars). Odorants presented are indicted below the dotted line in the figure. Odorants (or water as a control) were applied to a disc of filter paper. The order of application for cVA and yeast paste was varied: cVA + yeast indicates cVA was put on top of yeast paste; yeast + cVA indicates cVA was applied first and covered with yeast. Data are presented as mean ± SEM and analyzed by ANOVA with posthoc test for pair-wise comparisons. * indicates P < 0.05 for the comparison to the water control value.
Figure 1B shows results for males courting immature virgins (left) and mature virgins (right). When the courtship object is immature, only very high concentrations of cVA can cause the male to suppress courtship. Addition of yeast paste did not enhance the effects of the low dose of cVA, either presented underneath or on top of the cVA. When the female target is mature; however, the effects of cVA are quite different. Both high and low concentrations of cVA can, by themselves, suppress courtship. When only the yeast paste odor is present (with cVA covered up underneath) courtship is unaffected. When a small amount of cVA is placed on top of the yeast paste so that both odors are present, there is an enhancement of the courtship suppressing effects of cVA.
Three conclusions can be drawn from these results. The first is that the type of female pheromone (immature versus mature) can affect the sensitivity of the male to cVA. This suggests that the response to cVA is in some way gated by female pheromones, consistent with our previous findings.13 The second conclusion is that mature female pheromone can alter the hedonic perception of the cVA/food pair. In noncourting flies cVA/food is attractive, but in a courting male this pairing becomes even more aversive than cVA alone. The third conclusion that can be drawn from these data is that food odor increases cVA sensitivity during courtship, but only in the context of courtship toward a mature female. These are interesting and novel observations. The adaptive significance of mated female courtship conditioning in the wild has been postulated to be that it limits the amount of futile courtship a male will do toward an unreceptive female. In the wild, courtship often occurs at feeding sites, so the enhancement of courtship suppression by food odor fits with this idea. The importance of food for survival can make it a potent modulator of sensory perception. In the rat,20 for example, food has been shown to suppress the response to pain. Further studies on the mechanisms of interaction between food odors and courtship in the fly will provide new insights into how the brain uses context to sculpt behavior.
Footnotes
Conflicts of Interest The authors declare no conflicts of interest.
References
- 1.Spieth HT. Courtship behavior in Drosophila. Ann. Rev. Entomol. 1974;19:383–406. doi: 10.1146/annurev.en.19.010174.002125. [DOI] [PubMed] [Google Scholar]
- 2.Hall JC. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- 3.Lasbleiz C, Ferveur JF, Everaert C. Courtship behavior of Drosophila melanogaster revisited. Anim Behav. 2006;72:1001–1012. [Google Scholar]
- 4.Ferveur JF. Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav. Genet. 2005;35:279–295. doi: 10.1007/s10519-005-3220-5. [DOI] [PubMed] [Google Scholar]
- 5.Bray S, Amrein H. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron. 2003;39:1019–1029. doi: 10.1016/s0896-6273(03)00542-7. [DOI] [PubMed] [Google Scholar]
- 6.Kyriacou CP, Hall JC. Learning and memory mutations impair acoustic priming of mating behavior in Drosophila. Nature. 1984;308:62–65. doi: 10.1038/308062a0. [DOI] [PubMed] [Google Scholar]
- 7.Eberl DF, Duyk GM, Perrimon N. A genetic screen for mutations that disrupt an auditory response in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 1997;94:14837–14842. doi: 10.1073/pnas.94.26.14837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ejima A, Griffith LC. Measurement of courtship behavior in Drosophila melanogaster. CSH Protocols. 2007 doi: 10.1101/pdb.prot4847. doi: 10.1101/pdb.prot4847. [DOI] [PubMed] [Google Scholar]
- 9.Joiner MA, Griffith LC. CaM kinase II and visual input modulate memory formation in the neuronal circuit controlling courtship conditioning. J. Neurosci. 1997;17:9384–9391. doi: 10.1523/JNEUROSCI.17-23-09384.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ejima A, et al. Sequential learning of pheromonal cues modulates memory consolidation in trainer-specific associative courtship conditioning. Curr. Biol. 2005;15:194–206. doi: 10.1016/j.cub.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ejima A, Griffith LC. Courtship initiation is stimulated by acoustic signals in Drosophila melanogaster. PLoS ONE. 2008;3:e3246. doi: 10.1371/journal.pone.0003246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel RW, Hall JC. Conditioned responses in courtship behavior of normal and mutant Drosophila. Proc.Natl. Acad.Sci.USA. 1979;76:565–578. doi: 10.1073/pnas.76.7.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ejima A, et al. Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr. Biol. 2007;17:599–605. doi: 10.1016/j.cub.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butterworth FM. Lipids of Drosophila: a newly detected lipid in the male. Science. 1969;163:1356–1357. doi: 10.1126/science.163.3873.1356. [DOI] [PubMed] [Google Scholar]
- 15.Van Der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr. Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartelt RJ, Schaner AM, Jackson LL. cis-vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J. Chem. Ecol. 1985;11:1747–1756. doi: 10.1007/BF01012124. [DOI] [PubMed] [Google Scholar]
- 17.Schlief ML, Wilson RI. Olfactory processing and behavior downstream from highly selective receptor neurons. Nat. Neurosci. 2007;10:623–630. doi: 10.1038/nn1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J. Neurosci. 2006;26:8727–8733. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 20.Foo H, Mason P. Sensory suppression during feeding. Proc.Natl. Acad.Sci.USA. 2005;102:16865–16869. doi: 10.1073/pnas.0506226102. [DOI] [PMC free article] [PubMed] [Google Scholar]