Abstract
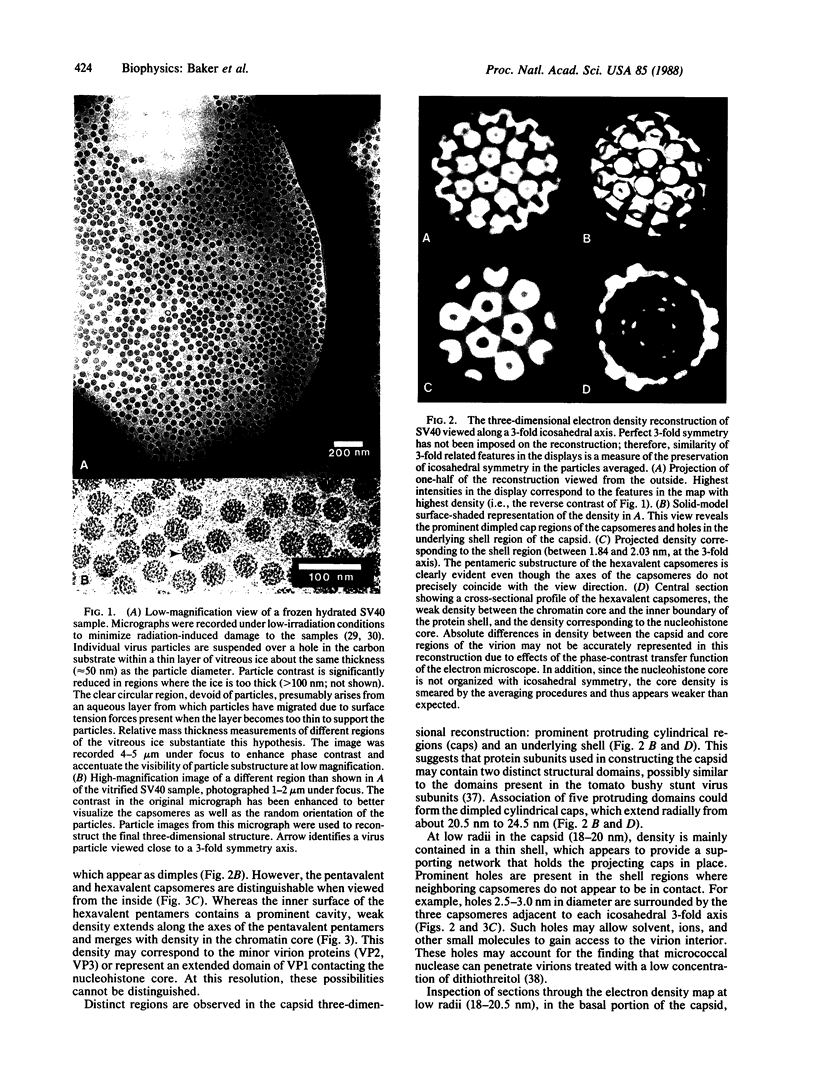
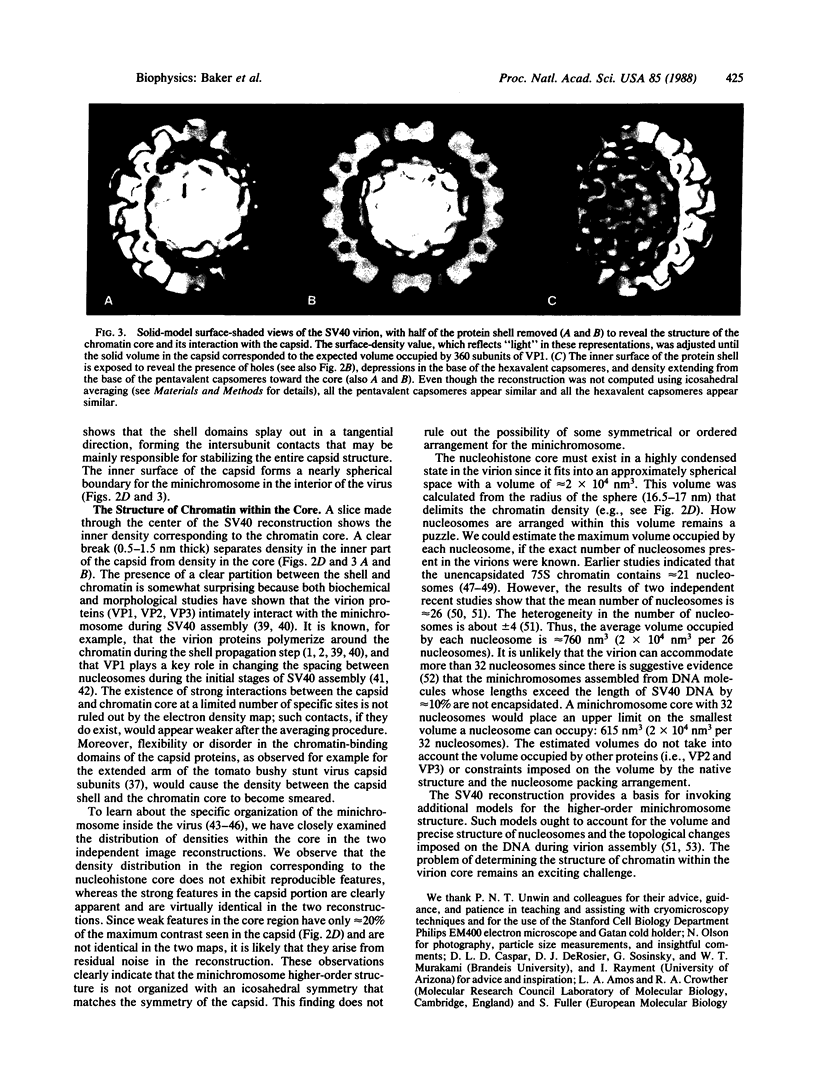
The three-dimensional structure of the capsid and the nucleohistone core of simian virus 40 (SV40) has been reconstructed by image analysis of electron micrographs of frozen hydrated samples. The 72 prominent capsomere units that comprise the T = 7d icosahedral surface lattice of the capsid are clearly resolved. Both the pentavalent and hexavalent capsomeres appear with pentameric substructure, indicating that bonding specificity in the shell is not quasi-equivalent. There is a remarkable similarity between the structure of the SV40 virion capsid and the structure reported for the polyoma empty capsid. This result establishes that (i) the unexpected pentameric substructure of the hexavalent capsomeres is also present in virions and (ii) the arrangement of the 72 pentamers in the capsid lattice may be a characteristic feature of the entire papova family of viruses. The center of the SV40 reconstruction reveals electron density corresponding to the nucleohistone core. This density is smeared, suggesting that the minichromosome is not organized with icosahedral symmetry matching the capsid symmetry. The visualization of the virion chromatin provides a basis for invoking new models for the higher order structure of the encapsidated minichromosome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian M., Dubochet J., Lepault J., McDowall A. W. Cryo-electron microscopy of viruses. Nature. 1984 Mar 1;308(5954):32–36. doi: 10.1038/308032a0. [DOI] [PubMed] [Google Scholar]
- Ambrose C., McLaughlin R., Bina M. The flexibility and topology of simian virus 40 DNA in minichromosomes. Nucleic Acids Res. 1987 May 11;15(9):3703–3721. doi: 10.1093/nar/15.9.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderer F. A., Schlumberger H. D., Koch M. A., Frank H., Eggers H. J. Structure of simian virus 40. II. Symmetry and components of the virus particle. Virology. 1967 Jul;32(3):511–523. doi: 10.1016/0042-6822(67)90303-0. [DOI] [PubMed] [Google Scholar]
- Baker T. S., Amos L. A. Structure of the tubulin dimer in zinc-induced sheets. J Mol Biol. 1978 Jul 25;123(1):89–106. doi: 10.1016/0022-2836(78)90378-9. [DOI] [PubMed] [Google Scholar]
- Baker T. S., Caspar D. L., Murakami W. T. Polyoma virus 'hexamer' tubes consist of paired pentamers. Nature. 1983 Jun 2;303(5916):446–448. doi: 10.1038/303446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bina M., Beecher S., Blasquez V. Stability and components of mature simian virus 40. Biochemistry. 1982 Jun 22;21(13):3057–3063. doi: 10.1021/bi00256a004. [DOI] [PubMed] [Google Scholar]
- Bina M., Ng S. C., Blasquez V. Simian virus 40 chromatin interaction with the capsid proteins. J Biomol Struct Dyn. 1983 Dec;1(3):689–704. doi: 10.1080/07391102.1983.10507475. [DOI] [PubMed] [Google Scholar]
- Blasquez V., Beecher S., Bina M. Simian virus 40 morphogenetic pathway. An analysis of assembly-defective tsB201 DNA protein complexes. J Biol Chem. 1983 Jul 10;258(13):8477–8484. [PubMed] [Google Scholar]
- Blasquez V., Stein A., Ambrose C., Bina M. Simian virus 40 protein VP1 is involved in spacing nucleosomes in minichromosomes. J Mol Biol. 1986 Sep 5;191(1):97–106. doi: 10.1016/0022-2836(86)90425-0. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Chang X. B., Wilson J. H. Formation of deletions after initiation of simian virus 40 replication: influence of packaging limit of the capsid. J Virol. 1986 May;58(2):393–401. doi: 10.1128/jvi.58.2.393-401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. S., Hsu M. T. Evidence for variation of supercoil densities among simian virus 40 nucleoprotein complexes and for higher supercoil density in replicating complexes. J Virol. 1984 Jul;51(1):14–19. doi: 10.1128/jvi.51.1.14-19.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu W. Electron microscopy of frozen, hydrated biological specimens. Annu Rev Biophys Biophys Chem. 1986;15:237–257. doi: 10.1146/annurev.bb.15.060186.001321. [DOI] [PubMed] [Google Scholar]
- Coca-Prados M., Yu H. Y., Hsu M. T. Intracellular forms of simian virus 40 nucleoprotein complexes. IV. Micrococcal nuclease digestion. J Virol. 1982 Nov;44(2):603–609. doi: 10.1128/jvi.44.2.603-609.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther R. A., Amos L. A., Finch J. T., De Rosier D. J., Klug A. Three dimensional reconstructions of spherical viruses by fourier synthesis from electron micrographs. Nature. 1970 May 2;226(5244):421–425. doi: 10.1038/226421a0. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Amos L. A., Finch J. T. Three-dimensional image reconstructions of bacteriophages R17 and f2. J Mol Biol. 1975 Nov 5;98(3):631–635. doi: 10.1016/s0022-2836(75)80091-x. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Amos L. A. Three-dimensional image reconstructions of some small spherical viruses. Cold Spring Harb Symp Quant Biol. 1972;36:489–494. doi: 10.1101/sqb.1972.036.01.062. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Geelen J. L., Mellema J. E. A three-dimensional image reconstruction of cowpea mosaic virus. Virology. 1974 Jan;57(1):20–27. doi: 10.1016/0042-6822(74)90104-4. [DOI] [PubMed] [Google Scholar]
- Crowther R. A. Procedures for three-dimensional reconstruction of spherical viruses by Fourier synthesis from electron micrographs. Philos Trans R Soc Lond B Biol Sci. 1971 May 27;261(837):221–230. doi: 10.1098/rstb.1971.0054. [DOI] [PubMed] [Google Scholar]
- DeRosier D. J., Moore P. B. Reconstruction of three-dimensional images from electron micrographs of structures with helical symmetry. J Mol Biol. 1970 Sep 14;52(2):355–369. doi: 10.1016/0022-2836(70)90036-7. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Adrian M., Schultz P., Oudet P. Cryo-electron microscopy of vitrified SV40 minichromosomes: the liquid drop model. EMBO J. 1986 Mar;5(3):519–528. doi: 10.1002/j.1460-2075.1986.tb04241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch J. T., Crowther R. A., Hendry D. A., Struthers J. K. The structure of Nudaurelia capensis beta virus: the first example of a capsid with icosahedral surface symmetry T-4. J Gen Virol. 1974 Jul;24(1):191–200. doi: 10.1099/0022-1317-24-1-191. [DOI] [PubMed] [Google Scholar]
- Finch J. T. The surface structure of polyoma virus. J Gen Virol. 1974 Aug;24(2):359–364. doi: 10.1099/0022-1317-24-2-359. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Haas M. Rapid concentration and purification of polyoma virus and SV40 with polyethylene glycol. Virology. 1970 Sep;42(1):248–250. doi: 10.1016/0042-6822(70)90263-1. [DOI] [PubMed] [Google Scholar]
- Fuller S. D. The T=4 envelope of Sindbis virus is organized by interactions with a complementary T=3 capsid. Cell. 1987 Mar 27;48(6):923–934. doi: 10.1016/0092-8674(87)90701-x. [DOI] [PubMed] [Google Scholar]
- Griffith J. D. Chromatin structure: deduced from a minichromosome. Science. 1975 Mar 28;187(4182):1202–1203. doi: 10.1126/science.187.4182.1202. [DOI] [PubMed] [Google Scholar]
- Jakobovits E. B., Bratosin S., Aloni Y. A nucleosome-free region in SV40 minichromosomes. Nature. 1980 May 22;285(5762):263–265. doi: 10.1038/285263a0. [DOI] [PubMed] [Google Scholar]
- Klug A., Finch J. T. Structure of viruses of the papilloma-polyoma type. IV. Analysis of tilting experiments in the electron microscope. J Mol Biol. 1968 Jan 14;31(1):1–12. doi: 10.1016/0022-2836(68)90050-8. [DOI] [PubMed] [Google Scholar]
- Koch M. A., Eggers H. J., Anderer F. A., Schlumberger H. D., Frank H. Structure of simian virus 40. I. Purification and physical characterization of the virus particle. Virology. 1967 Jul;32(3):503–510. doi: 10.1016/0042-6822(67)90302-9. [DOI] [PubMed] [Google Scholar]
- Lepault J., Booy F. P., Dubochet J. Electron microscopy of frozen biological suspensions. J Microsc. 1983 Jan;129(Pt 1):89–102. doi: 10.1111/j.1365-2818.1983.tb04163.x. [DOI] [PubMed] [Google Scholar]
- Lepault J., Leonard K. Three-dimensional structure of unstained, frozen-hydrated extended tails of bacteriophage T4. J Mol Biol. 1985 Apr 5;182(3):431–441. doi: 10.1016/0022-2836(85)90202-5. [DOI] [PubMed] [Google Scholar]
- MAYOR H. D., JAMISON R. M., JORDAN L. E. Biophysical studies on the nature of the simian papova virus particle (vacuolating SV40 virus). Virology. 1963 Mar;19:359–366. doi: 10.1016/0042-6822(63)90075-8. [DOI] [PubMed] [Google Scholar]
- Martin R. G. On the nucleoprotein core of simian virus 40. Virology. 1977 Dec;83(2):433–437. doi: 10.1016/0042-6822(77)90190-8. [DOI] [PubMed] [Google Scholar]
- Mellema J. E., Amos L. A. Three-dimensional image reconstruction of turnip yellow mosaic virus. J Mol Biol. 1972 Dec 30;72(3):819–822. doi: 10.1016/0022-2836(72)90195-7. [DOI] [PubMed] [Google Scholar]
- Milligan R. A., Brisson A., Unwin P. N. Molecular structure determination of crystalline specimens in frozen aqueous solutions. Ultramicroscopy. 1984;13(1-2):1–9. doi: 10.1016/0304-3991(84)90051-2. [DOI] [PubMed] [Google Scholar]
- Müller U., Zentgraf H., Eicken I., Keller W. Higher order structure of simian virus 40 chromatin. Science. 1978 Aug 4;201(4354):406–415. doi: 10.1126/science.208155. [DOI] [PubMed] [Google Scholar]
- Ng S. C., Bina M. Disulfide bonds protect the encapsidated chromosomes of Simian virus 40. FEBS Lett. 1981 Jul 20;130(1):47–49. doi: 10.1016/0014-5793(81)80662-x. [DOI] [PubMed] [Google Scholar]
- Ng S. C., Bina M. Temperature-sensitive BC mutants of simian virus 40: block in virion assembly and accumulation of capsid-chromatin complexes. J Virol. 1984 May;50(2):471–477. doi: 10.1128/jvi.50.2.471-477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I., Baker T. S., Caspar D. L., Murakami W. T. Polyoma virus capsid structure at 22.5 A resolution. Nature. 1982 Jan 14;295(5845):110–115. doi: 10.1038/295110a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salunke D. M., Caspar D. L., Garcea R. L. Self-assembly of purified polyomavirus capsid protein VP1. Cell. 1986 Sep 12;46(6):895–904. doi: 10.1016/0092-8674(86)90071-1. [DOI] [PubMed] [Google Scholar]
- Saragosti S., Moyne G., Yaniv M. Absence of nucleosomes in a fraction of SV40 chromatin between the origin of replication and the region coding for the late leader RNA. Cell. 1980 May;20(1):65–73. doi: 10.1016/0092-8674(80)90235-4. [DOI] [PubMed] [Google Scholar]
- Sogo J. M., Stahl H., Koller T., Knippers R. Structure of replicating simian virus 40 minichromosomes. The replication fork, core histone segregation and terminal structures. J Mol Biol. 1986 May 5;189(1):189–204. doi: 10.1016/0022-2836(86)90390-6. [DOI] [PubMed] [Google Scholar]
- Stewart M., Vigers G. Electron microscopy of frozen-hydrated biological material. Nature. 1986 Feb 20;319(6055):631–636. doi: 10.1038/319631a0. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Glaeser R. M. Electron microscopy of frozen hydrated biological specimens. J Ultrastruct Res. 1976 Jun;55(3):448–456. doi: 10.1016/s0022-5320(76)80099-8. [DOI] [PubMed] [Google Scholar]
- Taylor K. A. Structure determination of frozen, hydrated, crystalline biological specimens. J Microsc. 1978 Jan;112(1):115–125. doi: 10.1111/j.1365-2818.1978.tb01159.x. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Nedospasov S. A., Georgiev G. P. On the structure of eukaryotic, prokaryotic, and viral chromatin. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):457–473. doi: 10.1101/sqb.1978.042.01.049. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Fisher H. W. Electron microscopy of tobacco mosaic virus under conditions of minimal beam exposure. J Mol Biol. 1970 Aug 28;52(1):121–123. doi: 10.1016/0022-2836(70)90181-6. [DOI] [PubMed] [Google Scholar]