ER Stress – a Double-Edged Sword
The acquisition of the endoplasmic reticulum (ER) during evolution of eukaryotes represents one of the fundamental shifts in biochemical reactions, from the relics of prokaryotes in which biochemical processes occur in the cytosol, requiring the primordial, anaerobic reducing conditions, to the far more sophisticated metabolic pathways in which oxygen is an absolute necessity. In eukaryotes, the ER is recognised as the site of synthesis and folding of secreted, membrane-bound and some organelle-targeted proteins. Several factors are required for disulphide-bond formation, which is needed for optimal protein folding, including ATP, Ca2+ and an oxidizing environment.1 As a consequence of these special requirements, the ER is highly sensitive to stresses that perturb cellular energy levels, the redox state or Ca2+ concentration. Such stresses reduce the protein-folding capacity of the ER, which can result in the accumulation and aggregation of unfolded proteins and/or an imbalance between the load of resident and transit proteins in the ER and the organelle’s ability to process that load. This condition is referred to as ER stress. The ER stress response can promote cellular repair and sustained survival by reducing the load of unfolded proteins through global attenuation of protein synthesis and/or upregulation of chaperones, enzymes and structural components of the ER, which enhance protein folding.2 This response is collectively termed as the unfolded protein response (UPR) and it is mediated through three ER transmembrane receptors: pancreatic ER kinase (PERK), activating transcription factor 6 (ATF6) and inositol-requiring enzyme 1 (IRE1). In resting cells, all of these ER stress receptors are maintained in an inactive state through their association with the ER chaperone, GRP78 (also called BiP). Accumulation of unfolded proteins causes dissociation of GRP78 from PERK, ATF6 and IRE1, thereby initiating the UPR. Thus, the UPR is a pro-survival response to reduce the accumulation of unfolded proteins and restore normal ER function.3 In addition, the UPR plays a critical role in certain developmental processes that are associated with increased demand for protein synthesis and/or export, such as differentiation of immunoglobulin (Ig)-secreting plasma cells and myoblast formation.4,5 However, when misfolded-protein aggregation persists and the ER stress cannot be resolved, signalling switches from a pro-survival to a pro-apoptotic response. Thus lack of a UPR could be a mortal danger but an excessive response could be an absolute disaster!
ER Stress and Chemotherapeutic Drugs
Endoplasmic reticulum-associated degradation (also called ERAD) is an integral part of the ER quality assurance system and directs misfolded proteins for destruction by the cytoplasmic ubiquitin–proteasome pathway.6 The ERAD activity depends on the functions of the UPR; notably, several components of the ERAD system are under transcriptional control of the UPR.7 Thus, there is a regulatory loop connecting the ERAD with the UPR. Furthermore, since ERAD depends on the cytoplasmic protein degradation machinery, it appears likely that the UPR also depends on the proteasome machinery. The connection between UPR and cancer was first demonstrated in 1996 when it was reported that the major ER chaperone GRP78/BiP was highly induced in many tumours and molecular inhibition of this induction in the fibrosarcoma B/C10ME, while not affecting in vitro cellular proliferation, caused a dramatic increase in apoptotic cell death through ER stress and tumour regression in vivo.8 Similarly, Romero-Ramirez et al.9 had reported that XBP-1, the transcription factor involved in UPR, is essential for sustained tumour cell survival and cancer growth. Finally, it was shown that PERK-expressing tumours grow more rapidly than PERK-negative tumours in nude mice.10 Thus chemotherapeutic drug targeting of the UPR machinery offers a potential strategy for treating various forms of cancer. Indeed, this is reflected in several published studies on the mechanisms of action of chemotherapeutic drugs that target the protein degradation machinery.
NSAIDs
The non-steroidal anti-inflammatory drugs (NSAIDs) constitute one of the largest groups of drugs prescribed in the developed world.11 They act primarily as inhibitors of prostaglandin synthase but also have a number of other activities, including inhibition of neutrophil migration, mild immunosuppression and interference with cell membrane function. As well as being useful in a wide range of inflammatory arthropathies, they may also be beneficial in other types of pain, such as renal colic, bone pain due to cancer and in hypercalcaemia. Several studies have shown that NSAIDs can also inhibit tumour growth and the ability of tumours to metastasise in animal models.12,13 NSAIDs are currently in phase II clinical trials for treating lung cancer and precancerous malignancies (Clinical trial identifier no. NCT00368927, National Cancer Institute), and for treating squamous cell carcinoma of head and neck (Clinical trial identifier no. NCT00392665, Dana-Farber Cancer Institute).
While NSAID’s efficacy in treating pain, fever and inflammation is attributed to its cyclooxygenase (COX) inhibitory property, induction of apoptosis is known to be the major contributor to its antitumour activity14 as well as the gastrointestinal complications, such as gastric ulcers.15 Recent reports suggest that various NSAIDs, such as diclofenac, indomethacin, ibuprofen and celecoxib, trigger apoptosis through induction of ER stress.16,17 This has been corroborated by our recent results, which showed that indomethacin-induced apoptosis in macrophages requires the pro-apoptotic BH3-only Bcl-2 family member Bim, which is activated as a result of ER stress.18 Thus, NSAIDs represent a class of drugs that have been proven to be effective in treating various types of cancer, which appear to act by triggering ER stress-induced apoptosis of tumour cells.
Proteasomal Inhibitors
Bortezomib (Velcade; previously known as PS-341) is a peptide boronate inhibitor of the proteasome that recently received US Food and Drug Administration approval for the treatment of multiple myeloma19 and is currently being evaluated for treatment of certain solid tumours (CenterWatch, Clinical Trials Listing Service, 2007) and mantle cell lymphoma.20 The antineoplastic effects of bortezomib have been attributed, in part, to inhibition of IκB degradation, thereby preventing activation of Rel/NF-κB transcription factors, which are known to promote expression of antiapoptotic genes, such as bcl-2, bcl-xL and a1.21 However, recent findings demonstrated that inhibition of Rel/NF-κB activity accounts for only a small fraction of the anticancer activity of bortezomib.22 Furthermore, it has been shown that bortezomib kills cells through a process that is independent of the tumour suppressor p53,23 which involves activation of a pro-apoptotic ER stress response.24 It has been reported that bortezomib sensitises pancreatic cancer cells to ER stress-induced apoptosis and thereby strongly enhances the anticancer activity of cisplatin.24 Similarly, in head and neck squamous cell carcinoma cells, bortezomib was found to induce apoptosis by activating the ER stress response.25 Finally, bortezomib-induced apoptosis in multiple myeloma cells has been attributed to the activation of the apoptotic arm of the UPR, characterised by the stimulation of PERK, the ER stress-specific eIF-2 kinase; ATF4, an ER stress-induced transcription factor; and its pro-apoptotic target, CHOP/GADD153.26 Thus, similar to NSAIDs, proteasomal inhibitors represent another class of drugs that have been found to be effective in the treatment of certain cancer, which appear to act predominantly through their ability to induce ER stress-induced apoptosis of tumour cells.
HDAC Inhibitors
Histone deacetylase (HDAC) inhibitors are another group of chemotherapeutic drugs that have recently come to prominence. Several of these compounds are currently undergoing phase II clinical trial for refractory B-cell lymphoma by MethylGene Inc. While the exact mode of action of this class of drugs is yet unknown, it is generally believed that it relies on their ability to relieve transcriptional repression. However, recent reports suggest that HDACs are critically involved in proteasome-independent autophagic clearance of aggregated proteins27 and HDAC inhibitors have been reported to induce accumulation of misfolded proteins.28,29 Treatment of multiple myeloma cells with HDAC inhibitors, such as Tubacin, resulted in the accumulation of ubiquitinated proteins and this acted synergistically with proteasomal inhibitors to trigger cell death.27 Therefore, HDAC inhibitors represent yet another class of chemotherapeutic drugs, which can trigger ER stress-induced apoptosis of tumour cells by causing accumulation of misfolded proteins.
Conclusion
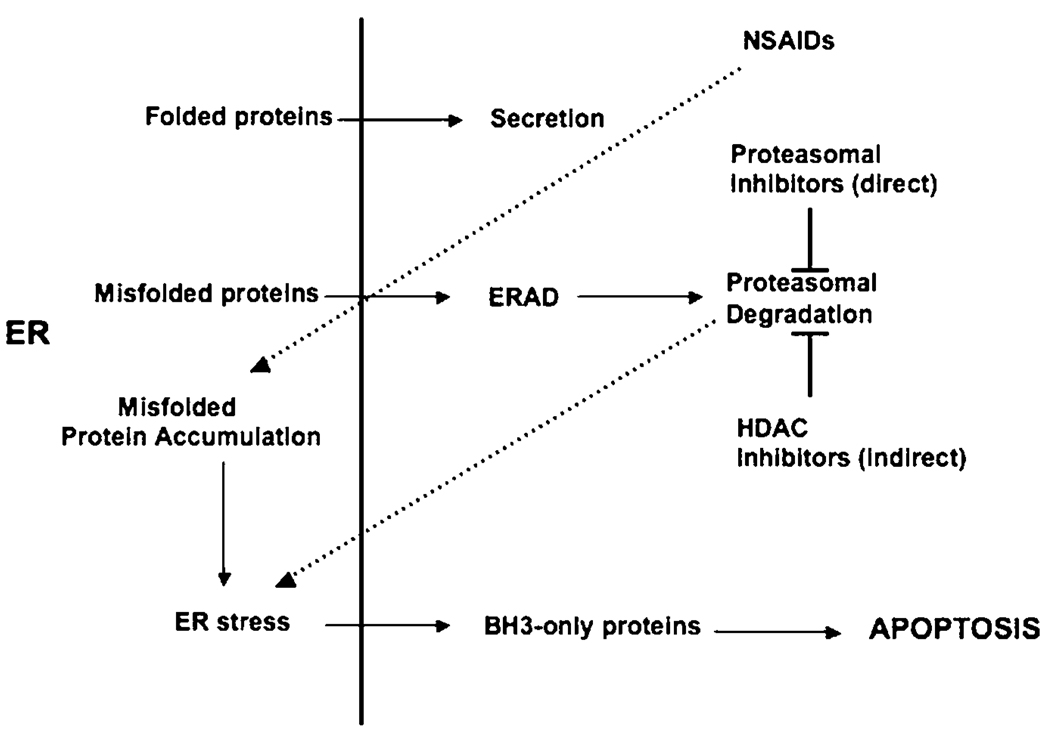
The converging point for various chemotherapeutic drugs, including NSAIDs, proteasomal inhibitors as well as HDAC inhibitors, appears to be the induction of ER stress (Figure 1). It is therefore tempting to suggest that future attempts to develop chemotherapeutic drugs should focus on eliciting ER-stress response-induced apoptosis in cancerous cells. This would be particularly relevant for tumour cells that secrete large amounts of protein. For example, one of the fundamental properties of multiple myeloma cells is the production and secretion of large amounts of antibodies. A substantial amount of newly synthesised antibodies in these cells are incorrectly folded and will therefore not be secreted. The build-up of misfolded antibodies elicits significant ER stress and the multiple myeloma cells must find ways to degrade these proteins to survive. Consequently, blocking the degradation of misfolded proteins by proteasomal inhibitory drugs will be potently toxic to these cells. A similar strategy could be used to treat endocrine tumours, which also secrete large amount of proteins, including hormones. The majority of human tumours are poorly oxygenated (hypoxic) and this is generally associated with poor prognosis, due to the protection it affords the tumour cells against γ-irradiation and treatment with certain chemotherapeutic drugs. The ability to survive and even proliferate under hypoxic conditions requires activation of the survival arm of the UPR pathway.30,31 Therapeutic inhibition of the ER stress pathway may therefore sensitise hypoxic tumours to γ-irradiation or chemotherapeutic drug treatment. The molecular mechanisms of ER stress-induced apoptosis have recently been clarified through the demonstration that transcriptional and posttranslational activation of pro-apoptotic BH3-only Bcl-2 family members, particularly Bim and Puma, are essential for initiation of this death pathway.18,32 Since this pathway is independent of the tumour suppressor p53, this approach may help supplement the emerging approach of using ‘BH3 mimetics’33 for treating cancers, particularly those in which p53 is mutated, accounting for approximately half of all human cancers.
Figure 1.
Induction of the ER stress response by various chemotherapeutic drugs. Effect of HDAC inhibitors on the proteasome is indirect, involving inhibition of ‘aggresomes’, which then results in abnormal accumulation of misfolded proteins and thus increased work load on the proteasome
Acknowledgements
This work is supported by fellowships and grants from the National Health and Medical Research Council (Australia; program no. 257502), the Cancer Council of Victoria, the Leukemia and Lymphoma Society (New York; SCOR Grant no. 7015) and the National Cancer Institute (NIH, US; CA 80188 and CA 43540).
References
- 1.Gaut JR, Hendershot LM. J Biol Chem. 1993;268:7248–7255. [PubMed] [Google Scholar]
- 2.Kauffmann RJ. J Clin Invest. 2002;110:1389–1398. [Google Scholar]
- 3.Schröder M, Kaufman RJ. Ann Rev Biochem. 2001;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 4.Gass JN, et al. Trends Immunol. 2002;25:2994–3003. [Google Scholar]
- 5.Nakanishi K, et al. FASEB J. 2007;21:2994–3003. doi: 10.1096/fj.06-6408com. [DOI] [PubMed] [Google Scholar]
- 6.Oyadomari S, et al. Cell. 2006;126:727–739. doi: 10.1016/j.cell.2006.06.051. [DOI] [PubMed] [Google Scholar]
- 7.Friedlander R, et al. Nat Cell Biol. 2000;2:379–384. doi: 10.1038/35017001. [DOI] [PubMed] [Google Scholar]
- 8.Jamora C, et al. Proc Natl Acad Sci USA. 1996;93:7690–7694. doi: 10.1073/pnas.93.15.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero-Ramirez L, et al. Cancer Res. 2004;64:5943–5947. doi: 10.1158/0008-5472.CAN-04-1606. [DOI] [PubMed] [Google Scholar]
- 10.Bi M, et al. EMBO J. 2005;24:3470–3481. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smalley W, et al. Arch Intern Med. 1999;159:161. doi: 10.1001/archinte.159.2.161. [DOI] [PubMed] [Google Scholar]
- 12.Rao KV, et al. Carcinogenesis. 1996;17:1435. doi: 10.1093/carcin/17.7.1435. [DOI] [PubMed] [Google Scholar]
- 13.Orner GA, et al. Carcinogenesis. 2003;24:263. doi: 10.1093/carcin/24.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rigas B, Shiff SJ. Med Hypothesis. 2000;54:210. doi: 10.1054/mehy.1999.0023. [DOI] [PubMed] [Google Scholar]
- 15.Hawkey CJ. J Cardiovasc Pharmacol. 2006;47 Suppl 1:S72–S75. doi: 10.1097/00005344-200605001-00013. [DOI] [PubMed] [Google Scholar]
- 16.Tsutsumi S, et al. Cell Death Differ. 2004;11:1009–1016. doi: 10.1038/sj.cdd.4401436. [DOI] [PubMed] [Google Scholar]
- 17.Pyrko P, et al. Mol Cancer Ther. 2007;6:1262–1275. doi: 10.1158/1535-7163.MCT-06-0629. [DOI] [PubMed] [Google Scholar]
- 18.Puthalakath H, et al. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 19.Richardson PG, et al. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 20.Weigert O, et al. Leukaemia. 2007;21:524–528. doi: 10.1038/sj.leu.2404511. [DOI] [PubMed] [Google Scholar]
- 21.Adams J. Cancer Cell. 2004;5:417–421. doi: 10.1016/s1535-6108(04)00120-5. [DOI] [PubMed] [Google Scholar]
- 22.Zheng B, et al. Clin Cancer Res. 2004;10:3207–3215. doi: 10.1158/1078-0432.ccr-03-0494. [DOI] [PubMed] [Google Scholar]
- 23.Strauss SJ, et al. Cancer Res. 2007;67:2783–2790. doi: 10.1158/0008-5472.CAN-06-3254. [DOI] [PubMed] [Google Scholar]
- 24.Nawrocki ST, et al. Cancer Res. 2005;65:11658–11666. doi: 10.1158/0008-5472.CAN-05-2370. [DOI] [PubMed] [Google Scholar]
- 25.Fribley A, et al. Mol Cell Biol. 2004;24:9695–9704. doi: 10.1128/MCB.24.22.9695-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obeng EA, et al. Blood. 2006;107:4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hideshima T, et al. Proc Natl Acad Sci USA. 2005;102:8567–8572. doi: 10.1073/pnas.0503221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawaguchi Y, et al. Cell. 2003;115:727–738. doi: 10.1016/s0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- 29.Nawrocki ST, et al. Cancer Res. 2006;66:3773–3781. doi: 10.1158/0008-5472.CAN-05-2961. [DOI] [PubMed] [Google Scholar]
- 30.Koumenis C, Wouters BG. Mol Cancer Res. 2006;4:423–436. doi: 10.1158/1541-7786.MCR-06-0150. [DOI] [PubMed] [Google Scholar]
- 31.Koumenis C. Curr Mol Med. 2006;6:55–69. doi: 10.2174/156652406775574604. [DOI] [PubMed] [Google Scholar]
- 32.Luo X, et al. Cell Death Diff. 2005;12:1310–1318. doi: 10.1038/sj.cdd.4401659. [DOI] [PubMed] [Google Scholar]
- 33.Adams J, Cory S. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]