Abstract
Various clinical populations display atypical volume asymmetry of language structures and also the auditory M100 source. Although such atypical volume asymmetries have also been observed in autism, M100 source asymmetries have not yet been investigated. We examined M100 asymmetry in autism and its relationship with language functioning. Evoked neural activity to a 1kHz tone was recorded using whole-cortex 151-channel magnetoencephalography in three groups of individuals. A single-dipole model identified the M100 generator in auditory cortex in each hemisphere. Healthy adults and control children displayed the expected right-sided M100 anteriority, whereas children with autism showed no such asymmetry. An association was found between language functioning and the degree of asymmetry across the two groups of children, suggesting a possible relationship between functional—structural asymmetry and language ability.
Keywords: asymmetry, auditory processing, autism, language, M100, magnetoencephalography
Introduction
One of the defining features of autism is language impairment. A number of studies have linked language impairment in autism with reversed or absent patterns of neural asymmetry as compared with the typically developing population. Such asymmetries have been shown in autism in evoked potentials to speech stimuli [1] and also volume of neural language structures, including frontal and temporal areas [2,3] and in particular, the planum temporale [4]. Although such neural asymmetries may be nonspecific indicators of pathology, or at least diagnosis, they may also be linked with language functioning in some way. A number of studies link atypical structural asymmetries with language functioning. The absent or reversed patterns of neural asymmetry found in autism are also observed in language disorders, such as dyslexia [5], developmental language disorder [4], and specific language impairment (SLI) [2]. Schizophrenia, although not a language disorder per se, is also characterized by language difficulties in addition to atypical asymmetry [6,7]; for reviews, see Ref. [8,9].
Another potential measure of asymmetry is the M100 source. The auditory M100, assessed using magnetoencephalography (MEG), localizes near Heschl’s gyrus and the planum temporale [10]. It is the magnetic counterpart to the N1 electroencephalographic component, occurring approximately 100ms after an auditory stimulus in adults. Healthy adults show M100 asymmetry with a more anterior right hemisphere (RH) than left hemisphere (LH) source [11]. This asymmetry is reversed or absent in adults with dyslexia or schizophrenia (e.g. Ref [12]), but has not to our knowledge been explored in autism. The above-mentioned comparable patterns of atypical structural asymmetries in dyslexia, schizophrenia, and autismsuggest that the M100 source may be similarly affected in autism. M100 source asymmetry differences have also been linked with language functioning [13].
The existence of atypical structural asymmetries in autism leads to our first hypothesis. We tested the idea that children with autism will show reduced or reversed M100 asymmetry similar to that showed in other disorders. As both atypical structural [2,4,5] and M100 source [13] asymmetries have been linked with language functioning, the second hypothesis we tested was that the degree of M100 source asymmetry would be associated with language abilities across controls and individuals with autism.
Methods
Participants
Eleven healthy adults, 13 children with typical development, and 13 children with autism were recruited from the Hospital for Sick Children, Toronto community. Participants were excluded from group analysis if any of the following is observed in either hemisphere: a lack of auditory response, residual variances greater than 20% (poor dipole model-data fits), or a dipole source located outside the superior temporal region as described below. Ten adults (five women, two left-handed) ranging in age from 21 to 53 years (M=33.5 years, SD=8.2), eight children with autism (one girl) ranging in age from 8 to 15 years (M=11.7 years, SD=1.9), and seven typically developing children (six girls) ranging in age from 9 to 16 years (M=11.4 years, SD=2.2) remained after exclusion criteria. There were no differences in age between the two groups of children [t(13)=0.29, P=0.78]. All non-adult participants were right-handed according to the Edinburgh Handedness Inventory [14]. All data were collected and informed consent was obtained with the approval of the local institutional research ethics board.
Autism spectrum diagnosis
Children with autism met the criteria for autism or autism spectrum disorder on the Autism Diagnostic Observation Schedule (ADOS) [15], with the possible exception of one participant who had a known diagnosis of autistic disorder, but for whom ADOS scores were unverifiable. Children with typical development scored in the normal range on this instrument.
Oral language ability
The Clinical Evaluation of Language Fundamentals — 4th edition (CELF-4) [16] was administered to all non-adult participants. On the basis of performance on four subtests, an age-based standardized Core Language score was obtained that provided a global representation of a child’s overall expressive and receptive language abilities. The CELF-4 [16] Core Language scores were lower in children with autism (M=66, SD=16) than children with typical development (M=119, SD=9; t(13)=–7.8, P<0.01).
Intelligence
All non-adult participants had a nonverbal intelligence standard score of at least 82. In most cases, this was measured using the Weschler Intelligence Scale for Children (WISC; [17]). One child with autism had unreliable performance on the WISC, and therefore the Leiter-R [18] was used to measure his nonverbal intelligence. Nonverbal intelligence standard scores did not differ between typically developing children (M=116, SD=12) and children with autism (M=102, SD=14), t(13)=2.13, P=0.05. Verbal or full-scale intelligence scores on the WISC were not used to estimate cognitive ability because of the potential for interference of language impairments in the children with autism.
Equipment and procedure
Non-adult participants first attended a standardized testing session, in which they completed the ADOS, CELF, and WISC. Then, participants completed a MEG session where they participated in two experimental procedures (one that reported in Ref [19]) in which stimuli were tone pairs consisting of two 1-kHz sinusoidal tones, 40ms in duration (with 10ms linear onset/offset ramps). The tone pairs in the two experiments had interstimulus intervals of 150 and 800ms, respectively. Stimuli were digitized at a 41.1-kHz sampling rate using SoundForge audio digitization software (Sonic Foundary Inc., Madison, Wisconsin, USA) and presented by using E-Prime experimental software (Psychology Software Tool Inc., Pittsburg, Pennsylvania, USA). Stimuli were digital-to-analog converted on an external 24-bit device (UA-5, Edirol Inc., Bellingham, Washington, USA) and processed through high-caliber amplification instrumentation (Series III, Tucker Davis Technologies Inc., Alachua, Florida, USA) with 1dB resolution. The auditory signal was delivered through a sound pressure transducer and sound conduction tubing (ER3A, Etymotic Research, Elk Grove Village, Illinois, USA) to the individual peripheral auditory canal through eartip inserts, which attenuated ambient environmental noise.
After determining each individual’s auditory threshold, at least 120 stimulus pairs were presented to the right ear at 50dB above threshold, with pseudorandom intertrial intervals of 0.5–1.5s for the first condition and 3–5s for the second. Evoked neural activity to the tone pairs was recorded at a sampling rate of 1250Hz using a whole cortex, 151-channel MEG system (CTF Systems, Inc., Vancouver, British Columbia, Canada). Data epochs, which include a 200-ms prestimulus baseline for determination of ambient brain activity and noise, of 1300 and 800ms were recorded for the first and second experimental conditions, respectively. To minimize fatigue and encourage an awake state during acquisition, participants viewed (but did not listen to) a movie projected on a video screen positioned at a comfortable viewing distance.
Analysis
All data were DC offset corrected with respect to the prestimulus baseline before artifact detection. After the removal of any inappropriately biased (‘bad’) channels and trials containing artifacts [>16pT based on a root mean square (RMS) vector analysis across all channels], data were averaged and band-pass filtered offline with 1 and 40Hz cutoffs. For each hemisphere, left and right, characteristic M100 peaks to the first tone of each averaged stimulus type were identified through calculation of RMS field strength at each sample point across the 70 channels defining each cerebral hemisphere. The M100 peak was defined as the point with the maximum RMS signal in the latency range 80–150ms for adults or 90–180ms for children (a wider and later latency window was used to allow for the known prolonged M100 latency at younger ages), which satisfied a single-dipole model and with a topographic distribution with maxima and minima emerging and entering fields approximately distributed on either side of the putative temporal cortex. Two children with typical development and four children with autism showed no identifiable M100 response by the above criteria in either the RH or LH and were excluded from further analysis.
A single-equivalent current dipole that best accounted for the magnetic flux distribution was calculated to determine the three-dimensional coordinates of the source of each M100. Dipole fits with residual variance exceeding 20% were rejected as unsatisfactory, as were dipole models as originating outside the ipsilateral superior temporal region. In some cases, the occipital sensors were excluded from the dipole fit source modeling when this provided a better fit. One adult and one child with typical development had residual variance at the M100 peak of greater than 20% and three children with typical development and one child with autism had a dipole model originating outside of the superior temporal region; all were excluded from further analysis. To calculate a measure of asymmetry, an M100 peak must have been apparent in both the RH and LH. To get the best possible measure of the M100, the response to the first tone (known to be a stronger, more consistent response) in the condition with the longest silent gap showing responses in both hemispheres was used to estimate the equivalent current dipole.
Anterior—posterior asymmetry was determined using paired sample t-tests of LH and RH M100 x-axis values (positive values extending anteriorly from the MEG origin and negative values posteriorly along the sagittal plane) for each group. To facilitate analyses of the correlation between asymmetry and language ability, an asymmetry index was calculated for each participant. This was derived by subtracting the LH x-axis value from the RH x-axis value, such that a positive number represented a more anterior RH source, and a negative number, a more anterior LH source.
Results
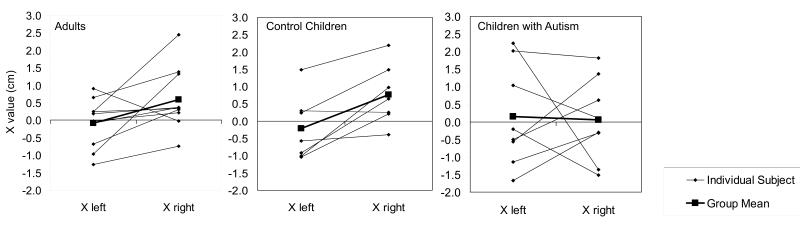
Analysis of anterior—posterior asymmetry revealed that, in control children, right M100 sources (mean x-axis=0.8cm, SD=0.9) were located anterior to left M100 (mean x-axis=—0.2cm, SD=0.9, t(6)=3.52, P=0.01). This pattern was also present in control adults (right mean x-axis=0.6cm, SD=0.9, left mean x-axis=—0.1cm, SD=0.7, t(9)=2.2, P=0.05), but absent in the children with autism (left mean x-axis=0.2cm, SD=1.5, right mean x-axis=0.1cm, SD=1.2, t(7)=—0.15, P>0.05). Figure 1 depicts individual x-axis values for each group, along with the group means.
Fig. 1.
Asymmetry data for the three participant groups. Adult and child controls show a clear rightward anteriority, whereas children with autism show no clear overall pattern.
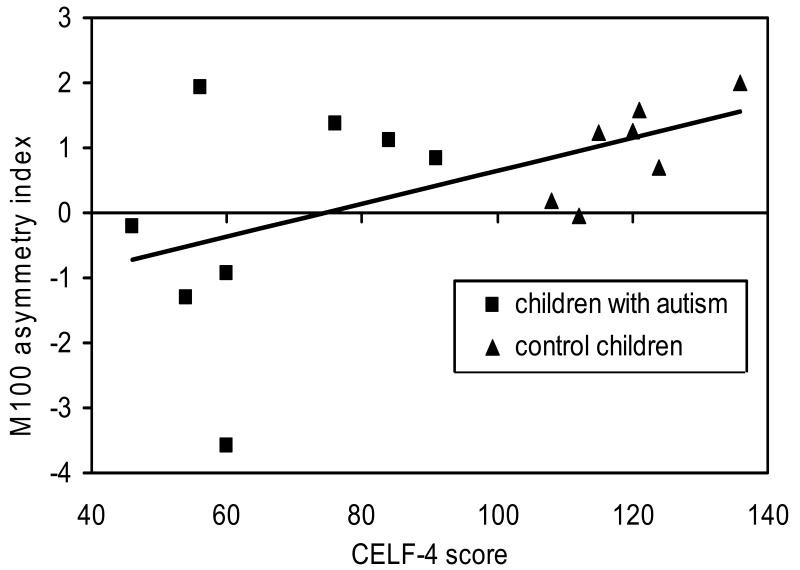
To examine the relationship between anterior—posterior asymmetry and language ability, the two groups of children (control children and children with autism) were combined. Pearson’s correlation showed that the M100 anterior—posterior asymmetry index was positively associated with the CELF-4 scores in the combined group (r=0.52, P<0.05; Fig. 2). There was no relationship between M100 asymmetry and nonverbal intelligence (P>0.05).
Fig. 2.
Correlation of asymmetry index and the Clinical Evaluation of Language Fundamentals — 4th edition (CELF-4 score). The line depicts the correlation for the combined group of children. X left, left hemisphere x-axis; X right, right hemisphere x-axis.
Discussion
In this study, we examined LH—RH anterior—posterior positional M100 asymmetry in healthy adults, children with typical development, and children with autism, and also the relationship between asymmetry and language abilities in children. Unlike children with typical development and adults, children with autism did not show evidence of the anterior—posterior M100 asymmetry previously reported in healthy adults [11] (Fig. 1), supporting our hypothesis. Rather, there was no consistent pattern of relationships between LH and RH M100 source, suggesting a heterogeneous pattern of asymmetry (or lack thereof) in autism. Lack of overall asymmetry is consistent with findings of volume asymmetry abnormalities of language structures in autism. These include atypical volume asymmetry of planum temporale (one source of the M100 [11]) and other language structures [3,4].
We suggest that the heterogeneity of this finding may be linked with the heterogeneity of language functioning in autism. Specifically, it may be that asymmetries in autism are specifically linked with language functioning rather than a diagnosis on the autism spectrum. This suggestion is consistent with a number of previously reported findings. For example, De Fossé et al. [2] report reversal of volume asymmetry of Broca’s area in boys with language impairment compared with those without language impairment, irrespective of the presence of an autism diagnosis. Neural rapid temporal processing deficits have also been reported in children with language impairment and children on the autism spectrum with language impairment, but not in those whose language was unimpaired [19], suggesting that it may be specifically language abilities that are tied to neurological signs of auditory dysfunction in autism and perhaps other disorders. Oram Cardy et al. [20] further report that M50 and M100 latencies, particularly in the RH, are more closely linked with language functioning than with an autism diagnosis.
This pattern of findings led us to examine the relationship between language functioning and our measure of M100 source asymmetry. We included the children with typical development because of previously reported associations of anatomical risk factors in language disorders and reading skills in normal children [21]. An association between language abilities (measured by the CELF-4) and M100 asymmetry in the combined group with typical development and children with autism was observed (Fig. 2). Visual inspection of the scatterplot indicates that this relationship is evident in the comparison children alone, ruling out the possibility that the association was an artifact of the differences in the CELF-4 scores between the two groups of children. Although atypical M100 positional asymmetry is often considered a general risk factor for various neurobiological disorders, the present findings show an association between M100 positional asymmetry and language ability in particular.
This finding is consistent with similar associations between other measures of brain asymmetry and language ability. Leonard et al.[21] report that children and adults with learning disabilities and a lack of planum temporale asymmetry had language comprehension deficits similar to those found in SLI. Dawson et al. [1] used the N100 event-related potential component to derive measures of asymmetry in children with autism, dysphasia, and matched controls. Typically developing children showed stronger LH than RH responses, whereas children with autism and dysphasia showed the opposite. In children with autism, lower language scores were associated with more pronounced asymmetry reversal. Thus, strong positional asymmetry may be a sign of healthy language functioning, whereas a reduction or lack of asymmetry could indicate weaker or abnormal language functioning.
Although these findings suggest a link between language functioning and atypical asymmetry in autism, results must be treated with caution because of the small sample size and the developmental heterogeneity found among children in general and in children with autism in particular. Present findings are promising and would benefit from extension to larger groups of participants, including a group of children with SLI. SLI has been directly compared with autism (for a review, see Ref. [22]), and SLI and autism may be phenotypically linked [3,19,20]. As a result of the link reported here between language ability within autism and between children with and without autism, a group of children with SLI would complete the picture.
Conclusion
Children with autism failed to show a right-sided M100 anteriority found in healthy adults and control children. Furthermore, an association was found between language functioning and the degree of asymmetry across the two groups of children.
Acknowledgements
The authors thank J. Christopher Edgar, Paul Ferrari, Elissa J. Flagg and Wendy Roberts for their contributions to this work. This study was supported by the NIH T32NS007413, NIH-R01 DC008871, Autism Speaks, and CIHR.
References
- 1.Dawson G, Finley C, Phillips S, Lewy A. A comparison of hemispheric asymmetries in speech-related brain potentials of autistic and dysphasic children. Brain Lang. 1989;37:26–41. doi: 10.1016/0093-934x(89)90099-0. [DOI] [PubMed] [Google Scholar]
- 2.De Fossé L, Hodge SM, Makris N, Kennedy DN, Caviness VS, Jr, McGrath L, et al. Language-association cortex asymmetry in autism and specific language impairment. Ann Neurol. 2004;56:757–766. doi: 10.1002/ana.20275. [DOI] [PubMed] [Google Scholar]
- 3.Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Kennedy DN, Filipek PA, et al. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain. 2005;128:213–226. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- 4.Rojas DC, Camou SL, Reite ML, Rogers SJ. Planum temporale volume in children with autism. J Autism Dev Disord. 2005;35:479–486. doi: 10.1007/s10803-005-5038-7. [DOI] [PubMed] [Google Scholar]
- 5.Shapleske J, Rossell SL, Woodruff PW, David AS. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res Rev. 1999;29:26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- 6.Langdon R, Coltheart M, Ward PB, Catts SV. Disturbed communication in schizophrenia: the role of poor pragmatics and poor mind-reading. Psychol Med. 2002;32:1273–1284. doi: 10.1017/s0033291702006396. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell RLC, Crow TJ. Right hemisphere language functions and schizophrenia: the forgotten hemisphere? Brain. 2005;128:963–978. doi: 10.1093/brain/awh466. [DOI] [PubMed] [Google Scholar]
- 8.Covington MA, He C, Brown C, Naçi L, McClain JT, Fjordbak BS, et al. Schizophrenia and the structure of language: the linguist’s view. Schizophr Res. 2005;77:85–98. doi: 10.1016/j.schres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 9.DeLisi LE. Speech disorder in schizophrenia: review of the literature and exploration of its relation to the uniquely human capacity for language. Schizophr Bull. 2001;27:481–496. doi: 10.1093/oxfordjournals.schbul.a006889. [DOI] [PubMed] [Google Scholar]
- 10.Teale P, Sheeder J, Rojas DC, Walker J, Reite M. Sequential source of the M100 exhibits inter-hemispheric asymmetry. Neuroreport. 1998;9:2647–2652. doi: 10.1097/00001756-199808030-00041. [DOI] [PubMed] [Google Scholar]
- 11.Elberling C, Bak C, Kofoed B, Lebech J, Saermark K. Auditory magnetic fields from the human cerebral cortex: location and strength of an equivalent current dipole. Acta Neurol Scand. 1982;65:553–569. doi: 10.1111/j.1600-0404.1982.tb03110.x. [DOI] [PubMed] [Google Scholar]
- 12.Edgar JC, Yeo RA, Gangestad SW, Blake MB, Davis JT, Lewine JD, Cañive JM. Reduced M100 asymmetry in schizophrenia and dyslexia: applying a developmental instability approach to assess atypical brain asymmetry. Neuropsychologia. 2006;44:289–299. doi: 10.1016/j.neuropsychologia.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Heim S, Kissler J, Elbert T, Rockstroh B. Cerebral lateralization in schizophrenia and dyslexia: neuromagnetic responses to auditory stimuli. Neuropsychologia. 2004;42:692–697. doi: 10.1016/j.neuropsychologia.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 15.Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 16.Semel E, Wiig EH, Secord W. Clinical evaluation of language fundamentals. 4th ed The Psychological Corporation; New York: 2003. [Google Scholar]
- 17.Wechsler D. Wechsler intelligence scale for children 3rd ed-Revised. 4th ed The Psychological Corporation; Toronto, Ontario: 1997–2003. [Google Scholar]
- 18.Roid GH, Miller LJ. Leiter International Performance Scale — revised. Stoelting; Wood Dale, Illinois: 1997. [Google Scholar]
- 19.Cardy JE Oram, Flagg EJ, Roberts W, Brian J, Roberts TPL. Magnetoencephalography identifies rapid temporal processing deficit in autism and language impairment. Neuroreport. 2004;16:329–332. doi: 10.1097/00001756-200503150-00005. [DOI] [PubMed] [Google Scholar]
- 20.Cardy JE Oram, Flagg EJ, Roberts W, Roberts TPL. Auditory evoked fields predict language ability and impairment in children. Int J Psychophysiol. 2008;68:170–175. doi: 10.1016/j.ijpsycho.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Leonard CM, Lombardino LJ, Walsh K, Eckert MA, Mockler JL, Rowe LA, et al. Anatomical risk factors that distinguish dyslexia from SLI predict reading skill in normal children. J Commun Disord. 2002;35:501–531. doi: 10.1016/s0021-9924(02)00120-x. [DOI] [PubMed] [Google Scholar]
- 22.Bishop DV. Autism and specific language impairment: categorical distinction or continuum? Novartis Found Symp. 2003;251:213–297. [PubMed] [Google Scholar]