Abstract
We compared the effectiveness of Seeking Safety (SS), an integrated cognitive behavioral treatment for substance use disorder (SUD) and post-traumatic stress disorder (PTSD), to an active comparison health education group (Women’s Health Education [WHE]) within NIDA’s Clinical Trials Network. We randomized 353 women to receive 12 sessions of SS (M = 6.2 sessions) or WHE (M = 6.0 sessions) with follow-up assessment at post-treatment and 3-, 6-, and 12-months post-treatment. Primary outcomes were the Clinician Administered PTSD Scale (CAPS) and PTSD Symptom Scale-Self Report (PSS-SR), and substance use (self-reported abstinence in the prior 7 days and days per week of any substance use). Intention-to-treat analysis showed large, clinically significant reductions in CAPS and PSS-SR symptoms (d = 1.94 and 1.12, respectively), but no reliable difference between conditions. Substance use outcomes were not significantly different over time between the two treatments and at follow-up showed no significant change from baseline, when 46% of participants were abstinent. Study results do not favor SS over WHE as an adjunct to SUD treatment for women with PTSD and reflect considerable opportunity to improve clinical outcomes in community-based treatments for these co-occurring conditions.
Keywords: PTSD, Substance Abuse, Comorbidity, Cognitive Behavioral Treatment, Randomized Control Trial
Twenty years of epidemiology confirms the high level of co-occurring trauma-stress related disorders, such as post-traumatic stress disorder (PTSD) and addictive disorders among women in community based drug treatment, revealing a significant need for therapeutic approaches that can address adverse psychiatric consequences (e.g., Shore, Vollmer, & Tatum, 1989; Breslau, Davis, Andreski, & Peterson, 1991; Resnick, Kilpatrick, Dansky, Saunders, & Best, 1993). Yet, treatment research in this area remains limited.
Quasi-experimental and small controlled studies (i.e., Finkelstein et al., 2004) suggest that a services model integrating cognitive behavioral treatment for trauma with substance abuse services can result in modest improvements in outcome (e.g., Amaro et al., 2007; Morrissey et al., 2005). For PTSD without co-occurring substance abuse, cognitive behavioral approaches have shown evidence of efficacy (e.g., Cloitre, Koenen, Cohen, & Han, 2002; Foa, Rothbaum, & Riggs, 1991). There has been concern, however, that discomfort aroused by focusing on the trauma could be harmful in substance dependent patients, who might escalate substance use or flee treatment. At the same time, the demand for specific interventions for patients with trauma and substance abuse has been mounting in community-based treatment systems (Cohen, Dickow, Horner, Zweben, & Balabis, 2003; Morrissey et al.).
To address this need, Najavits (2002) developed Seeking Safety (SS), anintegrated cognitive behavioral treatment of PTSD and substance use disorder. Thus far, SS has been researched in various studies including a multisite controlled trial with homeless women veterans (Desai, Harpaz-Rotem, Najavits, & Rosenheck, 2008); two randomized control trials with low-income urban women and adolescent girls (Hien, Cohen, Miele, Litt & Capstick, 2004; Najavits, Gallop, & Weiss, 2006); a controlled trial (Gatz et al., 2007); and eight uncontrolled pilot studies (e.g., Najavits, Weiss, Shaw, Muenz, 1998; Zlotnick, Najavits, & Rohsenow, 2003; Cook, Walser, Kane, Ruzek, & Woody, 2006). Overall, Seeking Safety has shown consistent positive outcomes on a variety of measures, superiority to treatment-as-usual, comparability to a gold standard treatment (relapse prevention), positive results in populations typically considered challenging (e.g., the homeless, prisoners, adolescents, public sector clients, and veterans), and high acceptability among diverse clients and clinicians.
One of the goals of NIDA’s Clinical Trials Network (CTN) is to conduct multisite studies to promote dissemination of promising evidence based treatments into the community using a blended research-to-practice model. Thus, SS appeared a logical choice for such evaluation given that it had already achieved positive results on various controlled and uncontrolled trials, and had been widely implemented in clinical practice, but had a limited number of randomized controlled trials, and no rigorous multi-site trials. Further, despite this existing literature on SS, the question remains how the model would fare when conducted with a fewer number of sessions more typical to community based programming and delivered by community practitioners.
With these questions in mind, the NIDA CTN undertook a multi-site clinical trial to test the effectiveness of SS when delivered by community-based clinicians across a range of substance abuse treatment programs to a broadly representative patient sample. Seeking Safety was adapted from 25 to 12 sessions. The active comparison group, Women’s Health Education (WHE), was intended to control for therapeutic time and attention, but may have also included other active therapeutic elements. It was important for our trial to address whether the specific elements of SS were responsible for observed treatment effects in order to inform future treatment development efforts. Although there were numerous prior studies of SS, the current project represents the first large-scale randomized controlled study using a high level of rigor (a combination of formal training of clinicians, adherence monitoring, interview-based diagnostic evaluation, and rigorous inclusion/exclusion criteria). It was hypothesized that SS delivered by community-based substance abuse counselors and their supervisors would produce superior outcomes to WHE when added to outpatient treatment. The main outcome variables were Posttraumatic Stress Symptom-Self Report (PSS-SR) severity, total Clinician Administered PTSD Scale (CAPS) severity, a continuous measure of the number of days using drugs or alcohol during the past seven days, and self reported abstinence in the prior seven days confirmed with urine and saliva tests, both during treatment and over a 12 month follow-up period. In addition, a priori subgroup analyses were planned to examine outcomes separately for minimal attendance (i.e., those who received 6 sessions or more of either active treatment).
METHOD
Studies conducted in the NIDA CTN attempt to replicate “real world” conditions in order to evaluate feasibility of intervention implementation in community clinics. To this end, this study used a hybrid model research design (Carroll & Rounsaville, 2003) which retained key elements of an efficacy trial: diagnostic assessment with blind clinician raters, randomization to an active treatment and credible comparison group, multiple longitudinal standardized assessments, and standards for therapist competence and adherence. Yet the design also allowed for certain elements to replicate real world conditions (effectiveness), namely, rolling group admissions and group format, broader inclusion criteria admitting participants with sub-threshold and full PTSD and in different stages of substance abuse treatment, treatments delivered by community-based counselors and supervisors, and multiple sites with varying treatment-as-usual.
All procedures were reviewed and approved by institutional review boards associated with the lead research team and each treatment site, and all patient-participants gave written informed consent. Because participating counselors and supervisors at each site were selected and randomly assigned to conduct one of the two treatments, they were also considered research participants, and gave written informed consent. A Certificate of Confidentiality, issued by NIDA, was obtained for each clinic participating in the study. The study was approved and periodically reviewed by a Data and Safety Monitoring Board convened for review of CTN studies. Rigorous quality assurance (QA) procedures, including local QA monitoring and regularly scheduled conference calls, were in place throughout the course of the study to ensure data collection integrity.
Participants
Participants were women enrolled in seven community-based substance abuse treatment programs (CTPs) across the United States. To be eligible, participants needed to have had at least one traumatic event in their lifetime and to have met DSM-IV-TR (APA, 2000) criteria for either full or sub-threshold PTSD. For sub-threshold PTSD, participants had to fulfill DSM-IV-TR criteria A (exposure to a traumatic stressor), B (re-experiencing symptoms), E (symptom duration of at least one month) and F (significant distress or impairment of functioning), and either C (avoidance and numbing symptoms) or D (symptoms of increased arousal), but not both as in full PTSD. This is a commonly used definition of subthreshold PTSD (Blanchard, Hickling, Taylor, Loos, & Gerardi, 1994; Grubaugh et al., 2005). Other inclusion criteria were: 1) between 18–65 years of age; 2) used alcohol or an illicit substance within the past six months and have a current diagnosis of drug or alcohol abuse or dependence; and 3) capable of giving informed consent.
Women were excluded if they had 1) advanced stage medical disease as indicated by global physical deterioration; 2) impaired cognition as indicated by a Mini-Mental Status Exam (Folstein, Folstein, & McHugh, 1975) score < 21; 3) significant risk of suicidal/homicidal intent or behavior; 4) history of schizophrenia-spectrum diagnosis; 5) a history of active (past two months) psychosis; 6) involvement in litigation related to PTSD; 7) non English-speaking; or 8) refused to be video- or audio-taped.
Community Treatment Programs
Seven CTPs participated in the study, with the number of participants randomized at each site ranging from 7 to 106. The site randomizing 7 participants dropped from the study due to slow recruitment but did complete assessments, randomization, and treatment as prescribed in the protocol. The sites were a mixture of urban (n = 5) and suburban (n = 2) settings, located geographically in the Western (n = 1), Midwestern (n = 1), Northeastern (n = 2), and Southeastern (n = 3) United States. All participating programs offered a combination of outpatient individual and group treatment components, reflecting varying orientations and philosophies of addiction treatment. All but one of the sites had mixed gender programs and three sites offered some gender specific, trauma-informed services, although participants in the study did not receive these services for the duration of the time in the study.
Procedures
Design
This study used a randomized, controlled, repeated measures design to assess the effectiveness of SS (Najavits, 2002) plus standard substance abuse treatment in comparison to an active comparison treatment, WHE, plus standard substance abuse treatment. Counselors and supervisors at each site were nested within treatment conditions; each site delivered each of the treatment conditions. After baseline assessment, participants were randomly assigned to one of the two conditions consisting of two sessions per week over approximately 6 weeks. Participants were assessed weekly during treatment, and at 1-week, 3-, 6-, and 12-months post treatment.
Recruitment and Baseline Assessment
The study was advertised via brochures, fliers, newspaper, and other print media, as well as through referrals from CTP treatment staff. A potential participant who was not already in treatment at the CTP and who responded to an advertisement needed to enroll in outpatient treatment at the CTP in order to participate. Recruitment occurred over a 21-month period in 2004 and 2005. Interested participants completed a brief in-person or telephone screen to ascertain likely eligibility, followed by an in-person screening assessment to confirm eligibility. All participants who completed a screening assessment first signed an informed consent which included appropriate HIPAA language. Finally, a third (baseline) interview was completed, with additional study consent, to further assess substance use, PTSD, and social characteristics. Baseline interviews lasted approximately 2.5 to 3 hours. Independent Assessors who remained blind to randomization assignment performed all baseline and post-treatment assessments. After completion of the baseline assessment, eligible participants were randomized to SS or WHE.
Randomization
Randomization was stratified by prescription psychotropic medication use and by whether the participant met criteria for only an alcohol use disorder (as opposed to a drug use disorder only or both drug and alcohol use disorders concurrently). A statistician generated one blocked randomization list (block size known only to this statistician) for the entire study. Each CTP received sets of 60 sealed, tamper evident, security envelopes, containing one randomization number and the corresponding treatment assignment.
Treatments
In consultation with the developer, Dr. Lisa Najavits, the SS treatment was abbreviated from 25 to 12 core sessions to better fit within a feasible time frame for community-based outpatient treatment programs. Seeking Safety is a structured cognitive-behavioral treatment with both safety/trauma and substance use components integrated into each session (Najavits, 2002). All sessions have the same structure: 1) check in, including reports of any “unsafe” behaviors and use of coping skills; 2) session quotation, a brief point of inspiration to affectively engage participants and link to the session topic; 3) relating the material to the participants’ lives, in which hand-outs are used to facilitate discussion and structured skill practice; and 4) check out, including a commitment to specific between-session skills practice. Each session covered a different topic, and were as follows: Safety, PTSD: Taking Back your Power; When Substances Control You; Honesty; Setting Boundaries in Relationships; Compassion; Healing from Anger; Creating Meaning; Integrating the Split Self; Taking Good Care of Yourself; Red and Green Flags, and Detaching from Emotional Pain (Grounding).
The WHE active comparison condition was adapted from a protocol developed to be an attention control group for a treatment grant for female partners of injection drug users (Miller, Pagan, & Tross, 1998). It is a psychoeducational, manualized health curriculum focused on topics such as understanding the female body, human sexual behavior, pregnancy and childbirth, sexually transmitted diseases, HIV, and AIDS. WHE was designed to provide equivalent therapeutic attention, expectancy of benefit, and an issue-oriented focus, but without theory-driven techniques (i.e., of SS, nor any explicit focus or psychoeducation specific to substance abuse or trauma). All WHE sessions followed a common format: 1) introduction of topic; 2) review of group rules and between session assignment; 3) topic presentation, 4) video, storytelling and/or text readings; and 5) topic exercises in a variety of formats to facilitate group discussion and application of session materials; and 6) setting between-session goals.
Each intervention consisted of an initial individual session with the therapist to discuss the result of the participant’s random assignment, intervention format, and group rules. Research staff contacted participants to schedule this session within one day of randomization which was done immediately after the baseline assessment. Participants had to attend the individual session before starting treatment. Both groups had an open, rolling enrollment format, lasted approximately 75–90 minutes, and ran as long as at least 3 women were enrolled (n = 20 for those who had to wait six weeks or longer to begin their group treatment). Due to the criterion of needing 2 women present to conduct the group, many women took longer than 6 weeks to complete the interventions. Even if women could not immediately enter the group, the individual session took place right after randomization.
Training and Fidelity
Therapists and therapist supervisors from each site (heretofore referred to as “local supervisors”) were selected based on willingness to be randomized and after submitting an audiotaped therapy session exemplifying their ability to deliver a cognitive behavioral style of therapy. All counselors were women. About 6% had less than a bachelor’s degree, 39% held a bachelor’s degree, and 56% had a master’s degree or greater. Half of the counselors were white, 28% black, and 22% Latina. Supervisors were more likely to be white (67%) and have a master’s degree or doctorate (83%). After signing informed consent, two counselors and two local supervisors per site were randomized to deliver one of the two study interventions. All counselors and local supervisors attended a comparable centralized three day workshop and local supervisors received another half day of training focused on how to carry out supervision. Following training, counselors and local supervisors became certified once they successfully completed a training group of at least 4 sessions in the treatment to which they were assigned. An expert from the lead training team [L.C., G.M, and two SS trainers] rated the videotaped certification sessions for adherence to the manual and competency in the delivery of the interventions. The local supervisors obtained inter-rater reliability with the lead expert trainers on the adherence measures using the certification sessions.
Once the trial was underway, all intervention sessions were videotaped and a proportion of the tapes rated by local supervisors (≥ 50%). Throughout the study therapists met weekly with local supervisors for supervision, and if adherence fell below competency criterion, additional supervision was provided. In order to ensure competency on an ongoing basis, local supervisors had weekly conference calls with lead node experts [L.C., G.M, and two SS trainers]. The lead node experts rated a randomly selected quarter (29%) of the therapist session tapes reviewed by the local supervisor, comparing their ratings with the local supervisors’ ratings to assure supervisor fidelity and inter-rater reliability. For both interventions during the study, supervisor fidelity was determined by whether or not lead node experts and site supervisor ratings were in agreement on fidelity at a 70% level using specific adherence measures for each treatment.
Treatment as usual
All study participants were enrolled in one of the participating CTPs and were asked to attend treatment as usual (TAU) at the program during the six week treatment phase of the study. As mentioned above, TAU was not kept constant across sites but allowed to vary. Outpatient treatment differed across sites in frequency and length of sessions per week, although most offered intensive outpatient services of 3 days per week or more. The treatment orientation of the programs also varied, but none of the programs provided trauma focused treatment to participants during the study. During the study treatment and follow-up phases, TAU data was collected and categorized as mental health, outpatient medical, inpatient substance abuse treatment, emergency room or hospitalization, and 12-step meeting attendance.
Participants who dropped from the CTP prior to completing treatment were removed from the treatment portion of the study, but continued with follow-up assessments.
Measures
After screening and baseline assessments, randomized participants met weekly with the Research Assistant throughout the treatment phase of the study. During these weekly visits, urine drug screen, saliva alcohol tests, adverse events, self-reported PTSD symptoms and substance use data were collected. The Research Assistant met with the women as a group to read and assure completion of the self report assessments. Following the intervention phase of the study, assessments were conducted by the Independent Assessor, blinded to randomization assignment, at 1 week, 3-month, 6-month and 12-month follow-ups.
Sociodemographics
Basic demographic data, including age and race/ethnicity, were collected at the screening assessment and marital status, monthly income, employment pattern (prior 3 years), domestic living situation (prior 3 years), and prior treatment episodes were collected at baseline.
Post Traumatic Stress Disorder
PTSD was assessed via the Clinician Administered PTSD Scale (CAPS) (Blake et al., 1995), a structured interview which measures traumatic life events and frequency and intensity of signs and symptoms of PTSD in the past 30 days and is used as a measure of DSM-IV PTSD diagnosis and treatment outcome. The scale has three symptom cluster subscales; re-experiencing, avoidance/numbing, and hyperarousal. Cluster severity scores are calculated by summing the frequency and intensity of scores for each of the three subscales; an overall total scale score is obtained by summing subscale scores. Independent assessors had weekly conference calls with the lead team to maintain competency and inter-rater reliability on the measure. A 30 point or greater improvement on the CAPS can be used to determine clinically significant improvement of PTSD symptoms (Weathers, Keane & Davidson, 2001). Questions ascertaining childhood vs. adult physical and sexual abuse were asked separately. Childhood sexual abuse was defined as any sexual activity against your will under the age of 18.
Substance Use Diagnosis
Substance diagnostic data were collected via the Composite International Diagnostic Interview for DSM-IV (CIDI) (Robins et al., 1989), a fully structured, interviewer-administered measure used to determine lifetime and current substance disorder diagnoses for alcohol, marijuana, stimulants, opioids, cocaine, and sedatives.
Several assessment instruments were administered at baseline, weekly throughout the treatment phase and at each follow-up assessment. The Substance Use Inventory (SUI) consists of a series of self report questions about quantity and frequency of substance use adapted from the Time Line Follow-Back measure (Weiss, Huffard, Najavits, & Shaw, 1995) and included alcohol, cocaine, heroin, marijuana, sedatives and stimulants. The Post Traumatic Stress Disorder Symptom Scale-Self Report (PSS-SR) is a self report inventory that assesses the frequency and intensity of PTSD symptoms (Foa, Riggs, Dancu, Constance, & Rothbaum, 1993). The PSS-SR was used to assess PTSD symptom severity throughout the treatment phase of the study. Biologically confirmed abstinence for drugs of abuse was obtained by use of the SureStep urine drug screen card, a rapid visual immunoassay for the qualitative detection of 10 drug and drug metabolites in human urine. Recent alcohol use was tested using the ALCO-Screen Saliva Alcohol Test, distributed by Jant Pharmacal Corporation, that uses a reactive pad to test for the presence or absence of alcohol blood content greater than 0.02%. The SUI and PSS-SR captured data from the past week at baseline and all follow-up time points and since the last assessment during the treatment phase so as to assess substance use during the entire treatment phase.
Participants were compensated with cash or vouchers valued at $20 for the completion of the screening and $20 for the completion of the baseline assessments. For the follow-up compensation, participants received $20 in cash or voucher for completion of the 1-week post-treatment follow-up, $30 for the 3-month follow-up, $40 for the 6-month follow-up and $50 for the 12-month follow-up assessments. In addition, they received $10 for completion of weekly treatment assessments. These amounts varied by site depending on local research study comparability.
Statistical Methods
The demographic information and severity of symptoms at baseline among the two treatment groups were compared using the t-test for continuous variables and the χ2-test for categorical variables.
The overall data analytic strategy used was applied similarly for each of four models examining each of the four main outcome variables. The main outcome variables were PSS-SR severity, total CAPS severity, a continuous measure of the number of days using drugs or alcohol during the past seven days, and self reported abstinence in the prior seven days confirmed with urine and saliva tests. First, generalized linear models (using identity link function for normal data) were used to examine the effect of treatment group (SS vs. WHE) on the primary outcome measures over time for the intention-to-treat (ITT) sample of all randomized participants. All outcome measures were obtained at baseline, weekly during the treatment, 1-week post-treatment and over the follow-up period with the exception of the CAPS which was not conducted during treatment. We modeled each of four outcomes as a function of treatment, time of assessment, and baseline value of that outcome (before randomization). All models included pre-selected baseline covariates: race/ethnicity, age, and education level. Preliminary analyses examined potential additional covariates (frequency of services utilization during treatment as a measure of TAU, medication use, and duration of time in the active treatment phase), but none were found to be significantly different across SS and WHE groups and were not included in the primary outcome analyses. The possible interactions between treatment, the baseline level of the outcome measure, and time were tested and included in the final model only if statistically significant (p < .05) using backward elimination procedures. Time was defined as the assigned week of the treatment not the actual week of treatment. Baseline was time 0. The first assigned treatment was week 1. The generalized estimating equations (GEEs) (Diggle, Liang, & Zeger, 1994) were used to estimate and test the models. The GEE methodology is able to handle correlated data arising from repeated measurements, requires no parametric distribution assumption, and provides robust inference with respect to misspecification of the within-subject correlation. This analysis also allows for examination of continuous and categorical data which may be missing for some subjects either because of a missed session or drop-out; thus complete information for all subjects is not needed. All inferences above from incomplete or missing data are presumed valid provided that the data are “missing at random” (Little & Rubin, 2002). Since the two treatments did not differ in treatment attendance, study retention, or follow-up completion, the inference for the treatment effect was valid.
The outcome measures were heterogeneous across the small number of sites and testing differences in treatment effects among the clinical sites was desired, thus the site was always tested as an additional fixed effect in each of the four models. The models also included an indicator of the study phase (during intervention vs. follow-up) and possible interactions with treatment and time of assessment, except for the model predicting total CAPS score since the CAPS was not assessed during treatment. Comparisons between the two groups during the intervention phase (Week 1 to 1-week post treatment) and during follow-up time points (3-, 6-and 12-months post-treatment) were made using contrast statements. The study was powered to detect small to medium primary outcome effects (d=.3) based on Brown & Prescott (1999) and Raudenbush & Liu (2000) and power analyses were performed using S-Plus 6 (Insightful, 2001) with s=0.8 and α =.05. No corrections were made for multiple dependent variables.
As an a priori subgroup analysis, models were also fit to examine the effect of treatment assignment on the participants who completed at least 6 of the intervention sessions, defined at the point of study design as having received at least minimal exposure to the intervention (minimal attendance analysis). The participants’ weekly PSS-SR severity score was added as a time-dependent covariate to the generalized linear model for the participants’ abstinence status of drug use described above. PROC GENMOD in SAS 9.1.3 (SAS, 2003) was used to conduct all analyses.
RESULTS
Study Sample
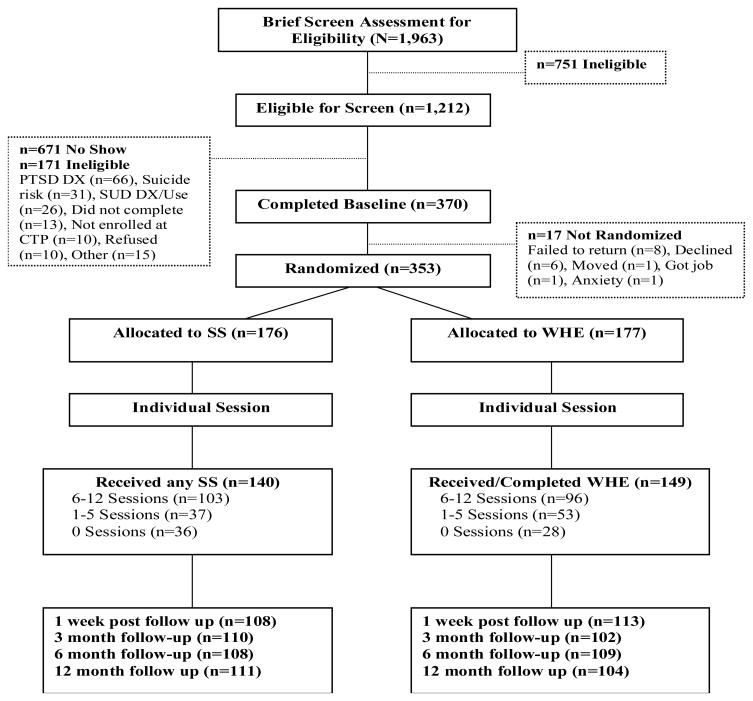
Figure 1 shows the patient flow from screening through 12-month follow-up. A total of 353 women met all eligibility criteria and were randomized into the study. Table 1 presents demographic, psychiatric, and trauma-related characteristics of the sample. The average age of the sample was 39.2 years. Forty five percent were Caucasian and 34.0% African American. Almost 18% were married and 41.1% lived with a partner. More than half (55.0%) were unemployed. They received an average of 5 previous courses of treatment for alcohol or drug abuse before enrolling in this study. About one quarter of participants (25.6%) were in a controlled environment in the 30 days prior to study enrollment. All participants met DSM-IV criteria for either full (80.4%) or sub-threshhold PTSD (19.6%). The most frequently diagnosed substance use disorder was cocaine dependence (70.5%), followed by alcohol (56.1%), marijuana (27.2%) and opioid dependence (25.6%).
Figure 1.
CONSORT diagram of participant flow through the protocol.
Table 1.
Baseline Participant and Diagnostic Characteristics by Treatment Group for the Intention-to-Treat Sample (N=353)
| Seeking Safetya | Women’s Health | ||
|---|---|---|---|
| Total | Educationa | ||
| N=176 | N=177 | ||
| Variables |
Mean(SD) or % |
||
| Age (years)b | 39.2(9.3) | 39.3(9.5) | 39.0(9.1) |
| Race/Ethnicityb | |||
| African American/Black | 34.0 | 33.0 | 35.0 |
| Caucasian | 45.6 | 47.16 | 44.1 |
| Latina | 6.5 | 3.98 | 9.0 |
| Multi-racial | 13.3 | 15.34 | 11.3 |
| Other | 0.6 | 0.6 | 0.6 |
| Marital Status | |||
| Married | 17.6 | 14.8 | 20.3 |
| Single | 36.8 | 37.5 | 36.2 |
| Divorced/Separated | 45.6 | 47.7 | 43.5 |
| Education (years)b | 12.5(2.4) | 12.7(2.3) | 12.4(2.6) |
| Employment | |||
| Employed | 40.2 | 40.3 | 40.1 |
| Unemployed | 55.0 | 54.6 | 55.4 |
| Student/Retired/Disabled | 4.8 | 5.1 | 4.5 |
| Prior Alcohol/Drug Treatment Episodes | 5.0(7.9) | 5.1(7.4) | 5.0(8.2) |
| Controlled Environment (past 30 days) | 25.6 | 28.2 | 23.0 |
| Currently Prescribed Psychotropic Medicationc,d | 44.8 | 45.5 | 44.1 |
| Current Substance Abuse or Dependence Diagnosis | |||
| Cocaine | 70.5 | 72.7 | 68.2 |
| Stimulants | 7.7 | 8.5 | 6.8 |
| Opiates | 25.6 | 25.6 | 25.6 |
| Marijuana | 27.2 | 27.8 | 26.6 |
| Alcohol | 56.1 | 59.7 | 52.5 |
| Current Alcohol Abuse or Dependence Diagnosis Onlyd | 8.8 | 8.5 | 9.0 |
| Baseline 7-day Abstinent Rate | 46.2 | 44.1 | 46.9 |
| PTSD Diagnosis (% full) | 80.4 | 76.7 | 84.2 |
| CAPS Severity (Total) | 62.9(19.4) | 61.6(19.36) | 64.2(19.4) |
| Lifetime Traumatic Experiences | |||
| Child Physical Abuse | 58.7 | 61.1 | 56.3 |
| Adult Physical Abuse | 84.8 | 83.4 | 86.2 |
| Child Sexual Abuse | 70.1 | 73.6 | 66.7 |
| Adult Sexual Abuse | 67.6 | 65.1 | 70.1 |
| Transportation Accident | 72.7 | 72.2 | 73.3 |
| Life Threatening Illness | 39.8 | 41.5 | 38.1 |
| Exposed to Violent Death | 19.3 | 16.5 | 22.2 |
There were no statistical differences between treatment groups on any variable.
The variables were used as covariates in the models.
Psychotropic medication was defined as medication prescribed for an emotional, psychological or psychiatric purpose to include depression, anxiety, psychosis, mood stabilization, or sleep disturbance.
Variables included in randomization stratification.
The average CAPS total score among all participants was 62.9 (SD = 19.4), consistent with a severe level of PTSD symptoms at baseline (Weathers et al., 2001). A summary of lifetime traumatic events exposure revealed that the majority of participants had experienced physical abuse (84.8%) or sexual abuse (67.6%) during adulthood. Very high rates of childhood abuse histories (70.1% sexual and 58.7% physical abuse) were also reported. Many of the participants reported other traumatic experiences including transportation accidents (72.7%) and a life threatening illness (39.8%). There was no significant difference between the two treatment groups on any demographic or baseline diagnostic characteristics.
Treatment Attendance and Study Retention
The median time from randomization to first treatment was 7 days. Overall, 81.9% (n = 289) of participants attended at least one group treatment session. Over half (n = 199, 56.4%) completed at least six treatment sessions (n = 43, 12.2% completed all twelve sessions). The average number of sessions completed was 6.2 (SD = 4.5) in the SS group and 6.0 (SD = 4.3) in the WHE group. Participants also received TAU during the treatment phase of the study. During treatment, participants attended on average about one and a half mental health visits per week (M = 1.3, SD = 1.6 for SS; M = 1.5, SD = 2.7 for WHE) and 3 12-step meetings (M = 3.4, SD = 4.1 for SS; M = 2.8, SD = 3.7 for WHE) in addition to study treatment. A total of 248 (70.3%) participants had at least one visit during the follow-up phase and 64 participants (18.1%) had no visits following randomization. Again, the two treatment groups did not differ on treatment attendance, other service utilization, study retention, or follow-up completion over the course of the study.
Counselor and Supervisor Fidelity
Seeking Safety supervisors rated a total of 257 counselor sessions. The mean, standardized score (based on a 5-point Likert scale) on the Seeking Safety Adherence Scale (Najavits & Liese, 2004) was 3.8, representing an acceptable level of counselor adherence. The internal consistency reliability of the total scale was .82, which is excellent and the average measure reliability (ICC) was good (.73). WHE supervisors rated a total of 193 counselor sessions. The mean adherence score was 4.0 out of a possible 5-point scale, corresponding to a rating of “good” on the adherence scale. The internal consistency reliability was .98, considered excellent, and average measure reliability (ICC) was good (.77).
PTSD Outcomes
PSS-SR severity
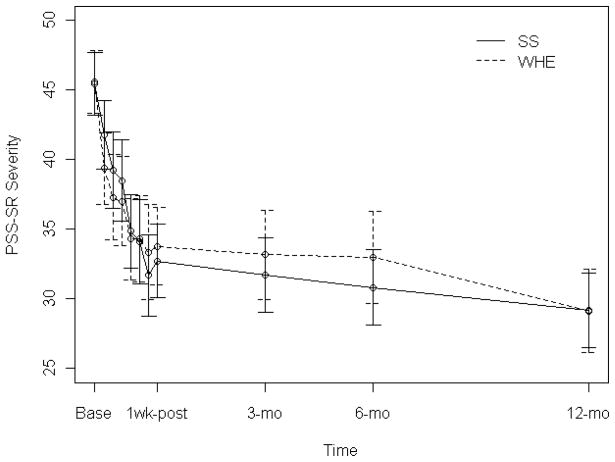
The PSS-SR severity score across the trial period is shown in Figure 2 and summarized in Table 2. In the final model, there was a significant study phase by treatment by time interaction effect (χ2(1) = 4.18, p-value < .05) on PSS-SR outcomes over the course of the study, which indicated that the treatment by time effect on participant’s PSS-SR score was different between the 6-week intervention period and the follow-up period. The final model for the PSS-SR severity included age, education, race/ethnicity, site, baseline PSS-SR severity, treatment, study phase (treatment week vs. follow-up), two-way interactions between PSS-SR severity and treatment week, treatment week and follow-up, and a three-way interaction between treatment week, follow-up, and treatment as a result of a backward elimination. Significant effects included education (χ2(1) = 4.62, p-value < .05) and site (χ2(6) = 27.29, p-value < .001; in one site baseline PSS-SR severity was significantly higher than in the rest of the sites who did not statistically differ from one another). During the six week treatment phase, the mean value of PSS-SR severity in both groups decreased. Participants in WHE group showed more reduction in PSS-SR severity than those in SS group during the first week. After the first week, the mean value of the PSS-SR severity in SS group decreased significantly more quickly than the WHE group (χ2(1) = 3.85, p-value = .05). By the end of treatment (1-week post treatment), there was no significant difference in mean PSS-SR severity between SS and WHE group (M = 32.7 (SD = 13.9) for SS vs. M = 33.8 (SD = 15.1) for WHE, p-value = .59). The PSS-SR severity score continued to decrease in both groups during the follow-up phase, but this was at a significantly slower rate compared to the treatment phase (χ2(1) = 7.99, p-value < .01). By the end of the 12-month follow-up period, the two groups showed no differences in mean PSS-SR severity (M = 29.2 (SD = 14.3) for SS vs. M = 29.1 (SD = 15.5) for WHE, p-value = .97). The interaction between the baseline PSS-SR severity and time was also significant (χ2(1) = 9.96, p-value < .01) such that participants with higher baseline PSS-SR severity improved more quickly than those with lower baseline PSS-SR severity during the treatment.
Figure 2.
Mean (with 95% CI) Post-Traumatic Stress Scale Self-report severity at baseline, 1-week post-treatment, 3-, 6- and 12-month follow-up for the intention-to-treat sample (N=353). Study phase refers to in-treatment (baseline to 1-week post-treatment) versus over the follow-up period (3-, 6- and 12-months post-treatment). There is a significant study phase by treatment by time interaction effect (χ2(1)=4.18, p-value = .04). PSS-SR=Post-traumatic Stress Scale Self-report (Foa et al., 1993).
Table 2.
Relevant Means, Standard Deviations, Odds Ratios, Confidence Intervals and Effect Sizes for the Primary PTSD and Substance Use Outcomes for the Intention-to-Treat (N=353) and Minimal Attendance Samples (n=199) at Baseline (based on raw data), 1-Week Post-Treatment and Over the Follow-up Study Period (model based)
| ITT Analysis | Minimal Attendance Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Outcome | Baseline | 1-wk Post- Treatment | Avg. over Follow-up | Baseline | 1-wk Post-Treatment | Avg. over Follow-up | |
| PSS-SR | SS | 45.4(15.3) | 32.7(13.9) | 30.0(13.0) | 45.8(15.7) | 32.6(14.1) | 29.8(12.9) |
| WHE | 45.6(15.3) | 33.8(15.1) | 32.0(15.0) | 47.0(15.8) | 34.9(15.8) | 31.7(14.7) | |
| ES | 0.01 | 0.07 | 0.15 | 0.08 | 0.15 | 0.14 | |
| CI | −.17,.18 | −.10,.26 | −.05,.30 | −.11,.24 | −.04,.32 | −.05,.30 | |
| CAPS | SS | 61.6(19.4) | 31.7(23.4) | 24.3(22.1) | 63.3(19.8) | 32.0(23.8) | 24.1(21.9) |
| WHE | 64.2(19.4) | 32.7(23.4) | 27.1(23.4) | 63.7(20.2) | 34.9(22.8) | 27.0(22.8) | |
| ES | 0.14 | 0.04 | 0.12 | 0.02 | 0.12 | 0.13 | |
| CI | −.05,.31 | −.14,.23 | −.05,.31 | −.15,.20 | −.06,.30 | −.04,.30 | |
| Abstinence | SS | 45% | 54% | 46% | 48% | 51% | 45% |
| Rate | WHE | 47% | 55% | 43% | 51% | 57% | 43% |
| OR | 0.96 | 1.05 | 1.04 | 1.15 | 1.28 | 1.04 | |
| CI | .71,1.65 | .62,1.78 | .94,1.16 | .66,2 | .69,2.34 | .93,1.16 | |
| Days of | SS | 1.7(2.6) | 0.8(1.8) | 1.4(2.1) | 1.5(2.5) | 0.78(1.7) | 1.4(2.2) |
| Drug use | WHE | 1.6(2.5) | 0.78(1.8) | 1.5(2.1) | 1.4(2.5) | 0.54(1.6) | 1.5(2.1) |
| ES | 0.02 | 0.01 | 0.07 | 0.02 | 0.15 | 0.03 | |
| CI | −.16,.20 | −.17,.19 | −.11,.24 | −.15,.19 | −.04,.32 | −.14,.2 | |
Note. SS=Seeking Safety Groups, WHE=Women’s Health Education Groups, PSS-SR=Post-traumatic Stress Scale Self Report (Foa et al., 1993), and CAPS=Clinician Administered PTSD Scale (Blake et al., 1995). Abstinence Rate=biologically confirmed yes/no for 1-week post-assessment, Days of Drug Use=number of days biologically confirmed self reported use over the previous seven days prior to assessment, ES=effect size, OR=odds ratio, and CI=confidence interval.
CAPS total
In the final model, the analysis revealed no treatment effect on CAPS total score (χ2(1) = 0.07, p-value = .78). The final model for CAPS total included age, education, race/ethnicity, site, baseline CAPS total, treatment and time as a result of the backward elimination method. Significant effects included site (χ2(6) = 43.08, p-value < .001), baseline CAPS total (χ2(1) = 42.27, p-value < .001), and time (χ2(1) = 57.87, p-value < .001). At baseline the average CAPS total score was 61.6 (SD = 19.4) for SS group and 64.2 (SD = 19.4) for WHE group. Post-treatment, the mean CAPS total score in both SS and WHE group decreased from baseline (31.7 (SD = 23.4) vs. 32.7 (SD = 23.4); t = 20.1, df = 215, p-value < .001); thus clinically significant reductions (i.e., 30 or more scale points) were attained in 47.7% of those in the SS group and 45.9% for those in WHE group. The baseline CAPS total score was a strong predictor of outcome whereby higher baseline CAPS total scores predicted higher CAPS total scores throughout the study. Site was also a significant predictor of outcome such that two of the sites statistically differed from the rest in CAPS scores at each time point (χ2(6) = 43.08, p-value < .001), one having higher and one having lower scores.
Substance Use Outcomes
Seven day abstinence from any illicit drug or alcohol use
In the final model, there were no treatment specific effects evident on abstinence rates. The final model for abstinence rates included age, education, race/ethnicity, site, baseline abstinence status, treatment, time, study phase (treatment week vs. follow-up), and study phase by time interaction. Significant effects included baseline abstinence status (χ2(1) = 48.59, p-value < .001), and site (χ2(6) = 45.28, p-value < .001; one was significantly lower and two were significantly higher than the remaining four sites). Throughout the trial, the seven day abstinence rate of any illicit drug or alcohol use was not significantly different between the two treatment groups. The baseline abstinence rates were 45% and 47% respectively for SS and WHE. By 1-week post treatment, these rates slightly increased to 54% and 55%, but this change was not statistically significant. At the 12-month follow-up, lower abstinence rates (43% for SS and 41% for WHE) were noted in comparison to baseline levels.
Percent of days using drugs or alcohol
In the final model, no treatment effects were observed on number of days using drugs or alcohol. The final model for days of use included age, education, race/ethnicity, baseline days of use, site, treatment, time, and baseline days of use by time interaction. Significant effects included a two-way interaction between baseline days of use and time (χ2(9) = 27.40, p-value < .001), whereby the effect of baseline drug use at week 1 was stronger than those in other weeks; and site (χ2(6) = 15.53, p-value= .02), whereby in one site baseline days of use was higher and in two sites lower. Since the number of days using drugs or alcohol was recorded as the number of days since last assessment, and the time intervals between two assessment times varied during treatment, the percent days of use was used in the model. As there was no clear linear time trend for the number of days using drugs or alcohol, time was treated as a categorical variable. At baseline, the average number of days using drugs or alcohol over the prior seven was 1.66 days (SD = 2.56) for the SS group and 1.60 days (SD = 2.52) for the WHE group. After receiving 6 weeks of treatment, fewer days using drugs or alcohol during the prior seven were reported in both groups with 0.80 days/week for SS group and 0.78 days/week for WHE, but this effect was not statistically significant. During the follow-up period, the number of days using drugs or alcohol increased, but again was not statistically significant. At the 12-month follow-up, the average number of days using drugs or alcohol in the prior seven returned to baseline levels, 1.65 days/week (SS) and 1.68 days/week (WHE), respectively. The most significant predictor of percent of days of drug or alcohol use at follow-up was the baseline number of days using.
Minimal attendance analysis
During the 6-week treatment, over half (n = 199, 56.4%) completed at least six treatment sessions (59% for SS and 54% for WHE). After fitting the same models as ITT analyses, the results were similar for all main outcomes except CAPS. For participants who attended at least six group sessions, a non-statistically significant but notable main treatment effect on CAPS total score was observed (χ2(1) = 3.13, p-value = .08, effect size = .22) after controlling for the baseline CAPS total scores (the corresponding result for ITT analysis was χ2(1) = 0.07 and p-value = .78). The SS group showed an overall lower CAPS total score compared with the WHE group throughout the follow-up period, but this difference was not at a level of statistical significance.
DISCUSSION
Implications of Main Effectiveness Findings
Despite the widespread recognition of the prevalence and adverse prognostic implications of trauma and trauma-related disorders among women in treatment for substance use disorders, treatment research on this problem has been limited. This study, the largest randomized clinical trial of a trauma-focused behavioral therapy for women with co-occurring substance use disorder, tested the effects of a trauma-focused group therapy, Seeking Safety (SS), in comparison to an attention control group, Women’s Health Education (WHE), among women enrolled in community-based substance abuse intensive outpatient treatment programs across the U.S. Both treatments were associated with large and clinically significant reductions in PTSD symptoms, which occurred rapidly during the acute treatment phase and were sustained over 12 months of follow-up. There were no overall differences in PTSD outcomes between the treatments, but among those who had a minimally adequate exposure (minimal attendance) to treatment, there was a trend toward lower symptom severity post-treatment on one clinician-rated measure (CAPS). It is important not to overstate the apparently different findings for clinician-rated vs. self-reported PTSD symptoms. The CAPS result, even for participants who received minimal treatment or greater was at a notable, but non-statistically significant level. In addition, the PSS-SR means were in the same direction, although not significant, and the effect sizes for SS vs. WHE differences post-baseline are similar. Previous treatment studies have varied regarding agreement between clinician- and patient-rated symptoms (Forbes, Creamer, & Biddle, 2001; Monson et al., 2008). In the current study, there were no overall effects of time or treatment on substance use outcomes.
This study was designed to contain the essential features of a rigorous efficacy trial. Both SS and WHE were manual-guided. The active comparison condition was intended to be clinically credible and to provide equivalent professional time and attention, isolating the specific elements of the focus on trauma, substance abuse, and their interaction from non-specific elements of treatment engagement and therapeutic alliance. Clinicians delivering the interventions were carefully trained, supervised, and monitored for fidelity to the treatment manuals. Although there had been various prior studies of SS (including four with control conditions), the current study extends the prior literature in its larger sample size and more rigorous methodology. Prior studies had consistently evidenced significant superiority of SS compared to TAU controls (Gatz et al., 2007; Hien et al., 2004; Najavits et al., 2006; Desai et al., 2008), high acceptability of the treatment, and significant improvements in trauma-related symptoms, substance use, and associated problem areas. In the only other RCT (Hien et al.) comparing SS to an active treatment control (cognitive-behavioral relapse prevention) both arms achieved equivalent positive outcomes and were superior to a TAU wait-list control.
Results of the present study are similar to the prior RCT trial (Hien et al., 2004) in that SS and the attention control were associated with substantial but similar reductions in PTSD symptoms. The present trial does show a very modest indication for superiority of the trauma focused approach in SS—PTSD symptom scores declined more rapidly in SS during treatment, and tended to be lower at a clinically significant level at the end of treatment among those with at least minimal treatment attendance. This should encourage further treatment development research in an effort to enhance the efficacy of the trauma-focused approach for women with PTSD and substance abuse. Steps to consider include: 1) incorporating additional behavioral interventions for PTSD such as exposure therapy (e.g., Foa et al., 1991); 2) combining behavioral treatment with medications shown to be effective for PTSD such as SSRI antidepressants (e.g., Brady et al., 2005); 3) incorporating strategies to improve adherence to treatment, such as voucher incentives for attendance (Petry & Martin, 2002); and 4) testing longer term treatment models to determine whether the impressive trajectory of improvement observed during the 6 week treatment (Figure 2) could be extended, resulting in even greater reductions in symptoms over follow-up. The latter would be consistent with McLellan, Lewis, O’Brien, & Kleber’s (2000) argument that substance abuse is a chronic illness for which long term treatment models are needed.
Considerations for Implementation and Study Effectiveness
Seeking Safety was originally developed as a 25 session treatment. For this study, SS was altered to 12 sessions, to create a treatment model considered to be feasible given the limitations of counselor time and reimbursement under which community-based treatment programs in the U.S. currently operate. However, this change could have attenuated the effectiveness of SS. Given that SS was designed for group or individual modality, our findings underscore that group modality is feasible and can produce comparable results to an active comparison condition.
The improvement in PTSD symptoms observed among participants in the WHE comparison group is intriguing and should not be overlooked. WHE may have been more than a nonspecific control, rather an active comparison group. The impact of trauma on the body is now well known (e.g., van der Kolk et al., 1996), and a number of treatments have proliferated that address body issues as a central feature of recovery from traumatic stresses (Fitch & Dryden, 2000; Price, 2005). It is plausible that the material presented in WHE was relevant to trauma-survivors’ understanding of their body and how to think about proper self care. WHE was also conducted in a group format and qualitative feedback suggested it enjoyed strong support from staff and participants. Future treatment development efforts should consider incorporating some of these potential strengths of the body-centered health model of WHE.
A prevailing clinical concern in regard to treating patients with combined PTSD and substance abuse has been that discussion of past trauma or PTSD symptoms could increase arousal and stress and either exacerbate substance use or cause patients to flee treatment. There was no evidence for such phenomena in the present data. PTSD symptoms improved during trauma focused treatment (SS), and there was no increase in either TAU dropout or adverse events on SS compared to the WHE active comparison (see Killeen et al., 2008, for a separate review of these findings). This should further encourage treatment development efforts on trauma-focused treatments, although providing effective coping skills for the dysphoria and arousal associated with traumatic memories should remain a priority. Seeking Safety, as currently designed, does not contain explicit elements of exposure therapy. If exposure techniques are tested among patients with comorbid PTSD and SUD there should be careful attention to the management of arousal and its impact on substance use and treatment retention.
Study Limitations
The absence of a TAU or minimal treatment control group is a design limitation that restricts causal interpretations regarding the impact of the specific elements of SS and WHE treatments upon PTSD outcomes. However, our prior RCT showed no improvement in PTSD symptoms over time in a wait list control (Hien et al., 2004). Other controlled and/or randomized trials of SS compared to TAU have favored SS (Desai et al., 2008; Gatz et al., 2007; Najavits et al., 2006). A large, quasi-experimental study that examined outcome for clinical programs that were implementing integrated treatment for trauma and substance abuse, compared to other programs selected as matched controls and providing TAU, showed only a 15% to 20% reduction in PTSD symptoms for the control programs over a 12 month follow-up (Morissey et al., 2005), compared to the 35% to 40% reductions observed here. (In that study, SS was used by four of the nine sites.) Notably, in the present study although participants were enrolled in intensive outpatient programs, most had a relatively low dose of TAU (on average one mental health or substance abuse session per week). Taken together, these findings suggest that the improvements in PTSD symptoms observed in SS and WHE are less likely attributable to contact with the research team, the passage of time, or TAU. In a context where TAU is more intensive, the effects of these groups may be attenuated.
A second possible explanation for the present findings is that observed improvements in PTSD symptoms were due to non-specific elements of manual-guided treatments, such as attention from therapists, treatment alliance, or membership in a supportive group of women who share a history of trauma and PTSD, rather than the specific elements of SS and WHE. However, as discussed above, it may be that SS and WHE each have unique active elements. Again, future research should examine whether a more powerful treatment would result from combining elements of both, or whether patient-level predictors can be found that would help match patients to these contrasting behavioral approaches.
Another important finding is the lack of improvement in substance use outcomes in either SS or WHE. Abstinence and lower levels of substance use at baseline predicted abstinence and level of substance use over treatment and follow-up. Substance use at baseline for participants in this study was not as high as typically seen at treatment entry, with nearly 50% of participants abstinent and a mean of two days of drug use in the week prior to baseline. However, in contrast to this study, all other studies of SS showed significant reductions in substance use. Participants in the current study were at various points in their treatment; some had just entered outpatient treatment from inpatient care, some from detoxification, some were beginning outpatient at approximately the same time they entered the study and others were already enrolled and receiving outpatient services. This variation and an inclusion criterion of substance abuse or dependence at some point in the prior six months may have contributed to the high level of recent (one week) abstinence. This criterion was adopted in order to allow for the variety of circumstances under which women may enter outpatient treatment (i.e., from detoxification, inpatient treatment or jail, or as a result of legal or employment consequences that are recently brought to bear even though the woman may have discontinued use at an earlier time point). While potentially limiting power to observe treatment effects on substance use outcomes, including women with recent abstinence does reflect real variation in the population seen in community-based treatment, and thus does not diminish ability to generalize from these results to community treatment populations. A conceptual model for this study was that substance use and PTSD are causally linked, and therefore effective treatment of PTSD would result in improved substance use. However, the absence of robust differences in PTSD outcome between SS and WHE limits the ability to assert this mediated model.
With seven study sites, the sites were treated as a fixed effect in the statistical analyses rather than as a random effect. As such, generalizations of the findings beyond the present sample of treatment programs to a larger “population” of community-based treatment programs must be made with caution. This study may also have limited generalizability to ethnic populations with less representation in this sample, such as Latina women, and also, since the predominant substances used by participants were cocaine and alcohol, findings may not generalize to those with different or less chronic SUDs. One additional limitation related to generalizability is the large number of participants who were eligible after the initial brief screen (assessing gross eligibility), but who did not attend the screening interview (n=671). Specific data was not available about why these participants did not attend the screening interview, but could perhaps shed light on the acceptability of the study for a sub-population of clients.
Four other limitations are worth noting. First, the study achieved lower follow-up rates relative to previous work with similar populations (Hien et al., 2004; Morrisey et al., 2005). There is no evident bias in attrition from follow-up (SS vs. WHE differences), but it is possible that there is an unmeasured source of bias relating to attrition. Second, it is unknown how the high level of monitoring (for an effectiveness trial) of intervention delivery in this trial affected outcome. A generally untested assumption is that fidelity monitoring improves outcome. If this is the case, interventions such as these would be expected to perform less well under the typical constraints found in community treatment programs (e.g., availability of supervisory time, videotaping treatment sessions). Third, a lack of information on duration of treatment involvement prior to study enrollment limited the ability to control for the effect of current treatment episode length on the association between study intervention and trauma and substance use symptoms. Finally, the analytic approach to potential group cohort effects did not account for clustering within groups, as has been addressed recently by Morgan-Lopez & Fals-Stewart (2006); such analyses may reveal findings which are not in line with the ones reported herein.
Future Directions
This study adds to an emerging body of literature showing the promise of community based treatments on psychiatric outcomes (e.g., SAMHSA’s Women, Co-Occurring Disorders and Violence study, Morissey et al., 2005). It also adds to the existing outcome literature on SS in representing the largest and most rigorous trial of that model. Future treatment development research with SS should consider conducting groups in varying contexts of TAU (i.e., less intensity versus high), testing the full 25 session dose of SS, examining SS in combination with other behavioral treatments for PTSD, and adding a pharmacological intervention to improve impact on PTSD and substance use. This study suggests that the addition of gender specific treatment for women with co-occurring substance use and post-traumatic stress disorders can have a significant effect on trauma symptom reduction for a subset of patients. The pattern of our findings (e.g., nearly half of all patients did not have clinically significant change on PTSD scores and there were no differences on abstinence rates overall) underscores that therapy groups such as these continue to require future treatment development to better understand the complex needs of this patient population.
Appendix 1.
CONSORT Statement Checklist for Reporting a Randomized Trial
| PAPER SECTION and topic | Item | Descriptor | Reported on Page # |
|---|---|---|---|
| TITLE & ABSTRACT | 1 | How participants were allocated to interventions (e.g., “random allocation”, “randomized”, or “randomly assigned”). | 1–2 |
| INTRODUCTION Background | 2 | Scientific background and explanation of rationale. | 3–5 |
| METHODS Participants | 3 | Eligibility criteria for participants and the settings and locations where the data were collected. | 6–7 |
| Interventions | 4 | Precise details of the interventions intended for each group and how and when they were actually administered. | 7–10 |
| Objectives | 5 | Specific objectives and hypotheses. | 4–5 |
| Outcomes | 6 | Clearly defined primary and secondary outcome measures and, when applicable, any methods used to enhance the quality of measurements (e.g., multiple observations, training of assessors). | 4–5, 12–14 |
| Sample size | 7 | How sample size was determined and, when applicable, explanation of any interim analyses and stopping rules. | 16 |
| Randomization -- Sequence generation | 8 | Method used to generate the random allocation sequence, including details of any restrictions (e.g., blocking, stratification) | 8 |
| Randomization -- Allocation concealment | 9 | Method used to implement the random allocation sequence (e.g., numbered containers or central telephone), clarifying whether the sequence was concealed until interventions were assigned. | 8 |
| Randomization -- Implementation | 10 | Who generated the allocation sequence, who enrolled participants, and who assigned participants to their groups. | 8, 12 |
| Blinding (masking) | 11 | Whether or not participants, those administering the interventions, and those assessing the outcomes were blinded to group assignment. If done, how the success of blinding was evaluated. | 12 |
| Statistical methods | 12 | Statistical methods used to compare groups for primary outcome(s); Methods for additional analyses, such as subgroup analyses and adjusted analyses. | 14–16 |
| RESULTS Participant flow | 13 | Flow of participants through each stage. Specifically, for each group report the numbers of participants randomly assigned, receiving intended treatment, completing the study protocol, and analyzed for the primary outcome. Describe protocol deviations from study as planned, together with reasons. | 39 |
| Recruitment | 14 | Dates defining the periods of recruitment and follow-up. | 8 |
| Baseline data | 15 | Baseline demographic and clinical characteristics of each group. | 16–17, 35 |
| Numbers analyzed | 16 | Number of participants (denominator) in each group included in each analysis and whether the analysis was by “intention-to-treat”. State the results in absolute numbers when feasible. | 17, 35 |
| Outcomes and estimation | 17 | For each primary and secondary outcome, a summary of results for each group, and the estimated effect size and its precision. | 18–21 |
| Ancillary analyses | 18 | Address multiplicity by reporting any other analyses performed, including subgroup analyses and adjusted analyses, indicating those pre-specified and those exploratory. | 21 |
| Adverse events | 19 | All important adverse events or side effects in each intervention. | 24 |
| DISCUSSION Interpretation | 20 | Interpretation of the results, taking into account study hypotheses, sources of potential bias or imprecision and the dangers associated with multiplicity of analyses and outcomes. | 21–28 |
| Generalizability | 21 | Generalizability (external validity) of the trial findings. | 25–27 |
| Overall evidence | 22 | General interpretation of the results in the context of current evidence. | 28 |
Acknowledgments
The authors wish to acknowledge the work and support of the six Regional Research Training Centers involved in the implementation of this study and the invaluable commitment of the patients and staff at the seven participating Community-based Treatment Programs. Staff from NIDA’s Clinical Trials Network collaborated in the design and conduct of the study, assisted with data management and quality assurance during the trial, and provided comments for consideration on this manuscript. The authors also wish to thank the two anonymous reviewers for their comments during the manuscript review process.
The research reported in this article was supported by the following grants from the National Institute on Drug Abuse (NIDA): U10 DA13035 (Edward Nunes, PI), U10 DA13714 (Dennis Donovan, PI), U10 DA13038 (Kathleen Carroll, PI), U10 DA13732 (Eugene Somoza, PI), U10 DA13727 (Kathleen Brady, PI), U10 DA013720 (Jose Szapocznik, PI), U10 DA013046 (John Rotrosen, PI), K24 DA022412 (Edward Nunes). The Clinical Trial Identification Number is NCT00078156 (NIDA).
Footnotes
Dr. Nunes has served on the advisory panel and speakers’ bureau of Alkermes/Cephalon, Inc. (resigned October 2007). All other authors report no competing interests.
Portions of this research were presented at the American Psychiatric Association Annual Meeting, Washington, DC (May 2008) and the 39th Society for Psychotherapy Research International Meeting, Barcelona, Spain (June 2008)
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/ccp.
Contributor Information
Denise A. Hien, City University of New York and Columbia University College of Physicians and Surgeons.
Elizabeth A. Wells, School of Social Work, University of Washington.
Huiping Jiang, New York State Psychiatric Institute, New York, New York.
Lourdes Suarez-Morales, University of Miami.
Aimee N. C. Campbell, Columbia University School of Social Work.
Lisa R. Cohen, New York State Psychiatric Institute and City College of New York.
Gloria M. Miele, New York State Psychiatric Institute, New York, New York.
Therese Killeen, Medical University of South Carolina.
Gregory S. Brigham, Maryhaven, Inc., Columbus, Ohio.
Yulei Zhang, New York State Psychiatric Institute, New York, New York.
Cheri Hansen, The VillageSouth, Inc., Miami, Florida.
Candace Hodgkins, Gateway Community Services, Jacksonville, Florida.
Mary Hatch-Maillette, University of Washington.
Chanda Brown, Charleston Center, Charleston, SC.
Agatha Kulaga, New York University.
Allison Kristman-Valente, School of Social Work, University of Washington.
Melissa Chu, Addiction Research and Treatment Corporation, Brooklyn, New York.
Robert Sage, Addiction Research and Treatment Corporation, Brooklyn, New York.
James A. Robinson, Nathan S. Kline Institute for Psychiatric Research, Orangeburg, New York.
David Liu, National Institute on Drug Abuse Center for Clinical Trials Network, Bethesda, Maryland.
Edward V. Nunes, New York State Psychiatric Institute and Columbia University College of Physicians and Surgeons.
References
- Amaro H, Dai J, Arevalo S, Acevedo A, Matsumoto A, Nieves R, et al. Effects of integrated trauma treatment on outcomes in a racially/ethnically diverse sample of women in urban community-based substance abuse treatment. Journal of Urban Health: Bulletin of the New York Academy of Medicine. 2007;84(4):508–522. doi: 10.1007/s11524-007-9160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text revision. [Google Scholar]
- Blake DB, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Hickling EJ, Taylor AE, Loos WR, Gerardi RJ. Psychological morbidity associated with motor vehicle accidents. Behavior Research and Therapy. 1994;32:283–290. doi: 10.1016/0005-7967(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Brady KT, Buonopane A, Draper JC, Kalbag AS, Levin FR, McCance-Katz EF, et al. The most critical contemporary unresolved issues associated with the pharmacotherapy treatment of substance users. Substance Use & Misuse. 2005;40(13):2043–2048. [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Archives of General Psychiatry. 1991;48:216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- Brown H, Prescott R. Applied mixed models in medicine. New York, NY: John Wiley & Sons; 1999. pp. 183–189. [Google Scholar]
- Carroll KM, Rounsaville BJ. Bridging the gap: A hybrid model to link efficacy and effectiveness research in substance abuse treatment. Psychiatric Services. 2003;54(3):333–339. doi: 10.1176/appi.ps.54.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloitre M, Koenen K, Cohen L, Han H. Skills training in affective and interpersonal regulation followed by exposure. Journal of Consulting and Clinical Psychology. 2002;70:1067–1074. doi: 10.1037//0022-006x.70.5.1067. [DOI] [PubMed] [Google Scholar]
- Cohen JB, Dickow A, Horner K, Zweben JE, Balabis J. Abuse and violence history of men and women in treatment for methamphetamine dependence. American Journal on Addictions. 2003;12(5):377–385. [PubMed] [Google Scholar]
- Cook JM, Walser RD, Kane V, Ruzek JI, Woody G. Dissemination and feasibility of a cognitive-behavioral treatment for substance use disorders and posttraumatic stress disorder in the Veterans Administration. Journal of Psychoactive Drugs. 2006;38:89–92. doi: 10.1080/02791072.2006.10399831. [DOI] [PubMed] [Google Scholar]
- Desai RA, Harpaz-Rotem I, Najavits LM, Rosenheck RA. Impact of the Seeking Safety Program on clinical outcomes among homeless female veterans with psychiatric disorders. Psychiatric Services. 2008;59:996–1003. doi: 10.1176/ps.2008.59.9.996. [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Liang K-Y, Zeger SL. Analysis of longitudinal data. Oxford, England: Oxford University Press; 1994. [Google Scholar]
- Finkelstein N, VandeMark N, Fallot R, Brown V, Cadiz S, Heckman J. Enhancing substance abuse recovery through integrated trauma treatment. Bethesda, MD: Substance Abuse and Mental Health Services Administration Center for Substance Abuse Treatment; 2004. [Google Scholar]
- Fitch P, Dryden P. Recovering body and soul from post-traumatic stress disorder. Massage Therapy Journal. 2000;39:41–62. [Google Scholar]
- Folstein M, Folstein S, McHugh P. ‘Mini-Mental State’: A practical method for grading cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:196–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Foa EB, Rothbaum BO, Riggs D. Treatment of post-traumatic stress disorder in rape victims: A comparison between cognitive behavioral procedures and counseling. Journal of Consulting and Clinical Psychology. 1991;59:715–723. doi: 10.1037//0022-006x.59.5.715. [DOI] [PubMed] [Google Scholar]
- Foa E, Riggs DS, Dancu CV, Constance V, Rothbaum BO. Reliability and validity of a brief instrument for assessing posttraumatic stress disorder. Journal of Traumatic Stress. 1993;6(4):459–473. [Google Scholar]
- Forbes D, Creamer M, Biddle D. The validity of the PTSD Checklist as a measure of symptomatic change in combat-related PTSD. Behaviour Research and Therapy. 2001;39:977–986. doi: 10.1016/s0005-7967(00)00084-x. [DOI] [PubMed] [Google Scholar]
- Gatz M, Brown V, Hennigan K, Rechberger E, O’Keefe M, Rose T, et al. Effectiveness of an integrated trauma-informed approach to treating women with co-occurring disorders and histories of trauma. Journal of Community Psychology. 2007;35:863–878. [Google Scholar]
- Grubaugh AL, Magruder KM, Waldrop AE, Elhai JD, Knapp RG, Frueh BC. Subthreshold PTSD in primary care: Prevalence, psychiatric disorders, healthcare use, and functional status. Journal of Nervous and Mental Disease. 2005;193(10):658–664. doi: 10.1097/01.nmd.0000180740.02644.ab. [DOI] [PubMed] [Google Scholar]
- Hien DA, Cohen LR, Miele GM, Litt LC, Capstick C. Promising treatments for women with comorbid PTSD and substance use disorders. American Journal of Psychiatry. 2004;161:1426–1432. doi: 10.1176/appi.ajp.161.8.1426. [DOI] [PubMed] [Google Scholar]
- Insightful Corporation. S-Plus for Windows. Seattle, WA: Author; 2001. (Version 6) [Computer software] [Google Scholar]
- Killeen T, Hien DA, Campbell AC, Brown C, Hansen C, Jiang H, et al. Adverse events in an integrated trauma-focused intervention for women in community substance abuse treatment. Journal of Substance Abuse Treatment. 2008;35:304–311. doi: 10.1016/j.jsat.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. New York, NY: John Wiley & Sons; 2002. [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: Implications for treatment insurance, and outcomes evaluation. JAMA. 2000;284(13):1693. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Miller S, Pagan D, Tross S. Peer activism for female partners of injection drug users. Columbia University; 1998. Women’s Health Education. Unpublished treatment manual. [Google Scholar]
- Monson CM, Gradus JL, Young-Xu Y, Schnurr PP, Price JL, Schumm JA. Change in posttraumatic stress disorder symptoms: Do clinicians and patients agree? Psychological Assessment. 2008;20(2):131–138. doi: 10.1037/1040-3590.20.2.131. [DOI] [PubMed] [Google Scholar]
- Morgan-Lopez AA, Fals-Stewart W. Analytic complexities associated with group therapy in substance abuse treatment research: Problems, recommendations, and future directions. Experimental and Clinical Psychopharmacology. 2006;14(2):265–273. doi: 10.1037/1064-1297.14.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey JP, Jackson EW, Ellis AR, Amaro H, Brown V, Najavits LM. Twelve-month outcomes of trauma-informed interventions for women with co-occurring disorders. Psychiatric Services. 2005;56:1213–1222. doi: 10.1176/appi.ps.56.10.1213. [DOI] [PubMed] [Google Scholar]
- Najavits LM. Seeking Safety: A treatment manual for PTSD and substance abuse. New York, NY: Guilford; 2002. [DOI] [PubMed] [Google Scholar]
- Najavits LM, Gallop RJ, Weiss RD. Seeking Safety therapy for adolescent girls with PTSD and substance abuse: A randomized controlled trial. Journal of Behavioral Health Services & Research. 2006;33:453–463. doi: 10.1007/s11414-006-9034-2. [DOI] [PubMed] [Google Scholar]
- Najavits LM, Liese BS. Seeking Safety Adherence Scale. McLean Hospital; Belmont, MA: 2004. Unpublished scale. [Google Scholar]
- Najavits LM, Weiss RD, Shaw SR, Muenz L. “Seeking Safety”: Outcome of a new cognitive-behavioral psychotherapy for women with posttraumatic stress disorder and substance dependence. Journal of Traumatic Stress. 1998;11:437–456. doi: 10.1023/A:1024496427434. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. Journal of Consulting and Clinical Psychology. 2002;70(2):398–404. doi: 10.1037//0022-006x.70.2.398. [DOI] [PubMed] [Google Scholar]
- Price C. Body-oriented therapy in recovery from child sexual abuse: An efficacy study. Alternative Therapies in Health and Medicine. 2005;11(5):46–57. [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Liu X. Statistical power and optimal design for multisite randomized trials. Psychology Methods. 2000;5(2):199–213. doi: 10.1037/1082-989x.5.2.199. [DOI] [PubMed] [Google Scholar]
- Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. Journal of Consulting and Clinical Psychology. 1993;61(6):984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, et al. The Composite International Diagnostic Interview: An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of General Psychiatry. 1989;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS software system for windows. Cary, NC: Author; 2003. (Version 9.1.3) [Computer software] [Google Scholar]
- Shore JH, Vollmer WM, Tatum EL. Community patterns of posttraumatic stress disorder. Journal of Nervous and Mental Disease. 1989;117:681–685. doi: 10.1097/00005053-198911000-00004. [DOI] [PubMed] [Google Scholar]
- van der Kolk BA, Pelcovitz D, Roth S, Mandel FS, McFarlane A, Herman JL. Dissociation, somatization, and affect dysregulation: The complexity of adaptation of trauma. American Journal of Psychiatry. 1996;153:83–93. doi: 10.1176/ajp.153.7.83. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-Administered PTSD Scale: A review of the first ten years of research. Depression and Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Hufford C, Najavits LM, Shaw SR. Weekly Substance Use Inventory. Harvard University Medical School; Boston, MA: 1995. Unpublished measure. [Google Scholar]
- Zlotnick C, Najavits LM, Rohsenow DJ. A cognitive-behavioral treatment for incarcerated women with substance use disorder and posttraumatic stress disorder: Findings from a pilot study. Journal of Substance Abuse Treatment. 2003;25:99–105. doi: 10.1016/s0740-5472(03)00106-5. [DOI] [PubMed] [Google Scholar]