Abstract
Anti-idiotypic antibodies (anti-Id) to autoantibodies are present in several autoimmune diseases and are hypothesized to have regulatory function. Recently we reported the presence of anti-Id to a major type 1 diabetes-associated autoantibody (GAD65Ab) in sera of healthy individuals. Our current assay for the detection of GAD65Ab-specific anti-Id requires the initial removal of anti-Id from the sera using immobilized monoclonal GAD65Ab, followed by detection of the now exposed GAD65Ab. However, anti-Id in samples that are GAD65Ab-negative cannot be detected in this assay. Furthermore, we cannot distinguish between serum GAD65Ab and the monoclonal GAD65Ab used in the absorption of anti-Id.
In this study we evaluated two novel detection assays for GAD65Ab-specific anti-Id. The biotin/streptavidin based absorption assay utilizes the strong interaction of biotin and streptavidin to prevent possible leakage of the immobilized antibody. Moreover, this assay format allows to identify the origin of the detected GAD65Ab. The ECL-based assay allows the direct detection of anti-Id independent of the presence of GAD65Ab.
We analyzed new-onset type 1 diabetes patients (n=133) and matched healthy controls (n=178) for the presence of GAD65Ab-specific anti-Id using both new detection assays and the original absorption assay.
We found that all three assays can distinguish between the type 1 diabetes cohort and the healthy control samples. The biotin/streptavidin assay allowed us to positively exclude the monoclonal GAD65Ab as the source of the detected GAD65Ab. While the original absorption assay showed the highest sensitivity and specificity, the ECL format showed the highest peak signal-to-noise ratio and excellent linear correlation, making this assay our first choice for quantification of anti-Id.
Keywords: anti-idiotypic antibodies, radioligand binding assay, glutamate decarboxylase, autoantibodies, type 1 diabetes, ECL
1. Introduction
Recently we reported the presence of concealed autoantibodies to the smaller isoform of glutamate decarboxylase (GAD65Ab) in the sera of healthy individuals (Oak et al., 2008). GAD65Ab are perceived as markers for the autoimmune response in type 1 diabetes (T1D) and latent autoimmune diabetes in adults (LADA) (for review see (Pihoker et al., 2005). We observed in healthy individuals GAD65Ab that are in complex with GAD65Ab-specific anti-idiotypic antibodies (anti-Id) blocking the autoantibodies from binding to GAD65. Therefore these antibodies are not detected in routine radioligand binding assays. Removal of anti-Id through absorption of sera to an excess of immobilized GAD65 monoclonal antibody allowed the detection of the now un-masked GAD65Ab in the serum. Sera that showed a significant increase in GAD65 binding after absorption of anti-Id were considered to be positive for GAD65Ab-specific anti-Id (Oak et al., 2008).
Anti-Id against autoantibodies are described also in other autoimmune diseases, such as systemic lupus erythematosus, myasthenia gravis, Sjorgren’s syndrome, and idiopathic thrombocytopenic purpura (Abdou et al., 1981; Dwyer et al., 1983; Zouali and Eyquem, 1983; Mehta et al., 2003) (Routsias et al., 2002). Their regulatory function has been suggested based on observations that anti-Id are found in healthy individuals and that in patients anti-Id levels are inversely related to disease severity (Williams and Isenberg, 1998; Mehta et al., 2003).
The previously employed assay allowed the detection of GAD65Ab/anti-Id complexes in the majority of healthy individuals and the specific lack of anti-Id masking T1D-associated GAD65Ab epitope specificities in sera from T1D patients. However, samples that were anti-Id-positive and GAD65Ab-negative would potentially be missed in this assay. Moreover, the use of an immobilized human monoclonal GAD65Ab to absorb the anti-Id prevented us from positively identifying the origin of the GAD65Ab detected.
To address these questions, we developed two novel detection assays. Direct detection of GAD65Ab-specific anti-Id is accomplished in an electrochemiluminescence (ECL)-based assay, while the utilization of the strong biotin-streptavidin interactions allows the identification of the source of the detected GAD65Ab.
In the present study we compared these two novel assays with the original absorption assay.
2. Material and Methods
2.1. Serum samples
We analyzed 178 healthy individuals and 133 T1D patients. These individuals were part of the Diabetes Incidence Study in Sweden (DISS) cohort, where newly diagnosed 15–35 year old T1D patients were registered in 1992–1993 (Törn et al., 2000). T1D patients were diagnosed according to WHO criteria. Age and region matched subjects were collected to serve as healthy controls. Sera in this study were selected at random regardless of their autoantibody status.
Characteristics of the participating subjects are shown in Table 1. All subjects or their legal guardians gave informed consent. Local institutional ethics committee approval and subjects’ consent was obtained prior to collection of all serum samples.
Table 1.
Subjects’ characteristics
| Ageatonset(years) | Gender | n | |
|---|---|---|---|
| Median/range | |||
| T1D | 24 (15–34) | 32% female | 133 |
| control | 24 (15–34) | 44% female | 178 |
2.2. Ruthenium and biotin conjugation of b96.11
Recombinant Fab (rFab) b96.11 was directly conjugated to ruthenium at a challenge ratio of 12:1 with Sulfo-tag NHS-ester (Meso Scale Discovery (MSD), Gaithersburg, MD) according to the manufacturers’ instructions. Subsequently the labeled antibody was buffer-exchanged to PBS using G-50 Sephadex Quick Spin High Capacity Columns (Roche Applied Science, Indianapolis, IN). Protein concentration was determined by Bradford, concentration of successfully sulfo-tagged protein was determined as absorption at 455 nm.
Purified monoclonal antibody or rFab were biotinylated using Biotin NHS Ester (MSD). Briefly, b96.11 (1mg/ml) was incubated with Biotin NHS Ester at a challenge ratio of 12:1. Recovery of the labeled protein was achieved as described above. Successful biotinylation was confirmed in a modified radioligand binding assay using streptavidin-agarose beads (Thermo Fisher Scientific Rockford, IL).
2.3. ECL assay
The Meso Scale Discovery (MSD) technology is based on ECL detection of proteins. The detecting protein is labeled with a sulfo-tag containing a ruthenium tris(bipyridine) complex that emits light when electrochemically stimulated. With a dedicated ECL plate reader, an electrical current is placed across the plate, resulting in the emission of the luminescent signal.
Biotinylated rFab b96.11 (0.3µg/reaction) and sulfo-tagged rFab b96.11 (0.6µg/reaction) were incubated with 10µl serum at 42°C for 10 min, followed by a 30 min incubation at 37°C, and a final incubation at room temperature for 15 minutes. These conditions were found to be optimal as determined by us previously, temperature dependency of the assay is discussed later in this study. The reaction was then transferred to a streptavidin-coated Multi-array 96-well plate (MSD) for 2 hours at room temperature. Unbound reagents were removed by 3 washes with PBS-T. Reading buffer (MSD T read buffer) (50µl/well) was added and the plate was analyzed using a dedicated ECL plate reader, the Sector Imager 2400 (MSD). Luminescence readings were converted into index by extrapolation using a control serum with a designated index of 1. Cut-Off for positivity was set at an index of 0.13 and was determined by displacement using unlabeled rFab b96.11 as discussed in this study.
2.4. B96.11-Biotin/Streptavidin assay
IgG b96.11-biotin (1mg) was allowed to bind to streptavidin beads (2ml) for 1 hour at room temperature. Subsequently any unbound antibody was removed. The beads (50µl of a 50% slurry) were incubated with 40µl of serum for 10 min at 55°C, 30 min at 37°C, and 15 min at room temperature. Beads were precipitated by centrifugation at 14,000 rpm for 10 min. The supernatant was carefully removed and transferred to a new tube. This centrifugation step was repeated to avoid any carry-over of b96.11-biotin/streptavidin beads. The final supernatant was assayed for the presence of GAD65Ab in a radioligand binding assay (RBA). Samples without serum were included in every assay and tested for GAD65Ab binding to ascertain that the monoclonal antibody did not leak off the beads.
2.5. B96.11-PAS assay
Purified b96.11 was crosslinked to Protein A Sepharose (PAS) (Invitrogen, Carlsbad, CA) using the Dimethylpimelimidate method (Harlow and Lane, 1988) as previously described (Oak et al., 2008). Briefly, serum samples (30µl) were incubated with b96.11-PAS (25µl of 50% slurry) for 1 hour. The bound fraction was separated from the unbound serum by centrifugation at 14,000 rpm for 15 min. The supernatant was carefully transferred to a fresh tube and centrifuged as before. This additional step was necessary to avoid carry-over of b96.11-PAS. The resulting supernatant was analyzed for GAD65Ab in a RBA. Change in volume was accounted for. Samples without serum were included in every assay and tested for GAD65Ab binding to ascertain that the monoclonal antibody did not leak off the beads.
Anti-Id in both absorption assays are calculated as the observed increase in GAD65Ab levels after absorption compared to the GAD65Ab level before absorption. Antibody levels were expressed as a relative index to correct for interassay variation using the World Health Organization standard for GAD65Ab (Mire-Sluis et al., 2000). To determine the relative index, positive and negative control subjects were included in all assays. Cut-off levels for positivity (index of 0.04) were defined as the 98th percentile of a healthy controls (n=50) (non-diabetic individuals without known autoimmune disease and no family history of diabetes) analyzed in traditional RBA without absorption.
2.6. Monoclonal GAD65-specific antibodies used in this study
Two human monoclonal antibodies and their recombinant Fab (rFab) specific to GAD65 were used in this study. B96.11 recognizes an epitope that is shared with GAD65Ab in T1D patients (Padoa et al., 2003). Anti-Id to this GAD65Ab are present in healthy individuals, but are specifically missing in T1D patients (Oak et al., 2008). B78 recognizes an epitope located at the C-terminus of GAD65 (Tremble et al., 1997; Fenalti et al., 2008). rFab were expressed in bacteria as previously described (Padoa et al., 2003).
2.7. Radioligand binding assay (RBA)
GAD65Ab were determined by RBA as previously described (Falorni et al., 1995). Briefly, recombinant [35]S-GAD65 was produced in an in vitro coupled transcription and translation system with SP6 RNA polymerase and nuclease treated rabbit reticulocyte lysate (Promega, Madison, WI). Sera were incubated with [35]S-GAD65 (25,000 of TCA precipitable radioactivity). After an overnight incubation at 4°C, antibody-bound [35]S-GAD65 was separated from unbound antigen by precipitation with PAS. The immunoprecipitated radioactivity was counted on a Wallac Microbeta Liquid Scintillation Counter (Perkin Elmer Life and Analytical Sciences, Inc, Boston, MA). In an alternative RBA, streptavidin beads were used to precipitate [35]S-GAD65 bound to biotinylated antibody or rFab.
3. Results
3.1. B96.11-biotin/streptavidin Assay
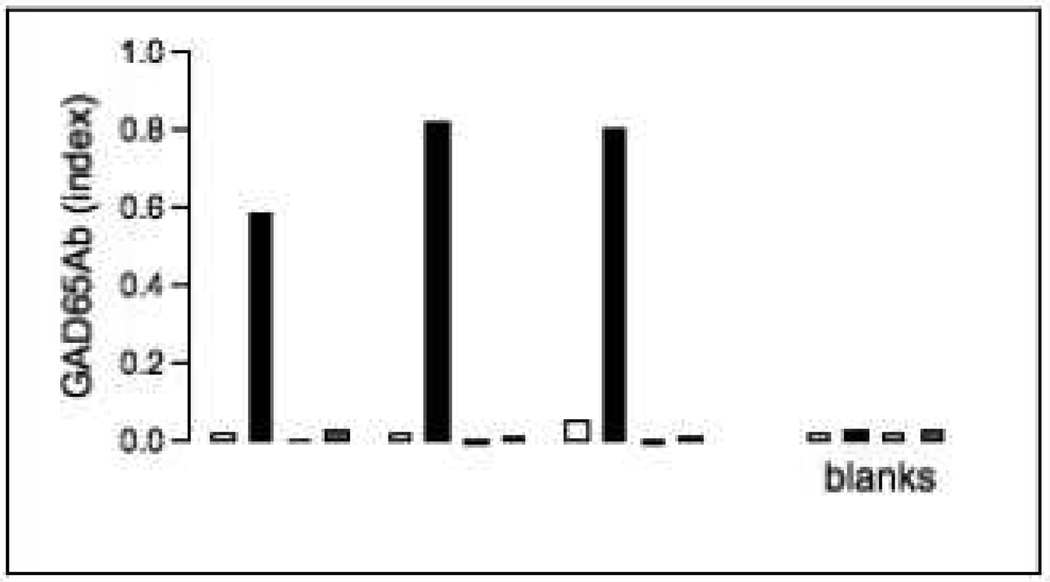
In our initial evaluation of the assay we ascertained that the GAD65Ab detected were not due to b96.11-biotin leaking off the column (Figure 1). While absorption of sera to b96.11-biotin resulted in a significant increase in GAD65Ab titer, PBS controls, that underwent the same procedure, but in the absence of serum, showed no increase of binding to GAD65 in the PAS- or Streptavidin- based RBA. We obtained identical results when we first incubated the serum sample with b96.11-biotin and subsequently absorbed the anti-Id-b96.11-biotin complexes to streptavidin-agarose (data not shown).
Figure 1. Assessment of the b96.11-biotin/streptavidin assay.
Three serum samples were analyzed for the presence of GAD65Ab-specific anti-Id in the b96.11-biotin/streptavidin assay. Samples were with b96.11-biotin/streptavidin-agarose complexes and subsequently separated by centrifugation. GAD65Ab were measured in serum samples prior to and after absorption by PAS-RBA (white and black, respectively), and streptavidin-agarose-RBA (grey columns). Samples that did not contain any human serum, but otherwise underwent the same procedure were analyzed as control samples to ascertain that GAD65Ab did not leak off the beads (blank). GAD65Ab levels are shown as index, the figure represents three independent experiments.
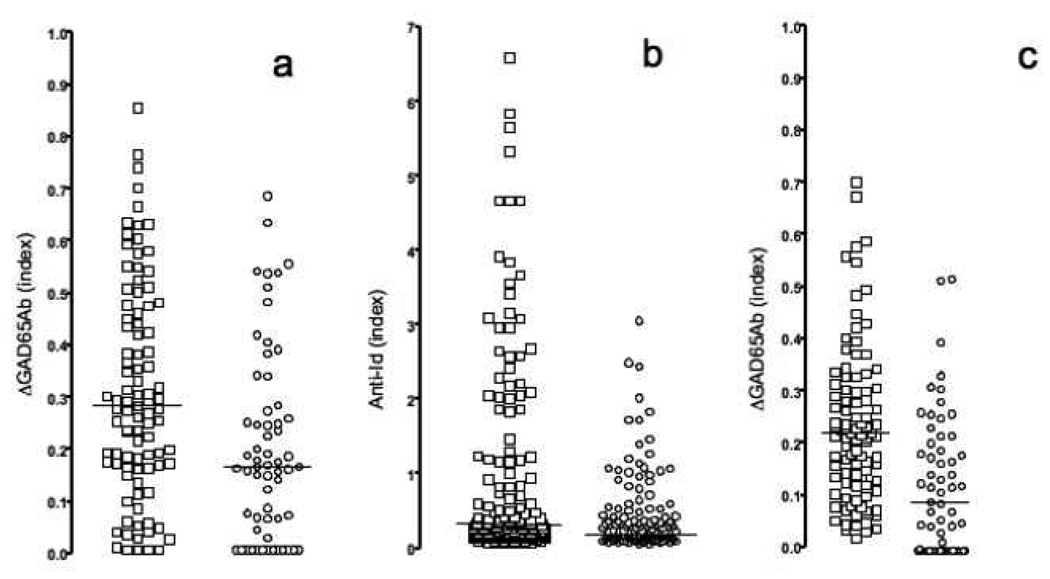
Subsequently we analyzed 61 T1D patient samples and 95 control samples for the presence of masked GAD65Ab (Figure 2a).
Figure 2. Anti-Id levels measured in different assays.
Samples obtained from healthy matched controls (squares) and new-onset T1D patients (circles) were tested for the presence of GAD65Ab-specific anti-Id in b96.11-biotin/streptavidin assay (a), ECL (b), and b96.11-PAS assay (c). Anti-Id detected in absorption assays (a and c) are reported as the difference in GAD65Ab index after absorption as compared to the index before absorption. Anti-Id detected in ECL are reported as relative index. Median binding is indicated.
The b96.11-biotin/streptavidin assay was able to distinguish samples obtained from T1D patients from those obtained from healthy controls. The median binding in the control group was significantly higher as compared to that in the T1D group (index of 0.3 vs 0.16, p=0.0003). The assay showed a sensitivity of 40% and a specificity of 86%.
3.2. ECL assay
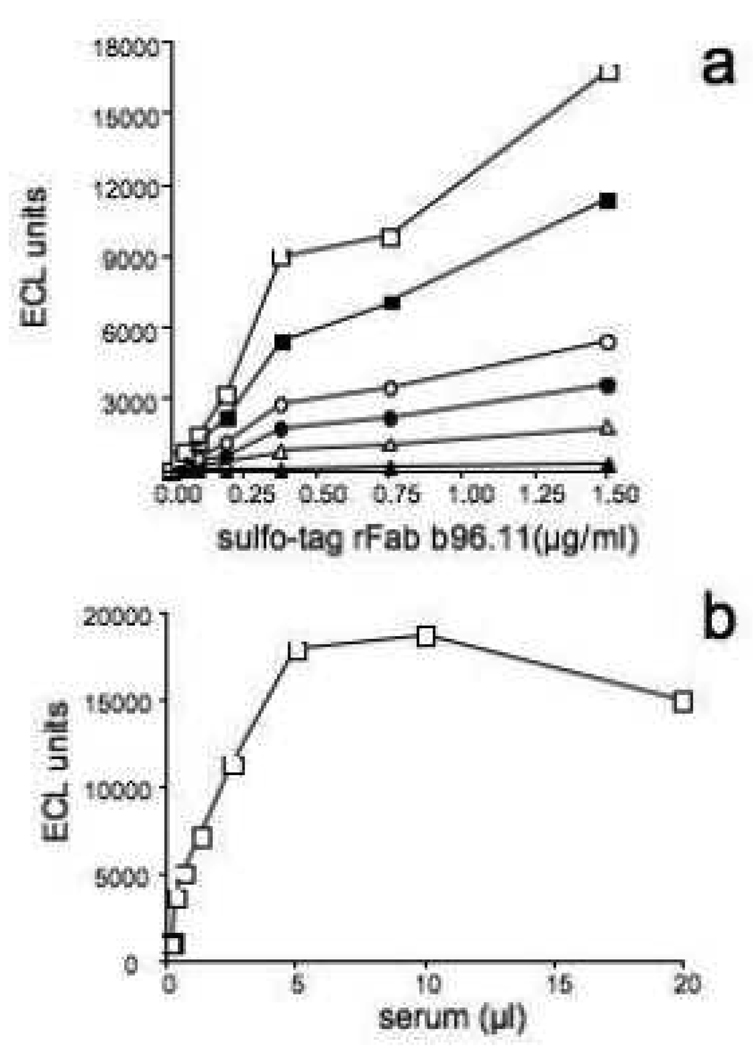
In initial experiments we determined the optimal conditions for the ELC assay. rFab-b96.11-biotin, rFab b96.11-sulfo, and serum samples were titrated to determine optimal assay concentrations (Figure 3a and b). Using seven control samples, we employed different conditions, performing the experiment at room temperature, or initially incubating the reaction for 10 min at 55°C, 42°C, or 37°C, followed by 30 min at 37°C and a final incubation of 15 min at room temperature (Figure 4a). Six/seven samples showed no significant effect of temperature on anti-Id detection. One samples showed significantly lower anti-Id levels when analyzed at 55°C. The peak-signal-to-noise ratio for the different conditions was 37, 40, 45, and 26 for room temperature, 37°C, 42°C, and 55°C, respectively. We chose 42°C as the optimal condition throughout our analysis.
Figure 3. Optimization of assay condition for ECL assay.
A positive serum was analyzed at stable dilution (10µl/well) with dilutions of biotinylated b96.11-rFab (0.75 (white squares), 0.37 (black squares), 0.18 (white circles), 0.09 (black circles), 0.05 (white triangles), and 0.02µg/ml (black triangles) and the indicated dilutions of sulfo-tagged b96.11 rFab.
B: The indicated dilutions of a positive serum were incubated with 0.75µg/ml biotinylated rFab b96.11 and 1.5µg/ml sulfo-tag rFab b96.11.
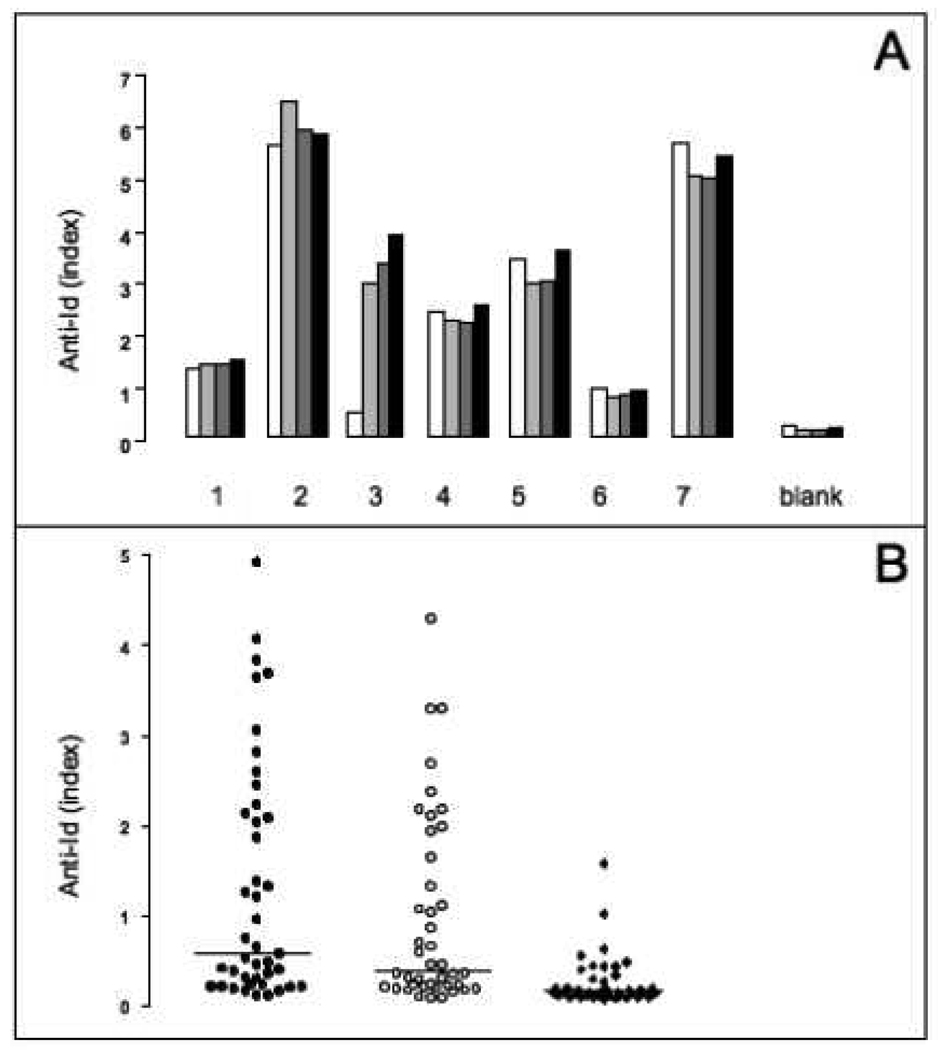
Figure 4. Temperature dependency and specificity of ECL.
A: Seven serum samples (1–7) and buffer blanks contained PBS instead of serum were analyzed at different temperature incubations in the ECL assay. Samples were first incubated for 10 minutes at 55°C (white), 42°C (light grey), or 37°C (dark grey), then for 30 minutes at 37°C, and finally for 15 minutes at room temperature. A fourth set of the samples underwent the assay at room temperature only (black). Anti-Id levels are shown as relative index.
B: serum samples (n=40) were tested for specificity of binding in the ECL assay in the absence of competitor (black circles), and in presence of unlabeled rFab b78 (white circles), or unlabeled rFab b96.11 (black diamonds). Anti-Id levels are shown as relative index. Median binding is indicated.
To determine the specificity of the ECL assay, we competed the binding of rFab b96.11-biotin with unlabeled rFab b96.11 (1µg/reaction) (Figure 4b). Samples (n=40) were incubated with an excess of unlabeled rFab b96.11 or rFab b78 in the presence of rFab b96.11-biotin and b96.11-Sulfotag. We found that only rFab b96.11 competed the detection of anti-Id, while rFab b78 had no significant effect on the binding of rFab-b96.11-biotin. The detection of the anti-Id in this assay is therefore specific to b96.11. This assay also served to determine the cut-off for positivity for the ECL assay as the index at which a >50% competition with b96.11 was observed (index of 0.13).
We analyzed 134 T1D patients and 177 healthy individuals for the presence of b96.11-specific anti-Id using the direct ECL detection method (Figure 2b). The direct detection assay was able to differentiate between samples obtained from healthy subjects and T1D patients (median index of 0.23 and 0.14, respectively, p=0.0004).
3.3. Comparison between assays
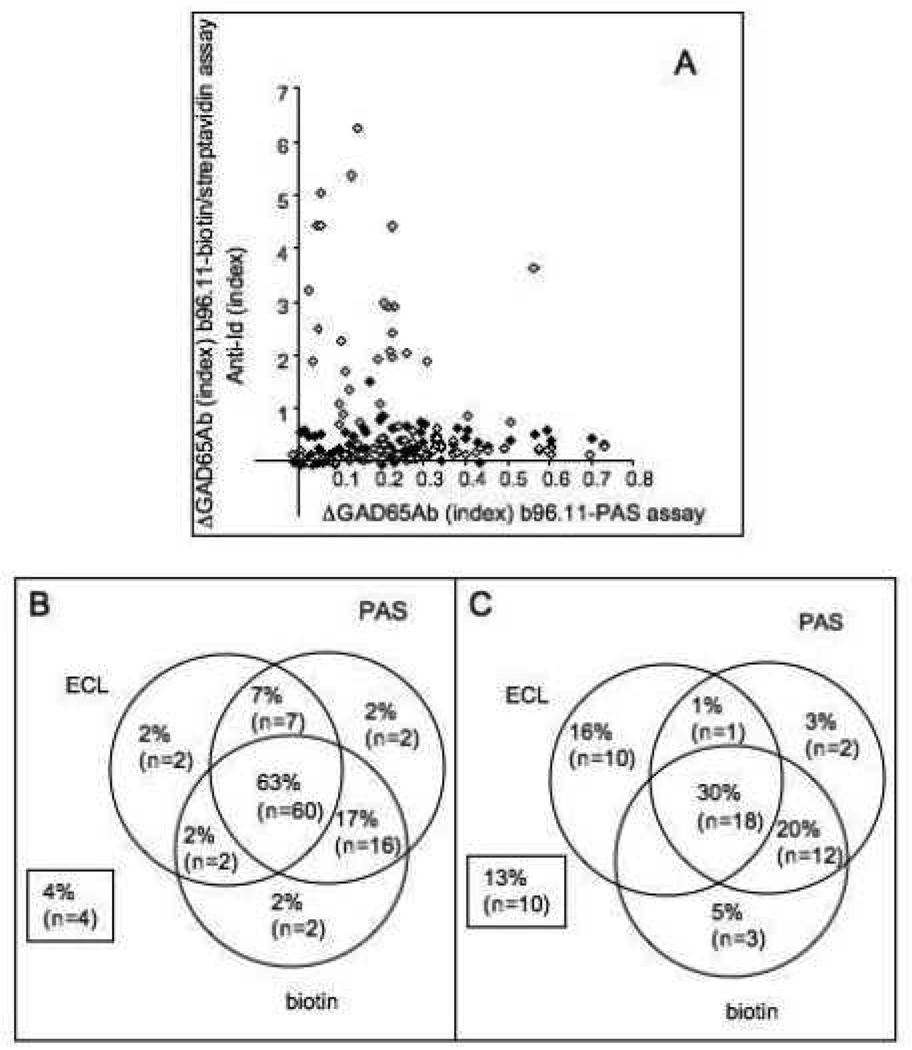
We analyzed 151 samples (95 controls and 56 T1D patients) for the presence of anti-Id using all three assays (Figure 5). While the b96.11-PAS and b96.11-biotin/streptavidin assays showed good correlation (p=0.0014), the ECL assay showed no correlation with either assay (Figure 5a). However, samples that tested negative in the ECL assay had significantly lower GAD65Ab levels in the b96.11-PAS assay (median index 0.1 vs. 0.25) (p<0.0001). We found that significantly more control samples tested positive in all three assays compared to T1D patients (63 vs 30%, p<0.0001). Samples that were negative for all three assays or tested positive in only one assay accounted for 10% of the healthy controls and 37% of the T1D patients (p<0.0001) (Figure 5b). The b96.11-PAS assay had the highest sensitivity and specificity, while the b96.11-biotin/streptavidin assay and the ECL assay showed similar sensitivities and specificities, and the ECL assay showed the highest peak signal-to-noise ratio (Table 2).
Figure 5. Direct comparison of three assays measuring GAD65Ab-specific anti-Id.
A: Anti-Id levels as determined by b96.11-PAS assay are correlated with anti-Id levels determined by b96.11-biotin/streptavidin assay (black) and ECL (white). Anti-Id levels determined in the absorption assays are shown as difference in GAD65Ab index prior and after absorption, while anti-Id levels determined in the ECL are shown as relative index.
Frequency (% and total numbers) of anti-Id positive samples as determined in the three assays is shown for control samples (B) and T1D patients (C). Samples that did not test positive in any of the assays are indicated in the boxes.
Table 2.
Assay characteristics
| B96.11-PAS | b96.11- biotin/streptavidin |
ECL | GAD65Ab RBA |
|
|---|---|---|---|---|
| Sensitivity (%) (anti-Id-/T1D patients) |
41 (23/56) | 40 (24/61) | 40 (53/134) | 99 |
| Specificity(%) (anti-Id+/controls) |
90 (85/95) | 86 (82/95) | 70 (123/177) | 72 |
| Peak signal-to-noise ratio | 28 | 23 | 51 | 28 |
3.4. Absorption to b96.11-PAS efficiently removes anti-Id from serum samples
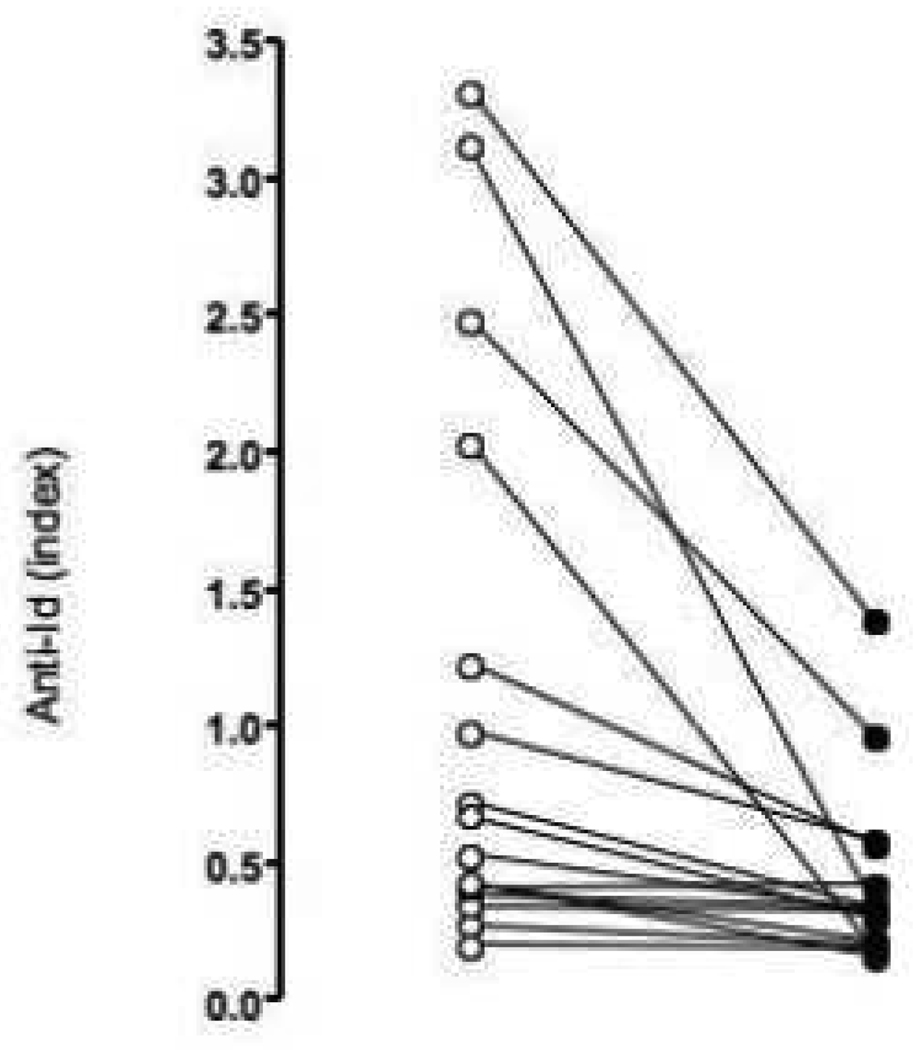
To test the efficiency of our absorption assay to remove b96.11-specific anti-Id from serum samples, we analyzed samples (n=16) before and after absorption to b96.11-PAS for the presence of anti-Id using the ECL assay (Figure 6). We found that absorption to b96.11-PAS significantly reduced the anti-Id levels detected in the ECL assay (median binding from an index of 0.49 to 0.23, p=0.0007). It is of note that the reduction was particularly obvious in the high binding samples, suggesting that the lack of reduction in the low binding samples may be an issue of sensitivity.
Figure 6. Effect of absorption of anti-Id by b96 11-PAS on anti-Id measurement by ECL.
Serum samples (n=16) were analyzed for GAD65Ab-specific anti-Id by ECL before (white) and after (black) absorption of the samples to b96.11-PAS. Anti-Id levels are shown as relative index.
4. Discussion
Our previous findings of GAD65Ab in healthy individuals have been met with understandable skepticism. GAD65Ab are perceived as markers for the autoimmune destruction of pancreatic beta cells. Our observations therefore challenge the current understanding that healthy individuals lack GAD65Ab. Detection of autoantibodies and their anti-Id in other autoimmune disease is often achieved only after dissociating the anti-Id/antibody complexes and subsequent blocking or absorption of the anti-Id (Stafford et al., 1995; Pan et al., 1998; Routsias et al., 2002). The use of immobilized human monoclonal GAD65Ab to absorb anti-Id from serum makes it difficult to rule out the possibility of monoclonal GAD65Ab contaminating the reaction, although precautions were taken to ensure that no GAD65Ab eluted from the column. Furthermore, the indirect measurement of anti-Id through the detection of un-masked GAD65Ab did potentially exclude the detection of anti-Id-positive, GAD65Ab-negative samples.
To address these concerns we compared three assays for the detection of b96.11-specific anti-Id. Two assays required the initial removal of anti-Id from the serum sample by immobilized monoclonal GAD65Ab. The serum sample was than analyzed for GAD65Ab in a radioligand binding assay. The third assay allowed the direct detection of anti-Id in a ECL-based assay. All three assays could significantly distinguish between T1D patients and controls.
The biotin-streptavidin based absorption assay allowed us to address the crucial question whether the GAD65Ab detected after absorption to immobilized GAD65Ab originate from the serum sample. We chose the streptavidin/biotin system because it provides the strongest noncovalent biological interaction (Kd~ 4×10-14M (for review see (Diamandis and Christopoulos, 1991)). Moreover, this complex has been demonstrated to be stable over a wide range of temperature (Tong and Smith, 1992) and we were able to show that no significant elution of the biotinylated antibody from streptavidin occurred. This finding verified that GAD65Ab detected in the radioligand binding assay do not stem from a leakage of the absorption column, and confirmed that the amount of streptavidin-agarose used to remove biotinylated-b96.11 in our assay was sufficient. No binding to GAD65 was observed when the controls were precipitated with PAS, indicating that the biotinylation of b96.11 was successful and complete.
A potential drawback of the ECL assay is that the rFab could recognize epitopes other than the paratope of the anti-Id. Our displacement experiments showed that only rFab b96.11 successfully competed the detection of b96.11-specific anti-Id, while the closely related human GAD65-specific rFab b78 did not. The ECL assay is therefore highly specific to b96.11. While the original b96.11-PAS assay showed the highest sensitivity and specificity, the ECL assay showed a peak signal-to-noise ratio of 50, exceeding that observed in our original assay (28). This observation and the finding that anti-Id levels detected in the ECL assay do not correlate with those in the b96.11- PAS assay suggest that GAD65Ab levels after removal of anti-Id do not necessarily reflect anti-Id levels.
We confirmed anti-Id in samples that are anti-Id-positive and GAD65Ab-negative that the absorption assay may fail to detect. We conclude that the original absorption assay provides the highest sensitivity and specificity, but is time consuming and cumbersome. The ECL assay requires only 1/3 of the time, does not involve radioactive labeled proteins and allows the quantification of anti-Id. The relative low sensitivity of the ECL assay needs to be addressed before this assay can replace the current b96.11-PAS assay. We optimized the amount of rFab and serum used in the assay and further optimization including buffer specifications are ongoing.
Our study confirms our previous finding of GAD65Ab in healthy individuals. The potential role of anti-Id in the treatment of autoimmune diseases has been suggested (DeKeyser et al., 1996; Blank et al., 2005) and studies suggest that anti-Id and autoantibodies arise concordantly (Pan et al., 1998), supporting a regulatory role of anti-Id in human health. Whether the lack of anti-Id to disease-specific GAD65Ab occurs gradually, or is an inherent characteristic in individuals who will later progress to T1D is currently under investigation.
Acknowledgements
The study was performed as independent research sponsored by the National Institutes of Health (DK53456, DK53004, DK26190, and DK17047), a Basic Science Award from the American Diabetes Association to CSH. We would like to thank Dr. G. Eisenbarth (Barbara Davis Center for Childhood Diabetes, University of Colorado) for his helpful comments and suggestions.
Glossary
- T1D
type 1 diabetes
- GAD65Ab
autoantibodies to the 65kDa isoform of glutamate decarboxylase
- ECL
electrochemiluminescence
- LADA
Latent autoimmune diabetes in adults
- WHO
World Health Organization
- anti-Id
anti-idiotypic antibodies
- PAS
protein A sepharose
- rFab
recombinant Fab
- MSD
Meso Scale Discovery
- RBA
radioligand binding assay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdou NI, Wall H, Lindsley HB, Halsey JF, Suzuki T. Network theory in autoimmunity. In vitro suppression of serum anti-DNA antibody binding to DNA by anti-idiotypic antibody in systemic lupus erythematosus. J Clin Invest. 1981;67:1297–1304. doi: 10.1172/JCI110158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank M, Nur I, Toub O, Maor A, Shoenfeld Y. Toward molecular targeting with specific intravenous immunoglobulin preparation. Clin Rev Allergy Immunol. 2005;29:213–217. doi: 10.1385/CRIAI:29:3:213. [DOI] [PubMed] [Google Scholar]
- DeKeyser F, DeKeyser H, Kazatchkine MD, Rossi F, Dang H, Talal N. Pooled human immunoglobulins contain anti-idiotypes with reactivity against the SLE-associated 4B4 cross-reactive idiotype. Clin Exp Rheumatol. 1996;14:587–591. [PubMed] [Google Scholar]
- Diamandis EP, Christopoulos TK. The biotin-(strept)avidin system: principles and applications in biotechnology. Clin Chem. 1991;37:625–636. [PubMed] [Google Scholar]
- Dwyer DS, Bradley RJ, Urquhart CK, Kearney JF. Naturally occurring anti-idiotypic antibodies in myasthenia gravis patients. Nature. 1983;301:611–614. doi: 10.1038/301611a0. [DOI] [PubMed] [Google Scholar]
- Falorni A, Örtqvist E, Persson B, Lernmark Å. Radioimmunoassays for glutamic acid decarboxylase (GAD65) and GAD65 autoantibodies using 35S or 3H recombinant human ligands. J Immunol Methods. 1995;186:89–99. doi: 10.1016/0022-1759(95)00139-2. [DOI] [PubMed] [Google Scholar]
- Fenalti G, Hampe CS, Arafat Y, Law RH, Banga JP, Mackay IR, Whisstock JC, Buckle AM, Rowley MJ. C-terminal clustering of autoantibody and T cell determinants on the structure of GAD65 provide insights into the molecular basis of autoreactivity. Diabetes. 2008 doi: 10.2337/db07-1461. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies : a laboratory manual. Harbor, N.Y.: Cold Spring; 1988. [Google Scholar]
- Mehta YS, Ghosh K, Badakere SS, Pathare AV, Mohanty D. Role of antiidiotypic antibodies on the clinical course of idiopathic thrombocytopenic purpura. J Lab Clin Med. 2003;142:113–120. doi: 10.1016/S0022-2143(03)00104-5. [DOI] [PubMed] [Google Scholar]
- Mire-Sluis AR, Das RG, Lernmark Å. The World Health Organization International Collaborative Study for Islet Cell Antibodies. Diabetologia. 2000;43:1282–1292. doi: 10.1007/s001250051524. [DOI] [PubMed] [Google Scholar]
- Oak S, Gilliam LK, Landin-Olsson M, Torn C, Kockum I, Pennington CR, Rowley MJ, Christie MR, Banga JP, Hampe CS. The lack of anti-idiotypic antibodies, not the presence of the corresponding autoantibodies to glutamate decarboxylase, defines type 1 diabetes. Proc Natl Acad Sci U S A. 2008;105:5471–5476. doi: 10.1073/pnas.0800578105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa CJ, Banga JP, Madec AM, Ziegler M, Schlosser M, Ortqvist E, Kockum I, Palmer J, Rolandsson O, Binder KA, Foote J, Luo D, Hampe CS. Recombinant Fabs of human monoclonal antibodies specific to the middle epitope of GAD65 inhibit type 1 diabetes-specific GAD65Abs. Diabetes. 2003;52:2689–2695. doi: 10.2337/diabetes.52.11.2689. [DOI] [PubMed] [Google Scholar]
- Pan ZJ, Anderson CJ, Stafford HA. Anti-idiotypic antibodies prevent the serologic detection of antiribosomal P autoantibodies in healthy adults. J Clin Invest. 1998;102:215–222. doi: 10.1172/JCI1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Autoantibodies in diabetes. Diabetes. 2005;54 Suppl 2:S52–S61. doi: 10.2337/diabetes.54.suppl_2.s52. [DOI] [PubMed] [Google Scholar]
- Routsias JG, Touloupi E, Dotsika E, Moulia A, Tsikaris V, Sakarellos C, Sakarellos-Daitsiotis M, Moutsopoulos HM, Tzioufas AG. Unmasking the anti-La/SSB response in sera from patients with Sjogren's syndrome by specific blocking of anti-idiotypic antibodies to La/SSB antigenic determinants. Mol Med. 2002;8:293–305. [PMC free article] [PubMed] [Google Scholar]
- Stafford HA, Anderson CJ, Reichlin M. Unmasking of anti-ribosomal P autoantibodies in healthy individuals. J Immunol. 1995;155:2754–2761. [PubMed] [Google Scholar]
- Tong X, Smith LM. Solid-phase method for the purification of DNA sequencing reactions. Anal Chem. 1992;64:2672–2677. doi: 10.1021/ac00046a004. [DOI] [PubMed] [Google Scholar]
- Törn C, Landin-Olsson M, Lernmark A, Palmer JP, Arnqvist HJ, Blohme G, Lithner F, Littorin B, Nystrom L, Schersten B, Sundkvist G, Wibell L, Ostman J. Prognostic Factors for the Course of beta Cell Function in Autoimmune Diabetes. J Clin Endocrinol Metab. 2000;85:4619–4623. doi: 10.1210/jcem.85.12.7065. [DOI] [PubMed] [Google Scholar]
- Tremble J, Morgenthaler NG, Vlug A, Powers AC, Christie MR, Scherbaum WA, Banga JP. Human B cells secreting immunoglobulin G to glutamic acid decarboxylase- 65 from a nondiabetic patient with multiple autoantibodies and Graves' disease: a comparison with those present in type 1 diabetes. J Clin Endocrinol Metab. 1997;82:2664–2670. doi: 10.1210/jcem.82.8.4171. [DOI] [PubMed] [Google Scholar]
- Williams WM, Isenberg DA. Naturally occurring anti-idiotypic antibodies reactive with anti-DNA antibodies in systemic lupus erythematosus. Lupus. 1998;7:164–175. doi: 10.1191/096120398678919958. [DOI] [PubMed] [Google Scholar]
- Zouali M, Eyquem A. Expression of anti-idiotypic clones against auto-anti-DNA antibodies in normal individuals. Cell Immunol. 1983;76:137–147. doi: 10.1016/0008-8749(83)90356-8. [DOI] [PubMed] [Google Scholar]