Abstract
In California, hard (Ixodidae) ticks transmit at least 8 zoonotic disease agents (1 virus, 6 bacteria, 1 protozoan) to humans or other animals. The correct taxonomic identification of all 3 parasitic stages (larvae, nymphs, adults) of ticks is integral to understanding host-tick associations and disease dynamics, but immature ticks, especially the larvae, can be difficult to identify. Here, we present larval keys to the 4 genera of Ixodidae (Dermacentor Koch, 1844; Haemaphysalis Koch, 1844; Ixodes Latreille, 1795; Rhipicephalus Koch, 1844) and to the 18 species of Ixodes known to be established in California. Several new diagnostic features, as well as photographs of microscopic structures, are provided to facilitate identification. Non-exclusive characters are utilized to separate the subgenera Ixodiopsis Filippova, 1957 and Pholeoixodes Schulze, 1942.
Keywords: Ixodidae, Ixodes, Ixodiopsis, Pholeoixodes, larvae
Introduction
Ticks (Acari: Ixodidae) are obligate, bloodsucking ectoparasites of reptiles, rodents, birds and mammals (Sonenshine 1991, 1993). In California, 27 species of hard (Ixodidae) and 20 species of soft (Argasidae) ticks are known to be established (Furman & Loomis 1984, Robbins 1989, Norris et al. 1997). At least 8 animal disease agents have been detected in or isolated from ixodid ticks, humans or other animals in the state, including those causing Colorado tick fever, human babesiosis, human granulocytic anaplasmosis, human monocytic ehrlichiosis, Lyme disease, Q-fever, Rocky Mountain spotted fever, and tularemia (Lane & Clover 2002, Hui et al. 2004). Several ticks belonging to the genus Ixodes Latreille, 1795 serve as enzootic or bridging vectors of Lyme disease spirochetes (Borrelia burgdorferi sensu lato [s.l.]) or the agent of human granulocytic anaplasmosis (Anaplasma phagocytophilum). These include the primary vector of both agents to humans, the western black-legged tick I. pacificus Cooley & Kohls, 1943 and at least 3 predominantly, if not exclusively, enzootic vectors of B. burgdorferi s.l., i.e., I. angustus Neumann, 1899; I. jellisoni Cooley & Kohls, 1938; and I. spinipalpis Hadwen & Nuttall, 1916.
All ixodid ticks have 3 parasitic stages, the larva, nymph and adult, each of which must secure a blood meal to develop to the next stage or, in the case of the adults, to reproduce. Although larval ticks occasionally acquire disease agents vertically via the eggs of infected females, a phenomenon known as transovarial transmission, most larvae that become infected typically ingest disease agents with the blood meal. After the transstadial molt, the resultant nymphs may transmit the disease horizontally to other vertebrate hosts. Similarly, nymphs that feed on an infective host may acquire an agent and pass it transstadially to adult ticks. The small size, <0.9 mm in length (Klompen et al. 1996) of unfed larvae can render them difficult to identify, nonetheless, accurate identification of larval ticks is crucial to delimiting the geographical host range of a given tick species and the transmission dynamics (i.e., vector-pathogen-host interrelationships) of tick-borne pathogens.
It has been 24 years since the publication of the seminal monographic work on the ticks of California (Furman & Loomis 1984), and 18 years since the latest taxonomic treatise on larval Ixodes spp. in this state (Webb et al. 1990). More recently, all stages of the soft tick Argas monolakensis (Schwan et al. 1992) and the larva of I. jellisoni were described for the first time (Keirans et al. 1996); I. holdenriedi Cooley, 1946 was synonymized with I. ochotonae Gregson, 1941 (Robbins 1989) and I. neotomae Cooley, 1944 with I. spinipalpis (Norris et al. 1997); and the genus Boophilus Curtice, 1891 was made a subgenus of Rhipicephalus Koch, 1844 (Murrell & Barker 2003). Furthermore, we discovered several useful diagnostic characters that facilitate identification of Ixodes larvae and differentiation of the subgenera Ixodiopsis Filippova, 1957 and Pholeoixodes Schulze, 1942. Here, we present updated larval keys, illustrated with photographs, to the 4 ixodid genera and to the 18 species of Ixodes known to be established in California.
Materials and Methods
Our keys borrow taxonomic information published previously by Serdyukova (1955), Clifford & Anastos (1960), Allred et al. (1960), Clifford et al. (1961, 1973), Filippova (1958, 1961, 1967, 1977), Furman & Loomis (1984), Webb et al. (1990), Robbins & Keirans (1992), Klompen et al. (1996, 1997), and Lindquist et al. (1999). We use diagnostic characters that can be visualized easily at either 100x or 400x magnification. They are illustrated (Fig. 1) by modified line drawings representing larvae in the genus Ixodes (Clifford et al. 1961) and with photographs that present tick structure as it is viewed under the microscope. Information on the hosts and seasonal occurrence of each species can be found in Furman & Loomis (1984) and Webb et al. (1990).
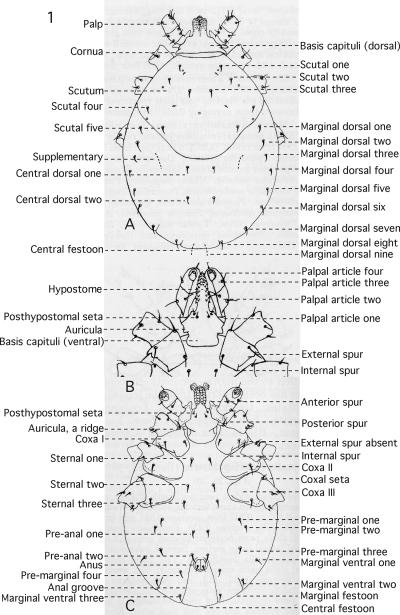
Figure 1.
Location of setae and other key characters are labeled, A. Ixodes angustus, dorsal view, B. I. brunneus, ventral view of gnatho-thorax, C. I. angustus, ventral view. Drawing modified from Clifford et al. (1960).
Larvae were cleared according to an extraction procedure used to prepare tissue for PCR (Qiagen, Foster City, CA). A tiny incision was made on one side of the abdomen, and the resultant mixture of ATL tissue lysis buffer, Proteinase K solution and tick larva were incubated for 24 hours at 34° C. Next, the exoskeleton was transferred to 5% acetic acid for ≈ 5 minutes to render the cuticle miscible in Hoyer's mounting medium that was used to mount specimens for dorsal or ventral examination. This process produces a reserve of lysed tissue for molecular studies while preserving the exoskeleton for subsequent morphological examination (Cruickshank 2002).
Photographs of larvae were taken with a Zeiss MC63 photo-micrographic camera mounted on a compound microscope, and printed with full-frame border options. Material examined included larval Ixodes spp. in the tick collection of the Orange County Vector Control District, Garden Grove, California (Webb et al. 1990). Some of these and the other specimens examined are listed below “Specimens examined”. Ticks photographed for inclusion herein, and those used to construct the keys, are archived in the collection of R. S. L. and recorded in the database of the Essig Museum, University of California, Berkeley. A few additional specimens were borrowed from the U. S. National Tick Collection located in the Institute of Arthropodology and Parasitology, Georgia Southern University, Statesboro, Georgia.
The Californian Genera of Ixodidae
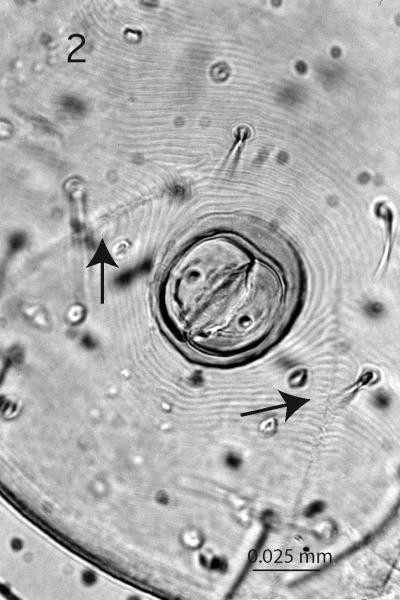
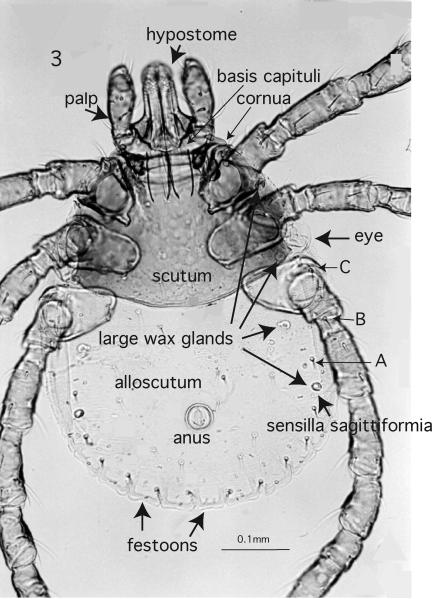
Traditionally, immature and adult Ixodidae have been grouped into two sections, the Prostriata and the Metastriata that are distinguishable by the anal groove (Fig. 2) (Nuttall & Warburton 1911). In the Prostriata (= the genus Ixodes), the anal groove is present in all 3 parasitic stages, but closed anteriorly only in nymphs and adults. It is not developed at all in larvae of the Metastriata, but nymphs and adults usually possess an anal groove posterior to the anus. The anal groove delineates segment XIV (Klompen et al. 1996) and it may form the centrally located of three festoons along the posterior margin. This character state also is unique to the genus Ixodes (Fig. 1). Contrariwise, sensilla sagittiformia (3 pairs of large ventral wax glands situated posterior to the coxae plus 1 pair located dorsally on the alloscutum) (Fig. 3) are present in the Metastriata, but absent in Ixodes.
Figure 2.
Prostriata, ventral view of anal groove, Ixodes pacificus, 400x.
Figure 3.
Metastriata, dorsal view of Dermacentor occidentalis Marx, 1892; 100x, key structures are labeled. A, B, C, three marginal dorsal setae anterior to the sensilla sagittiformia.
The family Ixodidae is composed of 12 genera, 4 of which are established in California (Dermacentor Koch, 1844; Haemaphysalis Koch, 1844; Ixodes Latreille, 1795; Rhipicephalus Koch, 1844). A member of a fifth genus, Amblyomma americanum Koch, 1844 has been introduced into the state repeatedly, but has not become established (Furman & Loomis 1984).
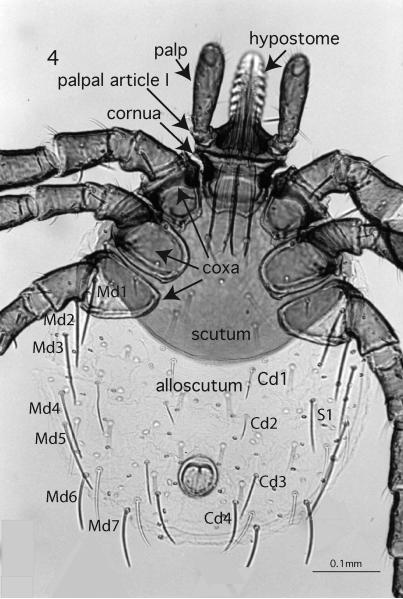
Larval ticks can be separated easily from the nymphs or adults by possessing 3 pairs of legs versus 4 pairs. Classification of larvae utilizes the arrangement of setae (Fig. 4) that are distributed symmetrically in pairs (Glashinskaya-Babenko 1949, Serdyukova 1955, Clifford & Anastos 1960). In contrast, nymphs have a greater number of setae that are distributed asymmetrically. The 4 palpal segments (Figs. 5-8) and posteromarginal festoons (9 or 11 in larval Metastriata, 3 in Prostriata) sometimes are fused and appear fewer in number in larval ticks than in nymphs and adults, but are consistent within species.
Figure 4.
Prostriata, dorsal view of Ixodes pacificus, 100x, indicating location of some key characters. Dorsal alloscutal setae are conspicuously long in the subgenus Ixodes, (Md 1-7) marginal dorsal setae 1-7; (Cd 1-4) central dorsal setae 1-4; (S1) supplementary setae.
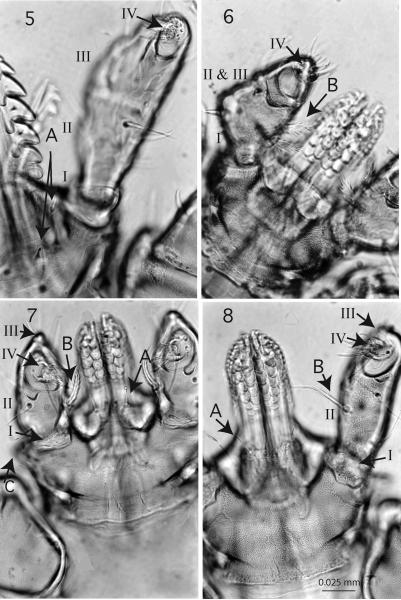
Figures 5 - 8.
Ventral view of capitulum, 400x, compares palps of the genera, articles I-IV, article III encloses article IV. Fig. 5. Ixodes texanus, A, 2 pairs of post-hypostomal setae; Fig. 6. Haemaphysalis leporispalustris, palps angled, B, 2 pairs of inner setae. Figures 7 - 8. A, 1 pair of post-hypostomal setae, B, palps with 1 pair inner setae. Fig. 7. Rhipicephalus sanguineus, palpal article I, II & III fused, article III with a pointed tip; Fig. 8. Dermacentor occidentalis, palpal article I visible, article III with a blunt tip.
A key to the genera of larval ixodids is included because larvae of Dermacentor and Ixodes frequently infest the same small mammals (e.g., rodents) in California, and lagomorphs (rabbits and hares) are parasitized by these genera as well as by Haemaphysalis leporispalustris Packard, 1869. The larval hosts of Rhipicephalus sanguineus Latreille, 1806 are less well known in California, but the available collection records suggest that they principally parasitize dogs and occasionally humans (Furman & Loomis 1984).
Key To The Genera of Ixodidae
1 Large wax glands absent; 2 pairs of posthypostomal setae, ventral view (Fig. 5); 5 pairs of scutal setae; eyes absent; anal groove present, obscure in some specimens (Fig. 2); (section Prostriata) . . . . . Ixodes Latreille Large wax glands present (Fig. 3); 1 pair of posthypostomal setae (Fig. 7); 3 pairs of scutal setae; eyes usually present (Fig. 3); anal groove absent; (section Metastriata) . . . . . 2
2(1) Palps triangular shaped, fused articles II and III form a lateral angular expansion (Fig. 6), 2 pairs of branched inner setae in ventral view (Fig. 6); eyes absent; 2 pairs of marginal dorsal setae present anterior to sensilla sagittiformia. . . . . Haemaphysalis Koch Palps not greatly angled laterally, 1 pair of inner ventral setae (Figs. 7, 8); eyes present (Fig. 3); more than 2 pairs of marginal dorsal setae anterior to sensilla sagittiformia (Fig. 3) . . . . . 3
3(2) Posterolateral extention of basis capituli pointed, but cornua undeveloped (Fig. 7); article I of palp fused with segments II and III; tip of palp (segment III) pointed (Fig. 7) . . . . . Rhipicephalus Koch Posterolateral extention of basis capituli with well developed cornua (Fig. 3) (exception D. albipictus Packard, 1869); article I of palp visible ventrally, tip of palp blunt (Fig. 8) . . . . . Dermacentor Koch
The Genus Ixodes in California
The key to Ixodes larvae is based upon the classification of subgenera in Clifford et al. (1973), except that Ixodiopsis is treated here as a separate subgenus (Filippova 1957, Robbins & Keirans 1992) and I. brunneus Koch, 1844 is included in the subgenus Trichotoixodes Reznik, 1961 (Filippova 1977). Seven subgenera and 18 species of Ixodes reportedly occur in California. Whenever possible, figures are organized to highlight the morphological similarities found among species belonging to a particular subgenus.
The subgenus Ixodes Latreille, 1795 consists of I. jellisoni Cooley & Kohls, 1938; I. pacificus Cooley & Kohls, 1943; I. peromysci Augustson, 1939 and I. spinipalpis Hadwen & Nuttall, 1916. Ixodes jellisoni and I. pacificus also belong to the medically important I. ricinus complex of vector ticks (Snow & Arthur 1970, Filippova 1991, 1999). Characteristics of this subgenus include the absence of anterior or posterior spurs on palpal article I (Fig. 4); auriculae present (Figs. 9, 10); a small external spur present on coxae I and II (Figs. 9, 10); alloscutal setae conspicuously long (often 0.08 mm – 0.10 mm) (Fig. 4), with typically 7 marginal dorsal setae, 2 or more central dorsal setae, and 1 pair of dorsal supplementary setae; and normally 3 setae on coxae III (Clifford & Anastos 1960, Snow & Arthur 1970). In addition, the anal groove usually is evident, but the 3 posterior marginal festoons are faint.
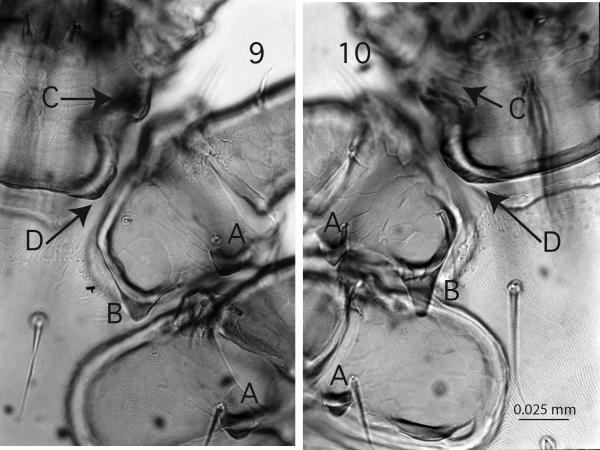
Figures 9 - 10.
Subgenus Ixodes, 400x, ventral view of basis capituli and coxa I & II, A, external spurs on coxae I and II. Fig. 9. Ixodes pacificus, B, inner spur of coxa I not overlapping coxa II, C, auricula well developed, D, posterior margin of basis capituli straight, extra chitin in corners; Fig. 10. I. spinipalpis, B, inner spur of coxa I overlapping coxa II, C, slightly bulbous auricula, D, posterior margin of basis rounded.
The subgenus Ixodiopsis Filippova, 1957 includes I. angustus Neumann, 1899; I. ochotonae Gregson, 1941; I. soricis Gregson, 1942; and I. woodi Bishopp, 1911; the subgenus Pholeoixodes Schulze, 1942 is represented by I. hearlei Gregson, 1941; I. kingi Bishopp, 1911; I. rugosus Bishopp, 1911; I. sculptus Neumann, 1904; and I. texanus Banks, 1909. The subgenera Ixodiopsis and Pholeoixodes can be separated from the subgenus Ixodes by the following combination of characters: 1 or more spurs present on palpal article I (Figs. 11-16); auriculae undeveloped, but a ridge usually is present (Figs. 11-13); external spurs absent on coxae I and II; alloscutal setae short (<0.05 mm) with 8 marginal dorsal setae, 2 central dorsal setae, and no supplementary setae; and 2 setae present on coxae III. The anal groove may be faint but in some species the 3 post-marginal festoons are evident.
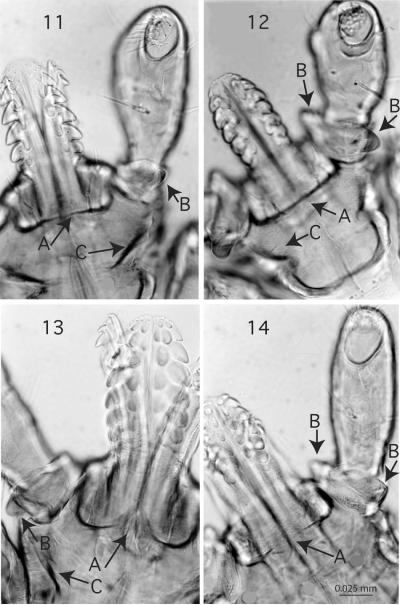
Figures 11-14.
Ventral view of capitulum, 400x A, ridge posterior to hypostome, B, spur on palpal article I, C, auriculae faint with a ridge present. Figures 11-12. Subgenus Pholeoixodes. Fig. 11. Ixodes hearlei; Fig. 12. I. sculptus. Figures 13-14. Subgenus Ixodiopsis. Fig. 13. I. woodi; Fig. 14. I. ochotonae.
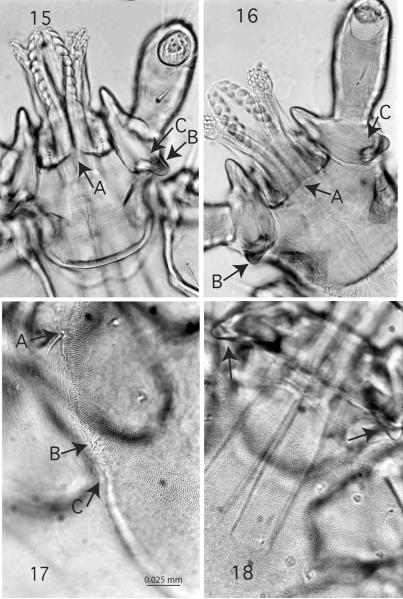
Figures 15-18.
Subgenus Ixodiopsis ventral view of capitulum, 400x, A, ridge posterior to hypostome, B, posterior spur, C, ventral projection. Fig. 15. Ixodes angustus; Fig. 16. I. soricis. Figures 17-18. Subgenus Pholeoixodes. I. sculptus, 400x, dorsal view. Fig. 17. Insertion of setae on the scutum, A, SC4 and B, SC5 at edge, C, indent; Fig. 18. Posterior margin of basis capituli with well developed cornua.
Immatures in the subgenera Pholeoixodes and Ixodiopsis traditionally have been difficult to separate (Klompen 1999), although they can be differentiated as adults by the shape of the palps, scutum, and 14 other characters selected by Robbins & Keirans (1992). Larvae can be distinguished by using several characters that are not exclusive for either subgenus. In Pholeoixodes larvae, we noted the presence of a taxonomically useful, uninterrupted chitinous substructural ridge posterior to the hypostome that extends horizontally (Figs. 11, 12). It is viewed optimally in ventral mounts. Filippova (1958, 1961, 1977) depicted similar ridges with dotted lines in ventral views of several Palearctic Pholeoixodes, i.e., I. crenulatus Koch, 1841; I. kaiseri Arthur, 1957; and I. subterraneus Filippova, 1961. In Ixodiopsis, this substructure is variable (Filippova 1967), but often cleaved medially (Figs. 13-15) as in most species belonging to other Ixodes subgenera (e.g., Ixodes) and in all genera of the Metastriata (Figs. 6-8).
In North America, species of Pholeoixodes usually display a spur or spurs on palpal article I, whereas they are absent among Palearctic species. When present, the spurs are very small (Fig. 11), and the anterior one is usually lacking or greatly reduced except for I. sculptus (Fig. 12). In Ixodiopsis, the anterior and posterior palpal spurs are usually prominent and may include both small dorsal and ventral projections (Figs. 15, 16). Other taxonomically utilitarian characters that have been observed among Pholeoixodes in the Nearctic and Palearctic regions (e.g., I. crenulatus) (Filippova 1958, 1977) include the insertion of setae SC4 inside the edge of the scutum, often with a paired dip in a somewhat elongated contour (Fig. 17) (Linquist et al. 1999). In Ixodiopsis (e.g., I. angustus) (Fig. 1), setae SC4 may be inserted well inside the edge of the scutum, and the contour is broadly rounded. Lastly, fused palpal segments II and III are wider in Pholeoixodes (Figs. 11, 12) and generally have a greater number of longer and stronger setae than in Ixodiopsis (Figs. 14-16).
The remaining four subgenera parasitize birds exclusively: Ceratixodes Neumann, 1902 with Ixodes uriae White, 1852; Multidentatus n. subgen. with I. auritulus Neumann, 1904; Scaphixodes Schulze, 1941 with I. howelli Cooley & Kohls, 1938, and I. signatus Birula, 1895; and Trichotoixodes Reznick, 1961 with I. brunneus Koch, 1844. The combinations of distinguishing characters for these subgenera are found in Table 1.
Table 1.
Character states distinguishing subgenera and species of Ixodes larvae that parasitize birds exclusively.
| Spurs palpal article I |
External spurs coxae |
Marginal dorsal setae |
Central dorsal setae |
Scutal setae |
Supplementary setae |
|
|---|---|---|---|---|---|---|
| Scaphixodes | ||||||
| I. howelli | 0 | yes | 7 | 5 | 4 | 2 |
| I. signatus | 0 | yes | 7 | 5 | 5 | 1 |
| Multidentatus | ||||||
| I. auritulus | 1 | yes | 8 | 3-6 | 5 | 2 |
| Ceratixodes | ||||||
| I. uriae | 0 | no | 7 | 5 | 4 | 0 |
| Trichotoixodes | ||||||
| I. brunneus | 0 | yes | 7 | 5 | 5 | 1 |
Specimens examined:
Demacentor albipictus 4 flagged 4-IX-92 Contra costa co., 5 flagged 22-IX-06 Alameda co., 5 flagged 5-XII-06 Marin co.; D. andersoni 2 reared; D. hunteri 5 reared; D. occidentalis 16 flagged 7-X-06 Mendocino co., 4 flagged 14-VI-96 Los Angeles co.; D. variabilis 12 reared ex. dog 28-VI-05 Contra Costa co.; Haemaphysalis 10 ex. turkey VIII-04 Sonoma co., 10 flagged VIII-92 Contra Costa co.; Rhipicephalus 1 ex. dog, 30-XII-59 Marin co., 6 24-III-90 Orange co.; Ixodes angustus 7 ex. Peromyscus 18-IV-00 Inyo co., 1 ex. Peromyscus. 26-VII-05 Mariposa co., 2 ex. Neotoma VI-03 ElDorado co., 5 ex. Peromyscus & Microtus VI-02 Mendocino co., 5 reared Marin co.; I. auritulus 1 ex. golden crowned sparrow 27-X-82 San Francisco co., 1 ex. fox sparrow 9-XI-78 Marin co.; I. brunneus 2 reared Anastos & Smith (1957) Orlando, Florida Ent. Res. Br.; I. crenulatus 1 ex. Red fox 23-II 83 Prymorskyi Krai; I. hearlei 2 ex. squirrel 16-VIII-66 Pend Oreille co. Wash. RML47129; I. howelli 1 ex. cliff swallow 7-VII-60 Granite co. Montana RML36515; I. jellisoni 10 reared Mendocino co., 1 ex. shrew 1-VII-94 Marin co.; I. kingi 2 ex. grasshopper mouse VIII-61 Esmeralda co., Nevada RML37761; I. ochotonae 1 ex. Pika 30-III-40 British Columbia RML56702; I. pacificus 10 flagged 13-II-04 Mendocino co., 25-III-05 Alameda co. 30-IV-07 Alameda co., 2 ex. Peromyscus 6-VI-02 Mendocino co.; I. peromysci ex. Peromyscus 2-X-78 Ventura co. RML10966 (Orange co. coll.); I. rugosus 1 ex. fox 27-II-07 Contra Costa co., 3 ex. fox 2-V-04 Humbolt co., 1 ex. opossum 27-VII-06 Mendocino co.; I. sculptus 17 ex. ground squirrel 15-VII-80 Sierra co., 5 ex. ground squirrel 7-VIII-05 Mariposa co.; I. signatus 1 ex. pigeon guillemot 23-VII-77 Santa Barbara co., 4 ex. pelagic cormorant 31-XII-02 Mendocino co.; I. soricis 3 ex. Sorex monticolus 24-IX-57 Clallan co., Washington RML 36554, 2 ex. shrew 8-IV-51 Marin co., 9 ex. broad-footed mole 5-XI-80 Contra Costa co., 5 ex. shrew 1-VII-86 Nevada co.; I. spinipalpis 10 flagged 6-V-04 Mendocino co., 6 flagged 14-VI-96 Los Angeles co.; I. texanus 2 ex. skunk 22-X-52 Orange co. RML31999, 1 ex. skunk 24-XI-53 Michoacan, Mexico, 2 ex. Fisher 4-I-95 New Hampshire; I. uriae 1 ex. storm petrel 23-VII-79 Curry co., Oregon; I. woodi 11 ex. Peromyscus & Neotoma 24-IV-02 Mendocino co., 3 reared Sonoma co., 2 ex. Neotoma VI-03 ElDorado co., 5 11-V-95 Mendocino co.
Key to the Californian species of Ixodes larvae
1 Spurs on palpal article I absent (Fig. 4) . . . . . 2 Spur or spurs on palpal article I present (Figs. 11-16) . . . . . 10
2(1) External spurs present on coxae I and II (Figs. 9, 10); supplementary setae usually present (Fig. 4); auriculae present (Figs. 9, 10) . . . . . 3 External spurs absent on coxae; supplementary setae absent; auriculae faint or with a ridge present (Figs. 11-13) . . . . . 9
3(2) Alloscutal setae conspicuously long (Fig. 4) (often 0.08 mm – 0.10 mm); hosts diverse. . . . . 4 Alloscutal setae short (<0.05 mm); parasitize birds exclusively . . . . . 8
4(3) Hypostome sharply pointed; outer contour of palps expanding sharply near junction of articles I and II (Fig. 1B); central dorsal setae 5 pairs; commonly found on ground-frequenting birds; subgenus Trichotoixodes . . . . . I. brunneus Koch Hypostome rounded; outer contour of palps continuous (Fig. 4); central dorsal setae 2 or 4 pairs; hosts diverse . . . . . 5
5(4) Central dorsal setae 4 pairs (Fig. 4); posterior ventral margin of basis capituli straight, thickened laterally at the corners (Fig. 9); internal spur on coxa I not overlapping coxa II (Fig. 9) . . . . . 6 Central dorsal setae 2 pairs; posterior margin of basis capituli rounded (Fig. 10); length of internal spur on coxa I variable . . . . . 7
6(5) Basis capituli ventrally with well developed auriculae (Fig. 9); base of hypostome ≈0.07 mm wide; cuticular pigment reddish brown (Fig. 22); found primarily on lizards, small rodents, and ground-inhabiting birds; subgenus Ixodes . . . . . I. pacificus Cooley & Kohls Basis capituli ventrally with bulbous auriculae; hypostome width at base ≈0.09 mm; cuticle light colored, with little pigmentation (Fig. 23); found on heteromyid rodents; subgenus Ixodes . . . . . I. jellisoni Cooley & Kohls
7(5) Supplementary setae 1 pair (Fig. 4); internal spur on coxa I acute and overlapping coxa II (Fig. 10); outer contour of palps slightly concave (Fig. 24); length of alloscutum less than the length of scutum in unfed ticks; subgenus Ixodes . . . . . I. spinipalpis Hadwen & Nuttall Supplementary setae absent; internal spur on coxa I just touching coxa II, outer contour of palps straight; length of alloscutum equal to scutal length; only records from Santa Barbara county and West Anacapa Islands; subgenus Ixodes . . . . . I. peromysci Augustson
8(3) Slightly bulbous auriculae; coastal species known primarily from Cormorants and the pigeon Guillemot (Fig. 19); subgenus Scaphixodes . . . . . I. signatus Birula Auriculae well-developed; inland species, high montane records from the gray-crowned Rosy Finch; subgenus Scaphixodes . . . . . I. howelli Cooley & Kohls
9(2) Marginal dorsal setae 9 pairs, 1 pair directly posterior to the anus (Fig. 1); 2 posthypostomal setae; 5 pairs of scutal setae (Fig. 27); found on skunks, opposum and raccoons; subgenus Pholeoixodes . . . . . I. texanus Banks Marginal dorsal setae 7 pairs; 1 pair of posthypostomal setae; 4 pairs of scutal setae; found on shore nesting marine birds (Fig. 20); subgenus Ceratixodes . . . . . I. uriae White
10(1) Prominent anterior spur only on palpal article I; auriculae distinct; prominent exterior spurs on coxae I, II and III (Fig. 21); 3-6 pairs of central dorsal setae; 2 or 3 pairs of supplementary setae; found on birds year-round; subgenus Multidentatus . . . . . I. auritulus Neumann Posterior spur, or posterior and anterior spurs, present on palpal article I (Figs. 11-16); auriculae undeveloped, but with a small ridge usually present (Figs. 11-13); external spurs absent on coxae; 2 pairs of central dorsal setae; supplementary setae absent . . . . . 11
11(10) Very small single posterior spur on palpal article I (Figs. 11, 13) . . . . .12 Anterior and posterior spur present on palpal article I (Figs. 12, 14, 15, 16) . . . . .14
12(11) Substructure posterior to base of hypostome cleaved as viewed in ventral mounts (Fig. 13); length of palps ≈0.170 mm; scutal length ≈ 0.321 mm (Fig. 28); found on woodrats or mice near woodrat nests; subgenus Ixodiopsis . . . . . I. woodi Bishopp Horizontal ridge posterior to base of hypostome entire as viewed in ventral mounts (Fig. 11); length of palps less than 0.150 mm and scutal length less than 0.300 mm . . . . .13
13(12) Marginal dorsal setae 9 pairs, 1 pair located on the central festoon behind the anus (Fig. 1); small spur on palpal article I directed laterally (Fig. 11), palpal length ≈ 0.141 mm; length of scutum ≈ 0.251 mm, posterior margin of scutum flattened or slightly emarginated (Fig. 25); hypostomal teeth separated slightly; found on ground and tree squirrels; subgenus Pholeoixodes . . . . . I. hearlei Gregson Marginal dorsal setae 8 pairs, marginal dorsal setae posterior to the anus absent; spur on palpal article I directed posteriorly, palpal length ≈ 0.096 mm long; length of scutum ≈ 0.270 mm (Gregson 1971), posteror margin rounded; hypostomal teeth retrose and tight fitting; hosts include kangaroo rats, pocket gophers, and sigmodontine mice; subgenus Pholeoixodes . . . . . I. kingi Bishopp
14(11) Palpal article I with both a very short anterior spur, and a small posterior projection (Fig. 14) . . . . . 15 Palpal article I with lengths of spurs variable (Figs. 15, 16) . . . . .16
15(14) Cornua present (Fig. 18), directed laterally; base of hypostome without teeth; horizontal structure below hypostome broken medially as viewed in ventral mounts; palp with a straight contour (Fig. 14); predominantly parasites of ochotonid lagomorphs; subgenus Ixodiopsis . . . . . I. ochotonae Gregson Cornua undeveloped; hypostomal teeth reach base; horizontal substructure below hypostome entire as viewed in ventral mounts; inner contour of palp indented near junction of articles I and II, expanding slowly from article I (Fig. 26); parasites of skunks, fox, weasels, and dogs; subgenus Pholeoixodes . . . . . I. rugosus Bishop
16(14) Cornua present, directed posteriorly (Fig. 18); inner and outer contours of palp indented at the junction of segments I and II, and expanding slowly (Fig. 12); setae SC4 and SC5 inserted at the edge of scutum, and an indentation often present in the contour at about SC5 (Fig. 17); structure below hypostome, as viewed in ventral mounts, forming a straight horizontal edge (Fig. 12); predominantly parasites of ground squirrels; subgenus Pholeoixodes . . . . . I. sculptus Neumann Cornua undeveloped; contour of palp straight (Figs. 15, 16); setae SC4 and SC5 inserted well inside the edge of the scutum, posterior contour of scutum broadly rounded; structure below the hypostome, viewed ventrally, variable, often cleaved . . . . . 17
17(16) Posterior spur on palpal article I usually passing lateral side (Fig. 15); subhypostomal ridge variable; length of scutum 0.226 – 0.324 (0.280 mm) (Robbins & Keirans 1992) (Fig. 29); hosts microtine and cricetid rodents; subgenus Ixodiopsis. . . . . I. angustus Neumann Posterior spur on palpal article I significant, but short, knob like and directed ventrally (Fig. 16), usually not passing lateral side of article I in dorsal view (Fig. 16); subhypostomal ridge entire, small tick, length of scutum 0.232 – 0.306 (0.263 mm) (Robbins & Kierans 1992) (Fig. 30); parasites of moles and shrews; subgenus Ixodiopsis. . . . . I. soricis Gregson
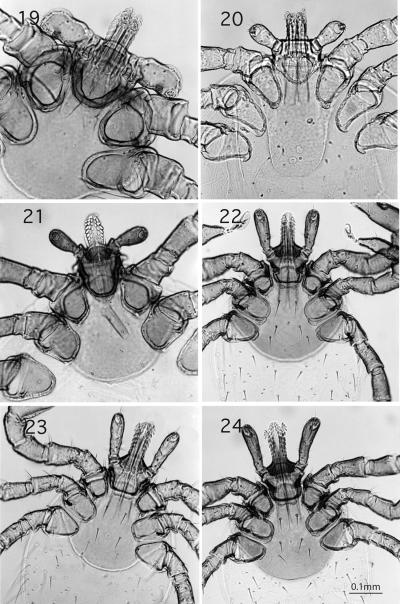
Figures 19-24.
Ventral view, 100x. Figures 19-21. Ixodes host-specific for birds. Fig. 19. I. signatus; Fig. 20. I. uriae; Fig. 21. I. auritulus. Figures 22-24. Subgenus Ixodes; Fig. 22. I. pacificus; Fig. 23. I. jellisoni, note lack of pigment; Fig. 24. I. spinipalpis, concave palps.
Figures 25-30.
Ventral view, 100x. Figures 25-27. Subgenus Pholeoixodes. Fig. 25. Ixodes hearlei; Fig. 26. I. rugosus; Fig. 27. I. texanus; Figures 28-30. Subgenus Ixodiopsis. Fig. 28. I. woodi; Fig. 29. I. angustus; Fig. 30. I. soricis.
Summary
Among the 18 species of Ixodes recorded from California only the larvae of I. pacificus, I. spinipalpis, and much less often, those of I. angustus or I. auritulus, have been collected while host-seeking atop leaf litter, logs, or along trails by dragging or flagging. Other Ixodes species that inhabit the nests or burrows of their avian or mammalian hosts rarely are collected aboveground, but instead must be removed directly from their vertebrate hosts. Therefore some larval species of Ixodes are rarely encountered, and consequently little is known about their taxonomic variation or relation to animal disease agents. Though Ixodes spp. recorded from California complied closely with their previous taxonomic descriptions, we detected variations, particularly for a few broadly distributed species that extend throughout North America and into the Palearctic region, such as I. texanus and I. angustus (Emel'yanova 1979, Filippova 1967). For example, I. texanus from California differs from other Pholeoixodes by lacking a spur on palpal article I (Fig. 5) (Allred et al. 1960, Webb et al. 1990), whereas I. texanus from coastal areas of the eastern United States has a small spur (Clifford et al. 1961). Ixodes texanus also differs from other Pholeoixodes in that the ridge posterior to the hypostome is cleaved.
Additionally, we found that the I. angustus-I. soricis species complex is highly variable with respect to the degree of subhypostomal cleavage and the lengths of spurs on palpal article I. Three taxonomic characters published earlier for distinguishing the larvae of I. angustus from those of I. soricis i.e., the anterior shape of the palps (Robbins & Keirans 1992), and the shape of coxal and palpal spurs (Webb 1990) were unreliable for differentiating Californian specimens. Unfortunately, the larvae of both species have not been reared and definitively associated with either the nymphs or adults in California. Therefore a larval specimen (RML 36554) from Washington identified as I. soricis in the U. S. National Tick Collection, and Californian specimens of I. soricis identified by D. P. Furman (Fig. 30) were utilized to select characters for our key. The most useful criterion proved to be host specificity (rather than morphology) because all stages of I. soricis are specific to shrews, and the immatures of I. angustus rarely infest these insectivores (Gregson 1942).
Ixodes woodi exhibits the greatest variety of overlapping characters between the subgenera Ixodiopsis and Pholeoixodes. The adults of I. woodi clearly qualify as Ixodiopsis (Robbins & Keirans 1992), but according to our observations the larvae of I. woodi (Fig. 28) contain more character states typical of Pholeoixodes. It has the robust appearance of the sympatric species I. rugosus (Pholeoixodes) (Fig. 26) including wider palps with longer and stronger palpal setae versus other Californian members of Ixodiopsis (Figs. 29, 30). It also displays SC4 inserted close to the edge of the scutal cuticle, lacks an anterior spur on palpal article I, and has a very small posterior spur, traits that are typical of Pholeoixodes. It does display the post-hypostomal cleavage observed in Ixodiopsis, however. We conclude that further morphological (Klompen 1999) and molecular analysis (Fang et al. 2002, Xu et al. 2003) of these two taxonomic constructs is warranted and might alleviate some of the taxonomic confusion that surround these distinct, but related taxa.
Acknowledgements
This research was funded by a gift from Annie Henry and by grant AI22501 from the National Institutes of Health to R. S. L. We thank Lorenza Beati, Steve Bennett, Richard Brown, Lars Eisen, Janet Foley, Lucia Hui, Marjorie Matocq, and Kerry Padgett for donating or lending us specimens. We thank Daniel Salkeld for helpful comments on the drafts. We gratefully acknowledge Deane Furman, whose careful notes and collections of larval ticks and literature facilitated this study, and we dedicate this paper to his memory.
Literature Cited
- Allred DM, Beck DE, White LD. Ticks of the genus Ixodes in Utah. Brigham Young University Science Bulletin, Biological Series. 1960;1(4):1–42. [Google Scholar]
- Anastos G, Smith CN. The male, nymph, and larva of Ixodes brunneus Koch (Acarina: Ixodidae) The Journal of Parasitology. 1957;43(5):535–541. [PubMed] [Google Scholar]
- Arthur DR. Two North African ixodes ticks: I. kaiseri sp. nov. from Egyptian desert fox cubs. A redescription of the female and a description of the male of I. festai, Rondelli, 1926 (Ixodoidea, Ixodidae) The Journal of Parasitology. 1957;43:578–585. [PubMed] [Google Scholar]
- Augustson GF. A new Ixodes (Acarina: Ixodidae) Bulletin of Southern California Academy of Sciences. 1939;38:141–147. [Google Scholar]
- Banks N. Three new ticks from the United States. Proceedings of the Entomology Society, Washington. 1909;10:170–173. [Google Scholar]
- Birula A. Ixodidae novi vel parum cogniti Musei Zoologici Academiae Caesareae Scientiarum Petropolitanae. Izviestiia Imperatorskoi Akademii Nauk series. 1895;2(4):353–364. 5. [Google Scholar]
- Bishopp FC. Some new North American Ixodidae with notes on other species. Proceedings of the Biological Society of Washington. 1911;24:197–208. [Google Scholar]
- Clifford CM, Anastos G. The use of chaetotaxy in the identification of larval ticks (Acarina: Ixodidae) The Journal of Parasitology. 1960;46:567–578. [PubMed] [Google Scholar]
- Clifford CM, Anastos G, Elbl A. The larval ixodid ticks of the eastern United States (Acarina-Ixodidae) Miscellaneous Publications of the Entomological Society of America. 1961;2(3):213–237. [Google Scholar]
- Clifford CM, Sonenshine DE, Keirans JE, Kohls GM. Systematics of the subfamily Ixodinae (Acarina: Ixodidae). 1. The subgenera of Ixodes. Annals of the Entomological Society of America. 1973;66(3):489–500. [Google Scholar]
- Cooley RA. Ixodes neotomae, a new species from California (Acarina: Ixodidae) The Pan- Pacific Entomologist. 1944;20(1):7–12. [Google Scholar]
- Cooley RA. Ixodes holdenriedi, a new species of tick from a pocket gopher in California (Acarina, Ixodidae) The Pan-Pacific Entomologist. 1946;22(3):103–104. [Google Scholar]
- Cooley RA, Kohls GM. Two new species of ticks (Ixodes) from California (Acarina: Ixodidae) U. S. treasury department public health service report. 1938;53(36):1616–1621. [Google Scholar]
- Cooley RA, Kohls GM. Ixodes californicus Banks, 1904, Ixodes pacificus n. sp. and Ixodes conepati n. sp. (Acarina: Ixodidae) The Pan-Pacific Entomologist. 1943;19(4):139–147. [Google Scholar]
- Cruickshank RH. Molecular markers for the phylogenetics of mites and ticks. Systematic and Applied Acarology. 2002;7:3–14. [Google Scholar]
- Curtice C. The biology of the cattle tick. Journal of Comparative Medical and Veterinary Archives. 1891;12:313–319. [PMC free article] [PubMed] [Google Scholar]
- Emel'yanova ND. Taxonomic status of ixodid ticks of the genus Pholeoixodes within the subfamily Ixodinae and its division into subgenera. In: Efimov MV, Pronin, editors. Zooparasitology of Lake Baikal Basin. N. M. Akademy Nauk SSSR, Siberyan Otdelenie Buryatia, Filial, Otdelenie Biology, Ulan-Ude; Siberia: 1979. pp. 5–27. [Google Scholar]
- Fang Q, Keirans JE, Mixson T. The use of the nuclear protein-encoding gene, RNA polymerase II, for tick molecular systematics. Experimental and Applied Acarology. 2002;28:69–75. doi: 10.1023/a:1025389914156. [DOI] [PubMed] [Google Scholar]
- Filippova NA. New tick species Ixodes stromi and its standing in the system of Ixodinae. Zoologicheskii Zhurnal. 1957;36(6):864–869. [Google Scholar]
- Filippova NA. A contribution to the morphology and systematics of the immature stages of the ticks. Parazitologicheskii Sbornik. 1958;18:10–77. [Google Scholar]
- Filippova NA. On the taxonomy of ticks of the group “crenulatus” (Ixodidae, Ixodes, Pholeoixodes) Parazitologicheskii Sbornik. 1961;20:226–247. [Google Scholar]
- Filippova NA. Species of the subgenus Ixodiopsis (Ixodoidea, Ixodinae, Ixodes) of the Soviet Union fauna. Parazitologicheskii Sbornik. 1967;23:100–123. [Google Scholar]
- Filippova NA. Ixodid ticks of the subfamily Ixodinae. (series 9).Paukoobraznye Fauna. 1977;4:393. 4. [Google Scholar]
- Filippova NA. Symposium 4. A hypothesis for the palaeogenesis of the distribution of the main vectors for Lyme disease. In: Dusbábek F, Bukva V, editors. Modern Acarology. Vol. 1. Academia, Prague & SPB Academic Publishing bv, The Hague; 1991. pp. 109–118. i + 651pp. [Google Scholar]
- Filippova NA. Section 9.4. Systematic relationships of the Ixodes ricinus complex in the Palearctic faunal region. In: Needham GR, et al., editors. Acarology IX. Vol. 2. Ohio Biological Survey; Columbus, Ohio: 1999. pp. 355–361. xvii + 507 pp. [Google Scholar]
- Furman DP, Loomis EC. The ticks of California (Acari: Ixodida) Bulletin of the California Insect Survey. 1984;25:1–239. [Google Scholar]
- Glashinskaya-Babenko LV. Chaetotaxy of the body of larval ticks of the family ixodidae and their taxonomic significance. Doklady Akademii Nauk SSSR. 1949;65:245–248. [Google Scholar]
- Gregson JD. Two new species of ticks from British Columbia (Ixodidae) The Canadian Entomologist. 1941;73:220–228. [Google Scholar]
- Gregson JD. A new species of tick found on shrews. The Canadian Entomologist. 1942;74:137–139. [Google Scholar]
- Gregson JD. Studies on two populations of Ixodes kingi Bishopp (Ixodidae) Canadian Journal of Zoology. 1971;49:591–597. doi: 10.1139/z71-095. [DOI] [PubMed] [Google Scholar]
- Hadwen S, Nuttall GHF. Notes on ticks, IV. Relating to the genus Ixodes and including a description of three new species and two new varieties. Parasitology. 1916;8:294–337. [Google Scholar]
- Hui LT, Castro MB, Clover J, Thompson MA, Husted S. Summary of Ixodes pacificus surveillance and testing for Borrelia burgdorferi in California. Proceedings of the California Vector Control Association. 2004;72:77–83. [Google Scholar]
- Keirans JE, Brown RN, Lane RS. Ixodes (Ixodes) jellisoni and I. (I.) neotomae (Acari: Ixodidae): Descriptions of the immature stages from California. Journal of Medical Entomology. 1996;33(3):319–327. doi: 10.1093/jmedent/33.3.319. [DOI] [PubMed] [Google Scholar]
- Klompen JSH. Section 9.3. Phylogenetic relationships in family Ixodidae with emphasis on the genus Ixodes (Parasitiformes: Ixodidae) In: Needham GR, et al., editors. Acarology IX. Vol. 2. Ohio Biological Survey; Columbus, Ohio: 1999. pp. 349–354. xvii + 507 pp. [Google Scholar]
- Klompen JSH, Keirans JE, Filippova NA, Oliver JH., Jr. Idiosomal lyrifissures, setae, and small glands as taxonomic characters and potential indicators of ancestral segmentation patterns in larval Ixodidae ( Acari: Ixodida) International Journal of Acarology. 1996;22(2):113–134. [Google Scholar]
- Klompen JSH, Oliver JH, Jr., Keirans JE, Homsher PJ. A re-evaluation of relationships in the Metastriata (Acari: Parasitiformes: Ixodida) Systematic Parasitology. 1997;38:1–24. [Google Scholar]
- Klompen JSH, Black WC, IV, Keirans JE, Norris DE. Systematics and biogeography of hard ticks, a total evidence approach. Cladistics. 2000;16:79–102. doi: 10.1111/j.1096-0031.2000.tb00349.x. [DOI] [PubMed] [Google Scholar]
- Koch CL. Ixodes crenulatus. 3. Vol. 39. 1841. Deutschlands Crustaceen, Myriapoden und Arachniden. Ein Beitrag zur deutschen Fauna. Herausg V. Herrich-Schäffer. 40 parts 1835-1844. Regensburg. [Google Scholar]
- Koch CL. Systematische Uebersicht über die Ordnung der Zecken. Archiv für Naturgesch (Berlin) 1844;10:232. [Google Scholar]
- Lane RS, Clover JR. Ticks. In: Meyer RP, Madon MB, editors. Arthropods of public health significance in California. Mosquito and Vector Control Association of California; Sacramento: 2002. pp. 171–183. Chapter 18. viii + 201 pp. [Google Scholar]
- Latreille PA. Observations sur la variété des organs de la bouche des tiques, et distribution méthodique des insectes de cette famille d'après les caractères établis sur la conformation de ces organes. Magasin Encyclopédique, ou Journal des Sciences, des Lettres et des Arts, Paris. 1795;4:15–20. [Google Scholar]
- Latreille PA. Genera crustaceorum et insectorum secundum ordinem naturalem in familias disposita, iconibus exemplisque plurimis explicata. Vol. 1. Amand Koenig; Paris: 1806. p. 303. P 157. [Google Scholar]
- Lindquist EE, King Wan Wu, H Redner J. A new species of the tick genus Ixodes (Acari: Ixodidae) parasitic on mustelids (Mammalia: Carnivora) in Canada. The Canadian Entomologist. 1999;131:151–170. [Google Scholar]
- Marx G, Curtice C. Parasites, being a list of those infesting the domesticated animals and man in the United States. Journal of Comparative Medical and Veterinary Archives. 1892;13:223–236. [PMC free article] [PubMed] [Google Scholar]
- Murrell A, Barker SC. Synonymy of Boophilus Curtice, 1891 with Rhipicephalus Koch, 1844 (Acari: Ixodidae) Systematic Parasitology. 2003;56:169–172. doi: 10.1023/b:sypa.0000003802.36517.a0. [DOI] [PubMed] [Google Scholar]
- Neumann LG. Révision de la famille des ixodidés (3e mémoire) Mémoires de la Société Zoologique de France. 1899;12:107–294. [Google Scholar]
- Neumann LG. Notes sur les Ixodides. I. Archives de Parasitologie, Paris. 1902;6:109–128. [Google Scholar]
- Neumann LG. Notes sur les Ixodides, II. Archives de Parasitologie, Paris. 1904;8:444–464. [Google Scholar]
- Norris DE, Klompen JSH, Keirans JE, Lane RS, Piesman J, Black WC., IV Taxonomic status of Ixodes neotomae and I. spinipalpis (Acari: Ixodidae) based on mitochondrial DNA evidence. Journal of Medical Entomology. 1997;34:696–703. doi: 10.1093/jmedent/34.6.696. [DOI] [PubMed] [Google Scholar]
- Nuttall GHF, Warburton C. Ticks: a monograph of the Ixodoidea, part 2. Cambridge at the University Press; London: 1911. pp. 105–348. [Google Scholar]
- Packard AS. List of hymenopterous and lepidopterous insects collected by the Smithsonian expedition to South America, under Professor James Orton. (Appendix to the report on Articulates) 1st Annual Report Trustees Peabody Academy Science (Salem, Mass.) 1869;1:56–69. [Google Scholar]
- Reznik PA. Contribution to study of immature stages of the tick family Ixodidae. Communication six. Description of Ixodes frontalis Panz. larvae and nymphs and Ixodes redikorzevi Ol. larvae. Trudy Nauchno-issledovatel'skii Protivochumnyi Institut Kavkaza i Zakavkaz'ia. 1961;5:276–286. [Google Scholar]
- Robbins RG. Ticks of the Subgenus Ixodiopsis: First report of Ixodes woodi from man and remarks on Ixodes holdenriedi, an new junior synonym of Ixodes ochotonae (Acari: Ixodidae) Proceedings of the Entomological Society of Washington. 1989;91:291–292. [Google Scholar]
- Robbins RG, Keirans JE. Systematics and ecology of the subgenus Ixodiopsis (Acari: Ixodidae: Ixodes) (Entomological Society of America) Thomas Say Foundation Monograph. 1992;14:1–159. [Google Scholar]
- Schulze P. Das Geruchsorgan der Zecken. Untersuchungen über die Abwandlungen eines Sinnesorgans und seine stammesgeschiehtliche Bedeutung. Zeitschrift für Morphologie und Okologie. Tiere. 1941;37:491–564. [Google Scholar]
- Schulze P. Die morphologische Bedeutung des-Afters und seiner Umgebung bei den Zecken. Zeitschrift für Morphologie und Okologie. Tiere. 1942;38:630–658. [Google Scholar]
- Schwan TG, Corwin MD, Brown SJ. Argas (Argas) monolakensis, new species (Acari: Ixodoidea: Argasidae), a parasite of California gulls on islands in Mono Lake, California: description, biology, and life cycle. Journal of Medical Entomology. 1992;29(1):78–97. doi: 10.1093/jmedent/29.1.78. [DOI] [PubMed] [Google Scholar]
- Serdyukova GV. On the question of differential characteristics of larvae and nymphs of Ixodidae. Zoologicheskii Zhurnal. 1955;34(5):1037–1051. [Google Scholar]
- Snow KR, Arthur DR. Larvae of the Ixodes ricinus complex of species. Parasitology. 1970;60:27–38. doi: 10.1017/s0031182000077222. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE. Biology of ticks. Vol. 1. Oxford University Press; New York, New York: 1991. pp. 1–447. [Google Scholar]
- Sonenshine DE. Biology of Ticks. Vol. 2. Oxford University Press; New York, New York: 1993. pp. 1–465. [Google Scholar]
- Webb JP, Jr., Bennett SG, Challet GL. The larval ticks of the genus Ixodes Latreille (Acari: Ixodidae) of California. Bulletin of the Society for Vector Ecology. 1990;15(1):73–124. [Google Scholar]
- White A, Sutherland PC. Journal of voyage in Baffin's Bay. 1852;2:210. Appendix. [Google Scholar]
- Xu G, Fang QQ, Keirans JE, Durden LA. Molecular phylogenetic analyses indicate that the Ixodes ricinus complex is a paraphyletic group. The Journal of Parasitology. 2003;89(3):452–457. doi: 10.1645/0022-3395(2003)089[0452:MPAITT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]