Abstract
Context: Dominant-negative GH1 mutations cause familial isolated growth hormone deficiency type II (IGHD II), which is characterized by GH deficiency, occasional multiple anterior pituitary hormone deficiencies, and anterior pituitary hypoplasia. The basis of the variable expression and progression of IGHD II among relatives who share the same GH1 mutation is poorly understood.
Objective: We hypothesized that the cellular ratios of mutant/normal GH1 transcripts would correlate with the severity of the IGHD II phenotype. We determined the relative amounts of mutant and normal GH1 transcripts in cell lines and correlated transcript ratios with severity.
Design and Patients: Members of the same IGHD II kindred were genotyped for the GH1 E3+1 G/A mutation by DNA sequencing. Ratios of their 17.5-kDa (mutant)/22-kDa (normal) GH1 transcripts were determined in cultured lymphocytes (CLs), and these ratios were correlated with height sd scores obtained before GH replacement therapy.
Results: Ratios of 17.5-/22-kDa GH1 transcripts in CLs from family members with the same IGHD II mutation correlated with differences in their height sd scores.
Conclusions: Our findings suggest that expression levels of both the mutant and normal GH1 allele are important in the pathogenesis of IGHD II, that the ratio of mutant/normal transcripts may be a predictive marker of the penetrance and severity of IGHD II, and that CLs may be useful as surrogates to study GH1 transcript expression of subjects whose anterior pituitary cells are not available.
Biallelic GH1 transcript ratios correlate with IGHD-II severity.
The human GH gene (GH1) contains five exons that are constitutively spliced to produce a full-length, 22-kDa protein that accounts for the majority of circulating GH. Aberrant splicing of normal transcripts gives at least five smaller isoforms, the most abundant of which are a 20- and a 17.5-kDa isoform (1,2). The 17.5-kDa isoform results from complete skipping of exon 3, and increased amounts can exert a dominant-negative effect that prevents secretion of normal GH in both tissue culture cells and transgenic mice (3,4,5,6). Patients with mutations that sufficiently increase 17.5-kDa levels can develop autosomal dominant familial isolated GH deficiency type II (IGHD II). Characteristics of IGHD II include GH deficiency, possible development of multiple hormone deficiencies, and anterior pituitary hypoplasia with variability in onset, severity, and progression, even among family members who share the same IGHD II mutation. Mullis et al. (7) concluded that the pituitary endocrine status of IGHD II patients should be monitored over time because further hormonal deficiencies may evolve.
The splice sites surrounding exon 3 of GH1 are relatively weak, and three splicing enhancers are required to ensure exon 3 inclusion (8). One of these exon splice enhancers (ESE1) comprises the first seven bases of exon 3, and it augments use of the upstream 3′ splice site and suppresses a downstream cryptic splice site (9). Disruption of ESE1 causes exon 3 skipping, which produces the smaller 17.5-kDa isoform that can have dominant-negative effects on production of 22-kDa GH (8). We reported a guanine to adenine transition in the first base of exon 3 (E3 + 1 G/A), which disrupts ESE1 sufficiently to cause exon 3 skipping and resultant IGHD II (9).
Here, we studied the ratios of the 17.5/22-kDa transcripts in cultured lymphocytes (CLs) derived from members of an IGHD II kindred caused by the E3 + 1 G/A mutation to identify a molecular basis of the variable expressivity of this disorder. Lymphocytes are one of the few tissues that express not only GH but also GH receptor (for a review, see Ref. 10). Our data show that the cellular ratios of 17.5/22-kDa GH1 transcripts of relatives with IGHD II correlated with their height sd scores (Ht SDS). Our findings suggest that: 1) expression levels of both the mutant and normal GH1 allele are important in the pathogenesis of IGHD II; 2) because the product of E3 + 1 A transcripts has a dominant-negative effect, the ratio of mutant/normal transcripts may be a predictive marker of the penetrance and severity of IGHD II; and 3) CLs may be useful as surrogates for anterior pituitary cells that are not available to study the relative expression of mutant and normal GH1 transcripts.
Subjects and Methods
Study subjects and Ht SDS
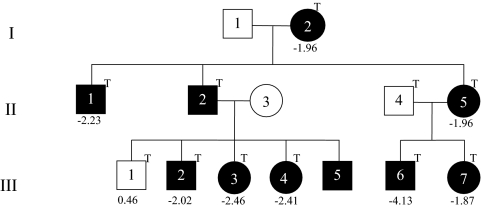
Study subjects were members of a Caucasian family with IGHD II (Fig. 1). Eleven family members were included, nine of whom were heterozygous for the E3 + 1 G/A transition that produces the 17.5-kDa transcript (11). Patient III-6 was diagnosed with GH deficiency at 27 months with a height of 75.7 cm (−4.13 SDS) and GH peaks of 1.12 and 1.14 ng/ml after clonidine and arginine, respectively (normal, >8 ng/ml). IGF-I was 28.2 ng/ml (normal, 51–303), and magnetic resonance imaging (MRI) showed a hypoplastic anterior pituitary (Fig. 1 and Tables 1 and 2). After GH replacement, his height was in the 25th percentile at 5 yr and 3 months. In contrast, his sister (III-7) was less severe, and her Ht SDS was −1.87 at diagnosis. Patients III-2, III-3, and III-4 are sibs diagnosed a 37, 14.5, and 16 months, respectively, with Ht SDS of −2.02, −2.46, and −2.41 at diagnosis. Patient III-5, who is 15 months old, has the E3 + 1 G/A mutation and a Ht SDS of −1.93 but has not yet had endocrine testing. Individuals I-2 and II-5, whose mutant/normal ratios are among the lowest of all the family members with IGHD II, have Ht SDS of −1.96 despite their never having received GH. Finally, II-2 was not treated until 12 yr of age (Fig. 1 and Tables 1 and 2). Ht SDS were calculated from heights obtained between 14.5 and 37 months of age at diagnosis and before treatment of GH deficiency, or adult heights for those not treated using http://www.phsim.man.ac.uk/SDSCalculator.
Figure 1.
Pedigree of the kindred with the E3 + 1 G/A dominant mutation that causes exon 3 skipping. Solid circles and boxes represent individuals with mutation. The letter “T” represents individuals whose CL-derived RNA was tested for GH1 transcripts by real-time PCR. Numbers below the boxes and circles represent Ht SDS before treatment.
Table 1.
Ht SDS and 17.5-/22-kDa transcript ratios
| Patient | Sex | IGHD II status | Ht SDS | 17.5/22-kDa ratio |
|---|---|---|---|---|
| I-2 | F | Affected | −1.96 | 0.56 |
| II-1 | M | Affected | −2.23 | 0.60 |
| II-2 | M | Affected | ? | 0.56 |
| II-4 | M | Unaffected | −2.23 | 0.11 |
| II-5 | F | Affected | −1.96 | 0.41 |
| III-1 | M | Unaffected | 0.46 | 0.02 |
| III-2 | M | Affected | −2.02 | 0.60 |
| III-3 | F | Affected | −2.46 | 1.04 |
| III-4 | F | Affected | −2.41 | 1.02 |
| III-6 | M | Affected | −4.13 | 1.54 |
| III-7 | F | Affected | −1.87 | 0.87 |
M, Male; F, female; ?, unknown.
Table 2.
Detailed information on height status and diagnostic tests at diagnosis of IGHD II
| Patient no. | IGHD II | Age (yr) | Height at Dx | Age at Dx (months) | Bone age (months) | Ht SDS before Rx | GH Peak (ng/ml) | IGF-I (ng/ml) | IGFBP-3 | TSH (μ U/ml) | T4 (μ g/dl) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| III-1 | No | 11 | ND | ND | ND | 0.46 | ND | ND | ND | ND | ND |
| III-2 | Yes | 8 | 88.6 | 37 | 28 | −2.02 | 2.2 | <5 | 0.4 | 2.49 | 8.9 |
| III-3 | Yes | 5 | 70.5 | 14.5 | 15 | −2.46 | ND | <5 | 0.5 | NA | NA |
| III-4 | Yes | 2 | 72 | 16 | NA | −2.41 | 3.3 | <10 | 0.8 | 1.59 | 8.8 |
| III-5 | Yes | 1 | 71.2 | 1.04 | ND | −1.93 | Pending | 33 at 12m | NA | NA | NA |
| III-6a | Yes | 6 | 75.7 | 27 | NA | −4.13 | 1.12/1.14 | 28.8 | 1.2 | 1.18 | 1.33 |
| III-7 | Yes | 2 | 80 | 18 | NA | −1.87 | ND | 31 | ND | 1.66 | 6.2 |
Dx, Diagnosis; Rx, treatment; IGFBP, IGF binding protein; ND, not determined; NA, not available.
MRI of III-6 showed pituitary hypoplasia.
Establishment of CL cell lines
Lymphocytes were isolated from anticoagulated whole blood within 48 h of collection and exposed to Epstein-Barr virus to induce cell immortalization as previously described (12).
Sequencing
DNA was isolated from whole blood collected in EDTA tubes using a QIAamp DNA Blood Midi Kit (QIAGEN, Valencia, CA). The GH1 gene from each patient was PCR amplified using Elongase Enzyme Mix (Invitrogen, Carlsbad, CA) in a 50-μl reaction consisting of 1 μl deoxynucleotide triphosphates, 5 μl Elongase buffer A and B, 2 μl Elongase Enzyme Mix, 2 μl each of 1 μm GH primer no. 1 F-(5′-CCAGCAATGCTCAGGGAAAG-3′) and 1 μm GH primer no. 2 R-(5′-TGTCCCACCGGTTGGGCATGGCAGGTAGCC-3′). Reactions were incubated at 94 C for 30 sec, then 35 cycles of 94 C for 30 sec, 65 C for 30 sec, and 68 C for 3 min, then a final incubation at 68 C for 10 min. The 2700-bp products were visualized on a 2% agarose gel and 5 μl of PCRs and 2 μl ExoSAP-it (USB Corp., Cleveland, OH) and were incubated at 37 C for 15 min, then 80 C for 15 min. Sequencing was done using Big Dye Terminator (Applied Biosystems, Foster City, CA) in 10-μl reactions containing 2 μl Big Dye Terminator, 2 μl Big Dye Buffer, 50 ng template, and 2.5 μl of primer. Reactions were heated to 95 C for 5 min, then cycled 30 times at 95 C for 30 sec, 55 C for 10 sec, and 60 C for 4 min. The following GH primers on the plus strand were used for sequencing (5′-CAGGACTGAATCGTGCTCAC-3′), (5′-TATCTCTGGCTGACACTCTGTGC-3′), (5′-GGCAACAGTGGGAGAGAAGG-3′), (5′-TGGGCACAATGTGTCCTGAGGGGA-3′), (5′-CCCTCTGTTGCCCTCTGGTT-3′), (5′-CTGGGAAATAAGAGGAGGAGA-3′), (5′-CTCAGAGTCTATTCCGACACCCT-3′), (5′-CACTGACTTTGAGAGCTGTGTTA-3′), and (5′-GAATGAATGAGAAAGGGAGG-3′). After ethanol precipitation, the products were resuspended in 10 μl formamide and analyzed on a 3100 Genetic Analyzer (Applied Biosystems).
Analysis of GH transcripts by real-time
cDNA was synthesized from 1 μg of total cellular RNA using Superscript III cDNA Synthesis Kit (Invitrogen). We used predesigned ABI Taqman assays for both 17.5-kDa (Hs00737954_g1) and 22-kDa (Hs00792200_g1) GH1 transcript detection. Real-time PCR analysis was done using Taqman Universal Master Mix and a 7500 Real-Time PCR system (Applied Biosystems) according to the manufacturer’s instructions. We used TaqMan human endogenous control plate (catalog no. 4309199) to analyze 13 genes as potential housekeeping genes [18S rRNA, acidic ribosomal protein, β-actin, cyclophilin, glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerokinase, β2-microglobulin, β-glucronidase, hypoxanthine ribosyl transferase (HPRT), transcription factor IID, TATA binding protein, transferrin receptor, and ABL] for data normalization. HPRT was chosen because it showed the least variability between tissues and was similar in abundance to GH1 isoforms (13). An initial denaturation at 95 C for 10 min was followed by 40 cycles of denaturation at 95 C for 15 sec and annealing and extension at 60 C for 1 min. Relative expression levels were calculated using the comparative cycle threshold method (14,15).
Segregation studies
Segregation of the wild-type GH1 gene inherited from the unaffected parent was determined by sequencing of a common single nucleotide polymorphism (SNP) (rs2005172) located in the 5′ untranslated region (E1-118 G→T) of GH1 gene.
Cell culture
Control CLs were obtained from the Coriell Cell Repositories (Camden, NJ), and all CLs were grown in 15% fetal bovine serum in RPMI 1640 with 2 mm L-glutamine.
Human subjects
Samples for establishment of CLs were collected with informed consent under a Vanderbilt University Institutional Review Board-approved protocol.
Statistics
Data are expressed as mean ± sem. Comparisons between groups were performed with one-way ANOVA. All experiments were repeated three times and done in replicates of three or more. Correlations between continuous variables were made using Spearman nonparametric and linear regression analyses. Assumptions of linear regression for the final model were evaluated graphically by examining partial residuals. Ninety-five percent confidence intervals (CIs) were reported. P values less than 0.05 were considered statistically significant, and all tests were two-tailed. Statistical analyses were performed with the statistical package SPSS for Windows (version 15.0; SPSS, Inc., Chicago, IL).
Results
DNA genotyping and sequencing
The exon 3 + 1 G/A mutation was detected in DNAs from patients I-2, II-1, II-2, II-5, and III-2 through -7 and excluded in DNAs from individuals II-3, II-4, and III-1 (Fig. 1). No other sequence variations other than previously reported SNPs were detected in any of the samples.
GH1 transcripts can be detected in CL cell lines
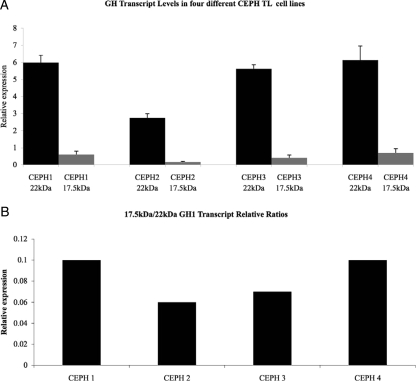
To determine whether GH1 transcripts can be detected in CLs, we analyzed four different control Centre d’Etude du Polymorphisme Humain (CEPH) CLs using TaqMan-based relative real-time PCR. Our results showed that CEPH CLs express both the 17.5- and 22-kDa GH1 transcripts (Fig. 2A) in ratios ranging from 5 to 10% of the total transcripts measured (Fig. 2B). This agrees with previous studies of GH1 transcript expression in pituitary and other cells (3,4,5,6,7,11).
Figure 2.
A, 17.5-kDa (gray) and 22-kDa (black) GH1 transcript levels as measured by relative real-time PCR analysis. B, The relative transcript ratios of 17.5-kDa/22-kDa in four different CEPH CL cell lines derived from normal individuals. Each sample was done in triplicate, and analysis was repeated three times. Error bars represent sem.
17.5- and 22-kDa GH1 transcript levels differ between IGHD II mutation carriers but do not reliably predict disease severity
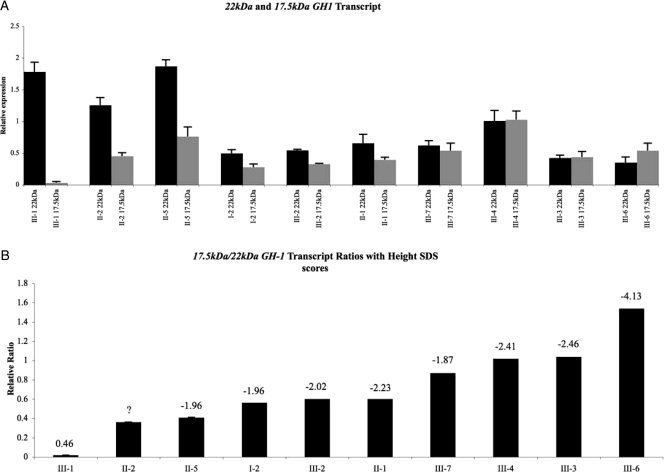
Affected members of the IGHD II kindred (Fig. 1) are all heterozygous for an E3 + 1 G/A transition that has a dominant-negative effect. Interestingly, family members sharing this mutation have differences in the severity (Ht SDS), age of onset, and presence of pituitary changes on MRI. We hypothesized that those with more 17.5-kDa transcripts would have a worse IGHD II phenotype. We tested our hypothesis by determining 17.5-kDa levels in CLs and found that noncarriers have significantly lower levels of 17.5-kDa expression than do mutation carriers (Fig. 3A). Although carriers with a more severe phenotype generally had higher levels of 17.5-kDa transcripts, this correlation was not consistent. For example, patients I-2, II-5, and III-7 had very different 17.5-kDa levels but similar Ht SDS. Thus, expression levels of 17.5-kDa transcripts alone did not correlate with disease severity (R2 = 0.269; P = 0.152; 95% CI, 0.88 to 0.22).
Figure 3.
A, 17.5-kDa (gray) and 22-kDa (black) GH1 transcript levels as measured by relative real-time PCR analysis. B, The relative transcript ratios of 17.5- kDa/22-kDa in CL cell lines derived from the kindred presented in Fig. 1. Numbers above the columns in panel B represent Ht SDS. Question mark indicates that Ht SDS of II-2 are not known. Family member II-4 was not included in the figure because we could not calculate his Ht SDS due to missing height information (his relative transcript ratio was 0.1, as would be expected for a normal person); additionally, because this person married into the kindred, we felt that he would not be an appropriate control. Each sample was done in triplicate, and analysis was repeated three times. Error bars represent sem.
We then hypothesized that 22-kDa transcript levels contributed to the variable expressivity seen in different mutation carriers. To test this, we determined the levels of the 22-kDa GH1 transcripts in mutation carriers (Fig. 3A). Although those with more severe phenotypes (III-3 and III-6) had the lowest levels of 22-kDa transcripts, III-4 had a similar Ht SDS to III-3 but a higher 22-kDa transcript level. Thus, expression levels of 22-kDa transcripts also did not show a statistical correlation with disease severity (R2 = 0.436; P = 0.053; 95% CI, −0.01 to 0.92).
The ratio of 17.5- vs. 22-kDa GH1 transcripts correlates with disease severity
We finally hypothesized that the relative ratios of mutant/normal transcripts in patient-derived CLs would correlate best with IGHD II severity because the 17.5-kDa transcript has a dominant-negative effect (9). As shown in Fig. 3B, there is a wide distribution in the relative ratio of 17.5/22-kDa transcripts. In addition, there is a statistically significant inverse correlation between the ratio and Ht SDS, with the lowest ratio among those with the highest Ht SDS and highest ratio among those with the lowest Ht SDS (R2 = 0.817; P = 0.001). Furthermore, data from CLs from members of two other kindreds (who carry a different GH1 mutation, IVS3 + 1G to A that causes more severe IGHD II) also showed a correlation between 17.5/22-kDa isoform transcript ratios and more negative Ht SDS (Supplementary Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). Our data thus show that 82% of the variability in the Ht SDS may be explained by variations in the relative ratio of 17.5/22 kDa. In fact, regression modeling predicts that the Ht SDS decreases by 2.43 U for every 1 U increase in the relative ratio (95% CI, −1.40 to −3.46; P = 0.001). We also attempted to derive Ht SDS for the individuals at roughly the same chronological age (see Supplementary Table 1). Regrettably, however, we were unable to get appropriate age-specific height information in more than half of the family members, including all the adults. Because the adults were nearly half of the individuals, a statistically valid regression analysis could not be done. Nevertheless, these data show that in family members where the corrected Ht SDS could be calculated, correlation between them and the isoform ratios remains. Furthermore, a regression analysis combining this information with the available information still produced a statistically valid correlation (P < 0.003) (Supplementary Table 1).
Variation in GH1 transcript expression is not secondary to CL immortalization
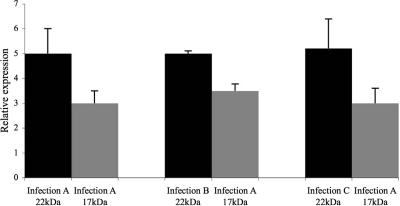
To eliminate the possibility that interpatient variation was secondary to the CL immortalization, we established multiple CLs using lymphocytes isolated from the same individual (I-2; see Fig. 1) but with different stocks of Epstein-Barr virus in three separate experiments. After immortalization, total cellular RNAs were isolated and used in cDNA reactions and then subjected to real-time PCR analyses for cellular GH1 transcript levels. The results showed that the 22- and 17.5-kDa transcript levels did not significantly vary between the three CLs established from the same patient (Fig. 4). The interindividual differences seen in GH transcript levels between IGHD II patients are greater than the intraindividual differences seen between different CLs from the same subject (Figs. 3 and 4) and likely represent true differences in transcript levels rather than artifacts of CL immortalization.
Figure 4.
17.5-kDa (gray) and 22-kDa (black) GH1 transcript levels as measured by relative real-time PCR analysis. The relative transcript ratios of 17.5- kDa/22-kDa in three different CL cell lines derived from the same individual (I-2) from the kindred shown in Fig. 1. Each sample was done in triplicate, and analysis was repeated three times. Error bars represent sem.
Variations in 22- and 17.5-kDa levels are not due to a cis-effect of a particular GH1 allele
To determine whether the variation in expression of GH1 transcripts is allele specific, we studied the segregation of a SNP identified in the GH1 gene. We found that III-2 through -4 and III-6 and III-7 inherited the same parental mutant and normal GH1 alleles (data not shown), suggesting that the variations seen in transcript levels may be a function of regulation by trans-acting factors, altered splicing efficiency, or both rather than cis changes that reside in their GH1 alleles.
Discussion
It has been hypothesized that the observed variable phenotypic expression commonly seen in IGHD II pedigrees is likely secondary to as yet unidentified “modifying” genes. Our data suggest, for the first time, that one of the important modifiers of IGHD II severity may be variation in the ratios of GH1 transcripts themselves. Our data further suggest that the expression of normal GH1 allele transcripts can vary and that the relative amounts of normal and mutant, rather than mutant transcript alone, determine severity and penetrance of IGHD II. Furthermore, these findings suggest that CLs may be useful as surrogates to study the cellular expression patterns of GH1 transcripts of subjects whose anterior pituitary cells are not available.
We initially hypothesized that differences in the amounts of mutant or normal transcripts alone could explain the variable expressivity in IGHD II. We found that the 17.5-kDa levels in CLs of noncarriers were significantly lower than those of mutation carriers (Fig. 3A). However, patient III-3, who had a Ht SDS of −2.46 before treatment, also had a 17.5-kDa expression level that was less than that of II-5 and III-7 (who had Ht SDS of −1.96 and −1.87, respectively). Thus, expression levels of 17.5-kDa transcripts alone did not correlate with disease severity (P = 0.152). On the other hand, although there was a difference between the levels of the 22-kDa transcripts seen in the most severely affected patients and normal controls, the 22-kDa transcript levels alone also could not predict severity of disease (P < 0.053). Thus, neither the amounts of mutant or normal transcripts alone independently explain the variable expressivity in IGHD II.
We subsequently hypothesized that the relative ratios of mutant/normal transcripts in patient-derived CLs would correlate with their IGHD II severity. This hypothesis was logical because the 17.5-kDa transcript has a dominant-negative effect, and increased levels of expression have been shown to be cytotoxic to pituitary cells (9). As shown in Fig. 3B, the relative ratio of 17.5/22-kDa transcripts statistically correlated with Ht SDS (P = 0.001), indicating that expression levels of both the mutant and normal GH1 allele are important in the pathogenesis of IGHD II. These results also suggest that more than approximately 80% of the variation in Ht SDS is attributable to differences in the isoform ratios. In agreement with this, we note that III-6 who has the highest mutant/normal ratio also has the lowest Ht SDS (Fig. 3B) and GH peak as well as a hypoplastic anterior pituitary (data not shown). In contrast, I-2 and II-5, who have among the lowest mutant/normal ratios of all the family members with IGHD II, also have Ht SDS of −1.96 despite having never been treated with GH replacement.
Variable expressivity is commonly seen in IGHD II pedigrees that contain affected individuals whose heights vary, and even without treatment, some may be of normal height. One hypothesis to explain this is that GH1 gene is not the only determinant of the final height, and thus other “modifying” genes may play an important role in the final phenotype. Knowledge about these modifying genes is still lacking. Our data suggest that one of the important modifiers of IGHD II severity may be variations in the ratios of GH1 transcripts themselves. Reports that variation of expression of the alleles modifies disease phenotype are rare but are noted for a small number of autosomal dominant diseases (16,17,18,19). Observing this variation raises the question of what regulates GH1 transcript/protein expression. Our segregation studies show that expression levels for the same mutant and normal GH1 alleles are not allele specific because sibs with disparate Ht SDS inherited the same parental mutant and normal GH1 alleles. Our segregation data suggest that transcript levels and ratios can differ between sibs such as III-6 and III-7, presumably due to differences in trans-acting factors and/or altered splicing efficiency rather than cis-acting factors.
Another significant finding of our study was that CLs may be useful as surrogates to study the cellular expression patterns of GH1 transcripts. Until now, the only in vitro method to study GH1 transcripts has been through transfection into cell lines such as the mouse pituitary AtT-20 cell line because patient pituitary cells are not available. Our results suggest that CLs derived directly from patients may be used to study some aspects of GH1 transcript expression. Several studies have shown that CLs have functional Golgi and endoplasmic reticulum late endosomal compartments that can normally traffic proteins. However, whereas neuroendocrine cell lines such as GH4C1 and AtT-20 can store translated and modified hormones and then release them in a controlled manner, nonendocrine cells such as CLs typically cannot. The dominant-negative effects of IGHD II mutations are likely to impact GH trafficking, and further work is needed to determine whether CLs can be used in studies of cellular GH trafficking.
In summary, our data offer the first molecular explanation of the variable expressivity seen in IGHD II kindreds and identify the usefulness of patient-derived CLs as a unique tool to study GH1 transcript expression. Variable expressivity of IGHD II can pose diagnostic and treatment dilemmas. Our finding that the ratios of the 17.5-/22-kDa GH1 transcripts correlate with disease severity must be validated in additional IGHD II cohorts to determine whether these ratios could be used to predict outcomes. Our finding that patient-derived CLs express GH1 transcripts may be useful in future studies of the molecular pathophysiology of familial GH deficiency.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grant R01 DK35592 (to J.A.P.).
Disclosure Summary: The authors have no conflicts of interests or disclosures regarding research presented in this manuscript.
First Published Online October 16, 2009
Abbreviations: CEPH, Centre d’Etude du Polymorphisme Humain; CI, confidence interval; CL, cultured lymphocyte; ESE, exon splice enhancer; Ht SDS, height sd score(s); IGHD II, isolated GH deficiency type II; MRI, magnetic resonance imaging; SNP, single nucleotide polymorphism.
References
- Procter AM, Phillips 3rd JA, Cooper DN 1998 The molecular genetics of growth hormone deficiency. Hum Genet 103:255–272 [DOI] [PubMed] [Google Scholar]
- Cogan JD, Phillips 3rd JA, Schenkman SS, Milner RD, Sakati N 1994 Familial growth hormone deficiency: a model of dominant and recessive mutations affecting a monomeric protein. J Clin Endocrinol Metab 79:1261–1265 [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Yamamoto M, Ohmori S, Kamijo T, Ogawa M, Seo H 1999 Inhibition of growth hormone (GH) secretion by a mutant GH-I gene product in neuroendocrine cells containing secretory granules: an implication for isolated GH deficiency inherited in an autosomal dominant manner. J Clin Endocrinol Metab 84:2134–2139 [DOI] [PubMed] [Google Scholar]
- Lee MS, Wajnrajch MP, Kim SS, Plotnick LP, Wang J, Gertner JM, Leibel RL, Dannies PS 2000 Autosomal dominant growth hormone (GH) deficiency type II: the Del32-71-GH deletion mutant suppresses secretion of wild-type GH. Endocrinology 141:883–890 [DOI] [PubMed] [Google Scholar]
- McGuinness L, Magoulas C, Sesay AK, Mathers K, Carmignac D, Manneville JB, Christian H, Phillips 3rd JA, Robinson IC 2003 Autosomal dominant growth hormone deficiency disrupts secretory vesicles in vitro and in vivo in transgenic mice. Endocrinology 144:720–731 [DOI] [PubMed] [Google Scholar]
- Shariat N, Ryther RC, Phillips 3rd JA, Robinson IC, Patton JG 2008 Rescue of pituitary function in a mouse model of isolated growth hormone deficiency type II by RNA interference. Endocrinology 149:580–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis PE, Robinson IC, Salemi S, Eblé A, Besson A, Vuissoz JM, Deladoey J, Simon D, Czernichow P, Binder G 2005 Isolated autosomal dominant growth hormone deficiency: an evolving pituitary deficit? A multicenter follow-up study. J Clin Endocrinol Metab 90:2089–2096 [DOI] [PubMed] [Google Scholar]
- Ryther RC, Flynt AS, Harris BD, Phillips 3rd JA, Patton JG 2004 GH1 splicing is regulated by multiple enhancers whose mutation produces a dominant-negative GH isoform that can be degraded by allele-specific small interfering RNA (siRNA). Endocrinology 145:2988–2996 [DOI] [PubMed] [Google Scholar]
- Ryther RC, McGuinness LM, Phillips 3rd JA, Moseley CT, Magoulas CB, Robinson IC, Patton JG 2003 Disruption of exon definition produces a dominant-negative growth hormone isoform that causes somatotroph death and IGHD II. Hum Genet 113:140–148 [DOI] [PubMed] [Google Scholar]
- Hattori N 2009 Expression, regulation and biological actions of growth hormone (GH) and ghrelin in the immune system. Growth Horm IGF Res 19:187–197 [DOI] [PubMed] [Google Scholar]
- Shariat N, Holladay CD, Cleary RK, Phillips 3rd JA, Patton JG 2008 Isolated growth hormone deficiency type II caused by a point mutation that alters both splice site strength and splicing enhancer function. Clin Genet 74:539–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh HM, Oh JM, Choi SC, Kim SW, Han WC, Kim TH, Park DS, Jun CD 2003 An efficient method for the rapid establishment of Epstein-Barr virus immortalization of human B lymphocytes. Cell Prolif 36:191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid R, Patterson J, Brandt SJ 2008 Genomic structure, alternative splicing and expression of TG-interacting factor, in human myeloid leukemia blasts and cell lines. Biochim Biophys Acta 1779:347–355 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Bustin SA 2002 Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol 29:23–39 [DOI] [PubMed] [Google Scholar]
- Wilmotte R, Maréchal J, Morlé L, Baklouti F, Philippe N, Kastally R, Kotula L, Delaunay J, Alloisio N 1993 Low expression allele α LELY of red cell spectrin is associated with mutations in exon 40 (α V/41 polymorphism) and intron 45 and with partial skipping of exon 46. J Clin Invest 91:2091–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vithana EN, Abu-Safieh L, Pelosini L, Winchester E, Hornan D, Bird AC, Hunt DM, Bustin SA, Bhattacharya SS 2003 Expression of PRPF31 mRNA in patients with autosomal dominant retinitis pigmentosa: a molecular clue for incomplete penetrance? Invest Ophthalmol Vis Sci 44:4204–4209 [DOI] [PubMed] [Google Scholar]
- Hamid R, Cogan JD, Hedges LK, Austin E, Phillips 3rd JA, Newman JH, Loyd JE 2009 Penetrance of pulmonary arterial hypertension is modulated by the expression of normal BMPR2 allele. Hum Mutat 30:649–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouya L, Puy H, Robreau AM, Bourgeois M, Lamoril J, Da Silva V, Grandchamp B, Deybach JC 2002 The penetrance of dominant erythropoietic protoporphyria is modulated by expression of wildtype FECH. Nat Genet 30:27–28 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.