Abstract
Context: Hutchinson-Gilford progeria syndrome (HGPS) and mandibuloacral dysplasia are well-recognized allelic autosomal dominant and recessive progeroid disorders, respectively, due to mutations in lamin A/C (LMNA) gene. Heterozygous LMNA mutations have also been reported in a small number of patients with a less well-characterized atypical progeroid syndrome (APS).
Objective: The objective of the study was to investigate the underlying genetic and molecular basis of the phenotype of patients presenting with APS.
Results: We report 11 patients with APS from nine families, many with novel heterozygous missense LMNA mutations, such as, P4R, E111K, D136H, E159K, and C588R. These and previously reported patients now reveal a spectrum of clinical features including progeroid manifestations such as short stature, beaked nose, premature graying, partial alopecia, high-pitched voice, skin atrophy over the hands and feet, partial and generalized lipodystrophy with metabolic complications, and skeletal anomalies such as mandibular hypoplasia and mild acroosteolysis. Skin fibroblasts from these patients when assessed for lamin A/C expression using epifluorescence microscopy revealed variable nuclear morphological abnormalities similar to those observed in patients with HGPS. However, these nuclear abnormalities in APS patients could not be rescued with 48 h treatment with farnesyl transferase inhibitors, geranylgeranyl transferase inhibitors or trichostatin-A, a histone deacetylase inhibitor. Immunoblots of cell lysates from fibroblasts did not reveal prelamin A accumulation in any of these patients.
Conclusions: APS patients have a few overlapping but some distinct clinical features as compared with HGPS and mandibuloacral dysplasia. The pathogenesis of clinical manifestations in APS patients seems not to be related to accumulation of mutant farnesylated prelamin A.
Eleven patients with atypical progeroid syndrome due to heterozygous lamin A/C mutations show marked phenotypic heterogeneity; the pathogenesis of clinical manifestations in these patients seems not to be related to accumulation of mutant farnesylated prelamin A.
Mutations in lamin A/C (LMNA) gene cause two well-defined, rare progeroid syndromes: Hutchinson-Gilford progeria syndrome (HGPS), a sporadic, autosomal dominant disorder, and mandibuloacral dysplasia (MAD), an autosomal recessive disorder (1,2,3,4,5,6,7,8,9,10,11,12). Besides HGPS and MAD, 13 patients have been reported to have an atypical progeroid syndrome (APS) due to heterozygous LMNA mutations, such as T10I, A57P, L59R, R133L, L140R, S143F, E145K, V169fsX176, D300N, E578V, and R644C (1,13,14,15,16,17,18,19,20,21). These patients have been variously referred to as HGPS, MAD, or atypical Werner’s syndrome. The phenotype of APS has not been well characterized, and patients have been reported to have variable progeroid features such as short stature, beaked nose, premature graying, partial alopecia, high-pitched voice, and skin atrophy over the hands and feet, besides having diabetes, generalized lipodystrophy, skin pigmentation, and mandibular hypoplasia. Therefore, the purpose of this study was to conduct in-depth phenotyping of patients presenting with APS who harbored heterozygous missense LMNA variants and investigate molecular mechanisms of progeroid manifestations. We also reviewed the literature to identify phenotypic differences among HGPS, MAD, and APS.
Case Reports
APS 200.5
This 27-yr-old Indian woman was born with normal weight (∼2.8 kg). She had normal growth and development until she was diagnosed with scoliosis at age 9 yr. She underwent extensive surgery for correction of scoliosis at age 13 yr, after which she lost about 5–7 kg weight. She has had no increase in her height since then. She attained menarche at age 11 yr and has had regular menstrual periods since then. Diabetes mellitus was diagnosed at age 16 yr, and at age 18 yr, she developed chylomicronemia-associated acute pancreatitis. At the time of our evaluation, she was taking pioglitazone, metformin, fenofibrate, and gliclazide but continued to have further episodes of acute pancreatitis and weight loss. She also had hepatic steatosis.
She was 147 cm tall and weighed 30.4 kg. Her blood pressure was 139/93 mm Hg. She had prominent fat loss over the face, extremities, trunk, back, palms, and soles (Fig. 1). She had a progeroid facies with loss of hair from the frontal region, prominent eyes, and pinched/beaked nose with thin lips. Axillary hair was scant and she had normal pubic hair. Skin was thin with prominent underlying veins. No breast tissue was palpable. She had hypopigmentation over the dorsum of metacarpophalangeal joints. She had no acanthosis nigricans or hirsutism. Fingers were thin and spindle-like. She had reduced muscle bulk. Liver was palpable 4 cm below the costal margin. Electrocardiography revealed borderline left ventricular hypertrophy.
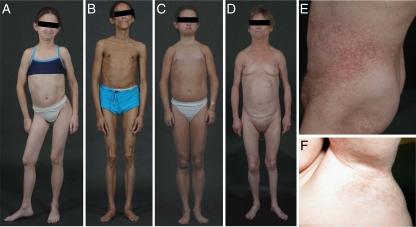
Figure 1.
Patients with APS. A, A 13-yr-old girl (APS 700.4) with Henoch-Schonlein purpura, spondylolisthesis, and avascular necrosis of right hip joint, dysplasia of heart valves, alopecia, and E159K mutation. B, A 27-yr-old woman (APS 200.5) with generalized lipodystrophy, diabetes, hypertriglyceridemia, and D136H mutation. C, A 16-yr-old female (APS 600.3) with diabetes, partial lipodystrophy and C588R mutation. D, A 51-year-old woman (APS 300.3) with partial lipodystrophy, diabetes, valvular anomalies, telangiectasias of the skin, and P4R mutation. E, Telangiectasias on the abdomen in a 51-yr-old woman (APS 300.3) with P4R mutation. F, A 7-yr-old boy (APS 400.5) with axillary freckles and C588R mutation.
APS 300.3
This is a 51-yr-old Caucasian woman who was normal at birth. However, a small mandible with a double chin was noted at age 3 yr, which became progressively worse. She also started losing fat from the extremities. She had problems with overcrowding and resorption of the teeth and had several teeth extracted. She attained menarche at age 12 yr and had normal menstrual periods, although her breast development was minimal. She also noticed progressive beaking of her nose. She was diagnosed with congestive heart failure secondary to mitral and aortic regurgitation during her first pregnancy at the age of 28 yr. She underwent aortic and mitral valve replacements at age 43 yr and had been on warfarin. She experienced palpitations and underwent atrioventricular nodal ablation with subsequent pacemaker insertion at 47 yr. She had dyslipidemia at 27 yr, diabetes mellitus at 38 yr, and hypertension at 41 yr of age. She was taking pravastatin, enalapril, furosemide, and pioglitazone. She has been progressively losing weight. Sensorineural hearing loss was noticed at age 31 yr, which required a hearing aid. She was taking alendronate for osteoporosis. Her younger brother had a similar phenotype but was not available for evaluation.
On physical examination, her height was 157 cm, and weight was 42.1 kg. She had a small face, with loss of fat from the cheeks, small chin, beaked nose, and prominent eyes. She had loss of sc fat from the extremities including the palms and soles (Fig. 1). However, she had excess fat accumulation in the abdomen and lower back. She had only 10 mandibular and 10 maxillary teeth. Auscultation revealed metallic sounds of the prosthetic aortic and mitral valves. Her breasts were small in size but not atrophic. She had excessive fat accumulation in the labia majora and mons pubis. Mottled hypopigmentation was noted on the arms and legs as well as telangiectasias on the waist, knees, and feet (Fig. 1). She had sclerodermatous skin on the legs. Radiographs of the chest, hands, and feet did not reveal acroosteolysis or clavicular resorption.
APS 300.4
He is the 25-yr-old son of APS 300.3, who noticed fat loss from the face and extremities at the age of 4 yr. He had a small chin along with a beaked nose. He had overcrowding of teeth, including external and internal resorption. Scalp hair started thinning since the age of 12 yr. He had aortic stenosis at birth and had valvuloplasty at age 12 yr, with subsequent aortic valve replacement at age 21 yr. He uses corrective lenses for myopia diagnosed at age 12 yr. He also had sensorineural hearing loss starting at the age of 23 yr with occasional tinnitus. He developed dyslipidemia and glucose intolerance at the age of 24 yr.
He was 165 cm tall and weighed 52.7 kg. He had prominent eyes, small chin, and a small pointed nose. He had mild frontal balding and graying of hair. He had normal facial fat but showed fat loss from the extremities, more striking distally. He had some fat accumulation in the abdomen and neck areas. He has 12 maxillary and 11 mandibular teeth after nine teeth had been extracted. Auscultation revealed metallic valvular sound with an ejection systolic murmur at left second intercostal space. The liver was palpable 1 cm below the right costal margin. Limitation of dorsiflexion of the feet and mild restriction of wrist extension were noted. Mottled hypopigmentation was noted over the trunk and extremities. He had spindle-looking tapering fingers and tight skin over the palms and soles. Also noted were telangiectasias over the ankles and abdomen over the waistband region. Radiographs showed mild osteoporosis around the metacarpophalangeal and interphalangeal joints but no acroosteolysis or clavicular resorption.
APS 300.5
She is the 22-yr-old daughter of APS300.3, who started losing fat progressively since the age of 2 yr. She attained menarche at age 13 yr with irregular menstrual periods but had normal menstruation during the last year. She has had poor breast development. She has no hearing or cardiac problems. She reported mottled skin pigmentation over her trunk since age 14 yr. She also had problems with overcrowding of teeth in both the upper and lower jaws.
She was 168 cm tall and weighed 51 kg. She had normal facial fat and had no beaking of the nose or small chin. She had decreased fat on her extremities. She had 12 mandibular and 12 maxillary teeth. She had minimal breast development at Tanner stage II. She had decreased flexion in the ankles and wrists. Skin showed mottled hypopigmentation over the extremities and trunk as well as telangiectasias over the waistband and groin region.
APS 400.5
This is a 7-yr-old boy of European origin, who was noticed to have axillary freckling at 5 months of age that progressively increased and spread to the groin, elbows, and knees. His height and weight had been in the second percentile. His skin was dry and hair growth was slow. He had dental crowding in the mandible.
He was 115.5 cm tall and weighed 17.3 kg. Facial features included prominent eyes, pinched nose with hypoplastic ala nasae, prominent vascularity on the tip of the nose, small mouth, and small mandible. He had 10 mandibular and 10 maxillary teeth with crowding in the mandible. His scalp hair was normal. He had no evidence of lipodystrophy. Liver was palpable 1 cm below the right costal margin. He had multiple bilateral pigmented macules, about 2–3 mm in diameter in the axillary, gluteal, and groin regions (Fig. 1). His skin was thin, atrophic, and sclerodermatous, especially over the hands and feet, with prominent blood vessels. His clavicles appeared short. Radiographs of the hands showed irregularity and fraying of the distal phalanges bilaterally, suggestive of acroosteolysis (Fig. 2). Chest radiographs did not show clavicular hypoplasia. Echocardiography and electrocardiogram were normal.
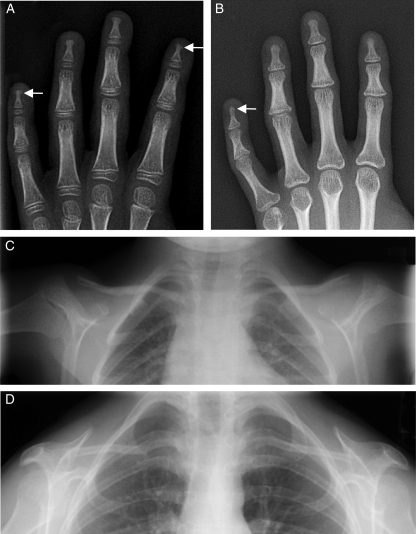
Figure 2.
Radiographs of the hands and upper part of the chest from the APS patients. Radiographs of the hands of APS 400.5 (C588R) (A) and APS 600.3 (C588R) (B) showing irregularity and fraying of the distal phalanges suggestive of acroosteolysis (arrows). Radiographic image of the upper chest from APS 400.5 showing no evidence of clavicular resorption (C), whereas that from APS 600.3 (D) shows features of clavicular hypoplasia.
APS 500.3
This is a 14-yr-old Caucasian boy with normal growth and development till age 10 yr when dysmorphic features like small chin, prominent eyes, and drooping shoulders were noted. He had significant overcrowding of teeth in both the jaws with an overbite, for which he underwent orthodontic treatment, including removal of two teeth. He noticed some pigmented freckles on the lateral aspects of his ankles.
His height was 157 cm and weight was 32.9 kg. He appeared to have reduced muscle bulk. He had prominent eyes, large nose, and underdeveloped maxilla and mandible. He had a high arched palate. He had 12 maxillary and 14 mandibular teeth. His hair was blond and fine in texture but no hair loss was noted. His thorax appeared bell shaped, with droopy shoulders. Auscultation revealed a grade 1 ejection systolic murmur at the right sternal border. External genitalia were normal male, at Tanner stage II. Joint examination revealed decreased extension at the knees, elbows, and shoulders as well as decreased flexion at the hips; however, muscle strength and neurological examination were normal. There was no evidence of lipodystrophy. He had a 1.5-cm round hypopigmented macule on the right upper back as well as pigmented freckles around the lateral aspect of his ankles. Chest radiograph showed bell-shaped contour of the rib cage with normal clavicles. Radiographs of the hands and feet did not show acroosteolysis. Echocardiography revealed mild pulmonary hypertension with thickened tricuspid valve and mild tricuspid regurgitation. Left ventricular ejection fraction was 56%.
APS 600.3
This is a 16-yr-old Caucasian girl from Bermuda who developed spotty pigmentation around the thighs at age 2 yr, which later spread to the neck, hands, and trunk. At the age of 6 yr, small mandible and beaked nose were noted, which progressed. She had poor growth. She attained menarche at age 11 yr but had menorrhagia and dysmenorrhea. She was initially treated with medroxyprogesterone acetate and then leuprolide for 8 months to suppress menstruation. Postclonidine peak GH level was 11.8 ng/ml (normal >7 ng/ml) and IGF-1 level was 525 ng/ml (normal 182–780 ng/ml). At age 13 yr, GH treatment was initiated but was discontinued after 1.5 yr because of inadequate increase in height. She was also diagnosed to have insulin resistance and glucose intolerance at the age of 15 yr and was treated with metformin.
She was 140 cm tall and weighed 36.7 kg. Her blood pressure was 140/90 mm Hg. Her face showed prominent eyes and beaked nose with some scarring at the tip and mandibular hypoplasia (Fig. 1). There were only 13 mandibular and 12 maxillary teeth erupted. Breasts were poorly developed at Tanner stage 1. Skin showed mottled hyper- and hypopigmentation on the extremities and abdomen. There was no axillary hair but pubic hair was normal. The scalp hair showed graying. There was slight hair loss from the temporal region. Skin was markedly thin with prominent veins and loss of sc fat was noted from the extremities. Hands and feet showed shortening of the digits and tapering. There was no hirsutism or acanthosis nigricans. Radiographs revealed mild osteolysis of the terminal phalanges of the hands and feet as well as a small mandible and hypoplastic clavicles (Fig. 2).
APS 700.4
This Caucasian girl presented to us at age 13 yr. She had joint pains in the ankles, knees, hips, wrists, and interphalangeal joints and purpura at the age of 6 yr and was diagnosed with Henoch-Schonlein purpura. She also had Raynaud’s phenomenon of her fingers and toes with cold exposure since she was a toddler. She was initially treated with naproxen and methotrexate for 9 months and then with etanercept for about 8 months. She then developed avascular necrosis of right femoral head requiring hip osteotomy at age 11 yr. At age 7 yr, mitral regurgitation was diagnosed, and at age 11 yr, moderate aortic regurgitation and severe tricuspid regurgitation were also noted. She was treated for congestive heart failure at age 10 yr, and a coronary angiogram at age 12 yr was normal. She developed hypertension at age 10 yr and was on atenolol. She had contractures of the wrist, elbow, knee, and ankle joints. She noted thinning of the scalp hair and crowding of her teeth, for which seven teeth were extracted. She attained menarche at the age of 12 yr but had irregular menstrual periods with menorrhagia.
She was 142 cm tall and weighed 29.1 kg. She had prominent eyes, pinched nose, small mandible, double chin, and thin hair (Fig. 1). There was no graying of hair. She had a high arched palate. She had 10 maxillary teeth and 11 mandibular teeth with braces. Auscultation revealed a grade 2 ejection systolic murmur. She had thin skin, with mottled hyper- and hypopigmentation on abdomen. Breasts were nearly absent. She had scant pubic hair and no axillary hair. She had scoliosis of the lower back as well as limited range of motion of elbows, wrists, and ankles. Her right leg was externally rotated due to femoral osteotomy. She had no hirsutism, acanthosis nigricans, or lipodystrophy.
Radiographic survey revealed no acroosteolysis but hypoplasia over both the lateral and medial ends of the clavicle. She underwent valve replacement at age 15 yr and shortly thereafter developed severe congestive heart failure requiring the support of ventricular assist device and died.
APS 800.3
This is a 15-yr-old boy from Kazakhstan, previously reported by us as having acquired generalized lipodystrophy (22). He had fat loss from the extremities at the age of 5 yr, which progressed to involve the trunk and face. Hyperglycemia- and hypertriglyceridemia-induced pancreatitis developed at the age of 10 yr. Aortic stenosis was detected on routine screening.
He was 161.5 cm tall and weighed 39.6 kg. There was generalized loss of sc fat involving the face, trunk, and extremities with prominent muscles and veins. He had mottled hyperpigmentation over the trunk but no acanthosis. He had mild retrognathia, but there were 28 teeth in total without any overcrowding. Cardiac examination revealed a grade 4/6 ejection systolic murmur with a diastolic component. The liver was palpable 5 cm below the right costal margin. Spleen was also palpable. Testes were of normal size with Tanner 4–5 pubic hair and a normal sized phallus. Hair was present over the upper lip, but there was no axillary hair. Radiographs of the hands and feet were normal. Electrocardiography revealed left ventricular hypertrophy with strain pattern. Echocardiogram revealed severe concentric left ventricular hypertrophy with hyperdynamic systolic function. Heavily calcified aortic valve with reduced leaflet excursion consistent with aortic stenosis was seen, although there was evidence of left ventricular outflow tract obstruction as well. Heavily calcified mitral valve apparatus and mild right ventricular hypertrophy were also seen.
During subsequent evaluation, we noted joint contractures at the ankle, knee, hip, and distal interphalangeal joints of the fourth and fifth fingers. Skin over the dorsolateral aspect of the feet appeared thin and atrophic. These features prompted us to consider the diagnosis of APS.
APS 1700.4
This is an 8-yr-old Caucasian male of Spanish origin who had onset of acanthosis nigricans in the axillae and inguinal regions at age of 6 months. Generalized lipodystrophy was diagnosed at age 3 yr. He had severe hyperlipidemia and insulin resistance and was taking metformin. He had voracious appetite. He also had muscle wasting and low exercise tolerance. He had severe hepatomegaly and liver biopsies showed marked fatty infiltration.
He was 140.3 cm tall and weighed 27.5 kg. There was generalized loss of sc fat involving the face, trunk, and extremities. Acanthosis nigricans was present on the neck, axillae, and inguinal regions. Spleen was palpable 4 cm below the costal margin. Cardiac examination revealed a grade 2/6 ejection systolic murmur. Testes were 5 ml bilaterally and he had Tanner 2 pubic hair and a normal-sized phallus.
Serum chemistry revealed markedly elevated alanine and aspartate aminotransferase levels. Electrocardiography revealed left anterior fascicular block with slight right ventricular hypertrophy and increased corrected QT interval of more than 440 msec. Echocardiography revealed a left ventricular ejection fraction of 31% with global hypokinesis. Mild aortic, mitral, and moderate tricuspid regurgitation were noted. There was mild pulmonary hypertension.
APS 1800.3
This is an 11-yr-old Italian girl with a birth weight of 3.7 kg. At age 8 yr, she had difficulty walking and had developed significant facial dysmorphy. She was 138.4 cm tall and weighed 36.8 kg (both in the 50th to 75th percentile range) at age 9 yr. She had beaked nose, marked retrognathia with a small mouth, high arched palate, and dental crowding of the lower jaw. Partial lipodystrophy was noted, with decreased sc fat in the lower extremities and hips and slight increase in fat deposition in the face, neck, and upper trunk. Muscle bulk was also reduced in the distal extremities. The skin was thin and dry with prominent veins. Radiological examination showed acroosteolysis of the distal phalanges in the hands.
Patients and Methods
All patients were evaluated at the General Clinical Research Center of the University of Texas Southwestern Medical Center at Dallas except APS 1700.4, who was evaluated at the National Institutes of Health, and APS 1800.3, who was evaluated at the Pediatric Department of the S. Orsola-Malpighi Hospital and at the University of Rome Tor Vergata. The research protocol was approved by the respective institutional review boards, and all the participants (patients, their parents, and normal subjects) gave written informed consent.
Anthropometry
Anthropometric measurements
Height and body weight were measured by standard procedures. Skinfold thickness was measured with a Lange caliper (Cambridge Scientific Industries, Cambridge, MD).
Magnetic resonance imaging (MRI)
MRI studies were performed using a 1.5 Tesla imaging device (Philips Medical Systems, Best, The Netherlands) and version 5.2–2 software. The patients were evaluated using 10-mm-thick T1 imaging technique with repetition time of 580 msec and echo time of 8 msec and a 384 × 512 matrix combined with a 45-cm field of view. Adipose tissue distribution and thickness were assessed by visual inspection of the films.
Dual-energy x-ray absorptiometry (DEXA)
Whole-body and regional fat in the head, trunk, and upper and lower extremities were determined using a multiple detector fan-beam QDR-2000 densitometer (Hologic, Inc., Waltham, MA).
Metabolic assessments and genetic analysis
Biochemical analyses
Plasma glucose was measured by the glucose oxidase method with a glucose analyzer (Beckman Coulter, Inc., Fullerton, CA). Serum insulin and leptin levels were determined by immunoassays using commercial kits (Linco Research, Inc., St. Charles, MO). Serum cholesterol, triglycerides, high-density lipoprotein cholesterol, and chemistry as well as blood hemoglobin A1C were analyzed as part of a systematic multichannel analysis (Synchron CX9 ALX clinical system; Beckman, Fullerton, CA).
Oral glucose tolerance test
After an overnight fast, a standard oral glucose tolerance test was performed in some patients who did not have diabetes mellitus using 75 g dextrose in adults and 1.75 g/kg body weight in children and fasting and postprandial serum glucose and insulin concentrations were measured.
Mutational analyses
The exons and exon-intron boundaries of the LMNA gene were sequenced according to Chen et al. (15).
Immunofluorescence microscopy
Immunofluorescence staining for lamin A/C was performed as described before (10,17). The chromatin was stained with 4′-6-diamidino-2-phenylindole.
Effects of pharmacological agents on nuclear morphology of fibroblasts
All experiments were performed by incubating fibroblasts with 10 μm inhibitors including farnesyl transferase inhibitors (FTI-277 and FTI-III), geranylgeranyl transferase inhibitor (GGTI-297), and histone deacetylase inhibitor, trichostatin A (EMD Biosciences, Inc., Gibbstown, NJ) for 48 h (10,17).
Immunoblotting for lamin A/C
Western blots were carried out as described before (10).
Results
LMNA genotyping
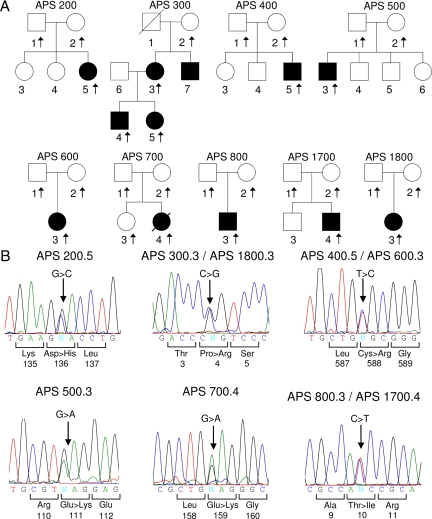
The pedigrees of the patients are shown in Fig. 3. Nine of the 11 patients had novel heterozygous missense LMNA mutations: C588R (two unrelated patients); P4R (three patients from the same family and in one unrelated patient); and E111K, D136H, and E159K in one patient each (Fig. 3). Two patients had a previously reported T10I heterozygous LMNA mutation. All the mutations were de novo except the P4R mutation in the APS 300 pedigree, which was transmitted in an autosomal dominant fashion. In addition, the following known heterozygous single-nucleotide polymorphisms were noted on sequencing of LMNA in the following patients: APS200.3, A287A; and APS300.3 and APS700.4, H566H. APS500.3, and his unaffected mother harbored a heterozygous variant IVS6-44C>T, which has not been previously reported in the LMNA single-nucleotide polymorphism database.
Figure 3.
Pedigrees of patients with APS and sequence analysis of LMNA. A, APS pedigrees. Circles denote female and squares represent males. Arrows indicate subjects from whom DNA was available. Filled symbols indicate affected and unfilled symbols indicate unaffected subjects. A diagonal line across a symbol indicates a deceased subject. B, Sequence electropherograms showing heterozygous mutations in probands from each pedigree. Arrows indicate the site of mutation. The numbers below the amino acids represent the number in lamin A protein.
Clinical assessment
Most patients had growth delay and short stature. One of the patients who was treated with GH therapy did not show a robust response to it. Other features included small mandible, overcrowding of teeth, high arched palate, progeroid appearance with a beaked nose, thin lips, loss of hair from the scalp, mild clavicular resorption, and acroosteolysis (Table 1). Mottling, telangiectasias, hypopigmentation, and sclerodermatous skin changes were the common skin findings. All postpubertal female patients had poorly developed breast tissue at Tanner stages 1 or 2 despite normal menstrual cycles in most of them (Table 1). Most of the patients had mild flexion contractures affecting the elbow, ankle, and wrist joints.
Table 1.
Phenotypic features and lamin A/C mutations in patients with atypical progeroid syndrome
| Age/sex |
LMNA mutation (heterozygous)
|
Lipodystrophy | Valvular anomalies | Small/atrophic breasts | Diabetes | Skin changes | Short mandible | Acroosteolysis | Clavicular resorption | Miscellaneous | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exon | Amino acid change | Nucleotide (cDNA) change | |||||||||||
| APS 200.5 | 27/F | 2 | D136H | 406G>C | Generalized | No | Yes | Yes | No | No | No | No | Scoliosis |
| APS 300.3 | 53/F | Partial | MVR, AVR | Yes | Yes | Yes | Yes | No | No | Osteoporosis, hearing loss | |||
| APS 300.4 | 25/M | 1 | P4R | 11C>G | Partial | MVR, AVR | NR | No | Yes | Yes | No | No | |
| APS 300.5 | 21/F | Partial | No | Yes | No | Yes | Yes | No | No | ||||
| APS 400.5 | 7/M | No | No | NR | No | Yes | Yes | Yes | No | ||||
| APS 600.3 | 16/F | 11 | C588R | 1762T>C | Partial | No | Yes | No | Yes | Yes | Yes | Yes | |
| APS 500.3 | 14/M | 1 | E111K | 331G>A | No | TR | NR | No | Yes | Yes | No | No | Pectus excavatum |
| APS 700.4 | 15/F | 2 | E159K | 475G>A | No | MVR, AVR | Yes | No | Yes | Yes | No | No | Avascular necrosis of hip; died postoperatively |
| APS 800.3 | 15/M | 1 | T10I | 29C>T | Generalized | AS | NR | Yes | Yes | Yes | No | No | |
| APS 1700.4 | 8/M | 1 | T10I | 29C>T | Generalized | MR,TR, AR | NR | Yes | Yes | No | NA | NA | |
| APS 1800.3 | 11/F | 1 | P4R | 11C>G | Partial | No | NA | No | Yes | Yes | Yes | No | Gait disturbance |
M, Male; F, female; MVR, mitral valve replacement; AVR, aortic valve replacement; AS, aortic stenosis; MR, mitral regurgitation; AR, aortic regurgitation; TR, tricuspid regurgitation; NA, information not available; NR, not relevant.
Five of the 11 patients had cardiac valvular anomalies including mitral regurgitation, aortic regurgitation, tricuspid regurgitation, and aortic stenosis. Whereas two patients belonging to APS 300 pedigree have undergone successful valve replacements, the patient APS 700.4 died in the postoperative period. Another patient APS 800.3 is awaiting surgery for severe aortic stenosis.
Of the eight patients with lipodystrophy, four had diabetes, four had hypertriglyceridemia, and three had hepatomegaly indicative of fatty liver (Table 2). Two patients had impaired glucose tolerance. Fasting and postprandial insulin values were elevated in only a few patients.
Table 2.
Biochemical evaluation in patients with APS
| Patient ID | LMNA mutation | HgbA1c (%) | Lipids
|
Leptin (ng/ml) | Glucose
|
Insulin
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TC (mg/dl) | TG (mg/dl) | LDLC (mg/dl) | HDLC (mg/dl) | Fasting (mg/dl) | 2 h PP (mg/dl) | Fasting (μU/ml) | 2 h PP (μU/ml) | ||||
| APS 200.5a,b | D136H | 7.8 | 246 | 360 | 149 | 43 | 1.2 | 124 | ND | 37.5 | ND |
| APS 300.3c,d | P4R | 5.3 | 175 | 126 | 118 | 41 | 5.2 | 94 | ND | 13.7 | ND |
| APS 300.4a | P4R | 5.1 | 196 | 116 | 140 | 44 | 3.0 | 91 | 144 | 18.0 | 96.6 |
| APS 300.5a | P4R | 4.8 | 120 | 34 | 79 | 36 | 5.8 | 102 | 135 | 14.7 | 88.9 |
| APS 400.5 | C588R | 5.7 | 133 | 51 | 96 | 45 | 1.2 | 89 | 128 | 4.9 | 29.1 |
| APS 500.3 | E111K | 5.0 | 114 | 56 | 68 | 39 | 1.2 | 88 | 103 | 11.4 | 37.8 |
| APS 600.3c,e | C588R | 5.2 | 175 | 78 | 118 | 50 | 4.4 | 91 | 173 | 1.9 | 24.1 |
| APS 700.4a | E159K | 5.0 | 167 | 160 | 113 | 36 | 8.6 | 84 | 193 | 7.0 | 55.9 |
| APS 800.3c | T10I | 10.7 | 164 | 445 | 34 | 42 | 0.1 | 176 | 274 | 15.6 | 13.0 |
| APS 1700.4 | T10I | 6.3 | 165 | 335 | 98 | 32 | 0.4 | 111 | 205 | 55.2 | 281.0 |
| APS 1800.3 | P4R | ND | 121 | 77 | 66 | 40 | ND | 93 | ND | 31.0 | ND |
HgbA1c, Hemoglobin A1c; TC, total cholesterol; TG, triglycerides; LDLC, low-density lipoprotein cholesterol; HDLC, high-density lipoprotein cholesterol; PP, postprandial; ND, not done.
Average of two fasting determinations;
On treatment with pioglitazone, fenofibrate, gliclazide, and metformin;
Average of three fasting determinations;
On treatment with pravastatin, pioglitazone, enalapril, and furosemide;
On treatment with metformin.
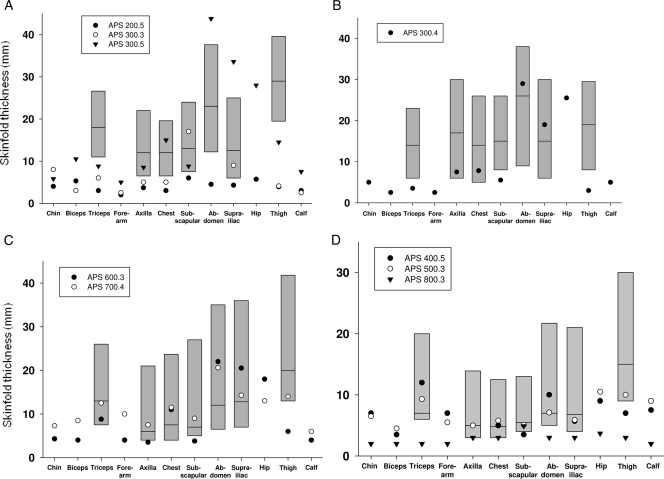
Measurement of skinfold thickness (Fig. 4) revealed varying degrees of lipodystrophy. APS 200.5 and 800.3 had reduced skinfold thickness at all the sites indicative of generalized lipodystrophy. On the other hand, patients belonging to the pedigree APS 300 and APS 600.3 had decreased skinfold thickness over the extremities suggestive of partial lipodystrophy. Three patients had normal skinfolds.
Figure 4.
Skinfold thickness at various anatomical sites in patients with APS. The shaded bars represent the 10th and 90th percentile of skinfold thickness values for normal women (A) aged 18–55 yr (37), men (B) aged 18–61 yr (38), prepubertal girls (C) aged 4–10 yr, and boys (D) aged 4–10 yr (39). No normal data are available for skinfold thickness in adolescents. Median valves are shown as horizontal lines. Patients APS 200.5 (D136H) and 800.3 (T10I) show generalized loss of sc fat, whereas patients belonging to pedigrees APS 300 (P4R) and 600 (C588R) show partial lipodystrophy, and those belonging to pedigrees APS 400 (C588R), 500 (E111K), and 700 (E159K) do not show significant fat loss.
All three patients with generalized lipodystrophy had markedly reduced total and regional body fat values on DEXA scans (Table 3). In those with partial lipodystrophy, fat in the arms and legs was reduced in comparison with the normal gender matched values. MRI studies confirmed the pattern of lipodystrophies in the affected patients. Both APS 200.5 and APS 800.3 had generalized reduction of sc fat (Figs. 5 and 6). Some intraabdominal fat was evident in APS 200.5 but APS 800.3 had none. Retroorbital fat was preserved in both of them. APS 300.3, 300.4, and 300.5 had partial loss of fat from the extremities with slightly increased sc fat in the dorsocervical region (Figs. 5 and 6). Bone marrow fat was well preserved in all patients. Similarly, APS 600.3 showed mild loss of sc fat from the calf and thighs (except medially) but with no areas of excess fat deposition. Three patients, APS 400.5, 500.3, and 700.4, had no evidence of fat loss on DEXA scan or MRI.
Table 3.
Body composition in our patients with APS as determined by DEXA
| Fat (percent of regional mass) | Females
|
Males
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| APS 200.5 (D136H) | APS 300.3 (P4R) | APS 300.5 (P4R) | APS 600.3 (C588R) | APS 700.4 (E159K) | Normal values (young adults) | APS 300.4 (P4R) | APS 400.5 (C588R) | APS 500.3 (E111K) | APS 800.3 (T10I) | Normal values (young adults) | |
| Whole body | 8.7 | 23.3 | 23.5 | 18.2 | 23.7 | 30.3 ± 1.5 | 17.2 | 17.1 | 12.3 | 6.6 | 13.8 ± 1.4 |
| Trunk | 8.1 | 27.8 | 24.8 | 19.0 | 22.6 | 29.0 ± 1.6 | 21.6 | 16.5 | 8.7 | 4.7 | 13.5 ± 1.4 |
| Arms | 12.3 | 23.0 | 26.3 | 21.8 | 26.9 | 30.2 ± 1.8 | 11.8 | 16.7 | 13.4 | 7.2 | 11.2 ± 1.5 |
| Legs | 5.2 | 13.6 | 21.7 | 15.7 | 26.9 | 33.1 ± 1.5 | 10.9 | 17.3 | 14.2 | 5.3 | 15.2 ± 1.7 |
Normal values for regional body fat distribution for children were not available. Normal values (mean ± sd) for young adults are from Mazess et al. (41).
Figure 5.
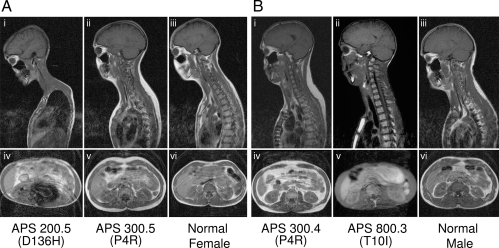
MRI scans of the head, neck, thorax, and abdomen from various APS patients and normal control subjects. In these T-1-weighted images, adipose tissue is seen as areas of bright signal intensity. Sagittal MRIs (A, i–iii, and B, i–iii) through the head, neck, and thorax and axial MRIs through the abdomen (A, iv–vi and B, iv–vi) of two female APS patients, 200.5 (D136H) and 300.3 (P4R), and a normal female and two male patients, 300.4 (P4R) and 800.3 (T10I), and a normal male subject, respectively. APS 200.5 (D136H) shows generalized reduction of sc fat from the head, neck, thoracic, and abdominal regions. Some intraabdominal fat is evident. APS 300.3 (P4R) and 300.4 (P4R) show slightly increased sc fat in the dorsocervical region and normal fat in the head, thorax, and abdomen. APS 800.3 (T10I) shows extreme loss of sc fat from the head, neck, thorax, and abdominal regions. He also has no intraabdominal fat. Retroorbital fat is preserved.
Figure 6.
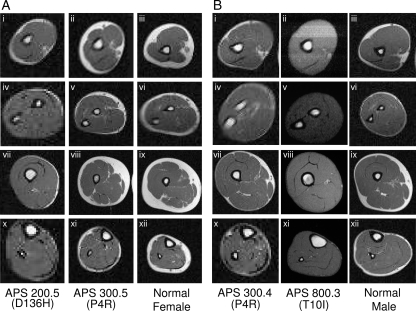
MRI scans of the arm, forearm, thigh, and calf from various APS patients and normal control subjects. A, Axial MRIs through the arm (i–iii), forearm (iv–vi), thigh (vii–ix), and calf (x–xii) of two female APS patients, 200.5 (D136H) and 300.3 (P4R), compared with a normal female subject. Fat loss is generalized in APS 200.5 (D136H) and partial in APS 300.3 (P4R) with relative preservation of medial thigh fat. Bone marrow fat is well preserved. B, Axial MRIs through the arm (i–iii), forearm (iv–vi), thigh (vii–ix), and calf (x–xii) of two male APS patients, 300.4 (P4R) and 800.3 (T10I), compared with a normal male subject. Fat loss is complete and generalized in APS 800.3 (T10I), whereas APS 300.4 (P4R) shows only partial fat loss in the extremities. Bone marrow fat is well preserved.
Nuclear morphology of skin fibroblasts
Skin fibroblasts from these patients were from early (3,4) passages. Indirect immunofluorescent studies revealed normal localization of the lamin A/C protein in the nuclear envelope of fibroblasts from all the subjects studied. The nuclear morphological abnormalities varied from being mild to severe. There were several deformed nuclei, showing multilobulations and nuclear membrane invagination (Fig. 7, A and B). Incubation of the fibroblasts from two patients, APS 500.3 and 700.4 with FTIs, GGTI, or trichostatin, however, did not improve nuclear morphological abnormalities. Fibroblasts from two other patients, APS 400.5 and 600.3, were treated with an FTI alone, and again no improvement in nuclear morphological abnormalities was observed.
Figure 7.
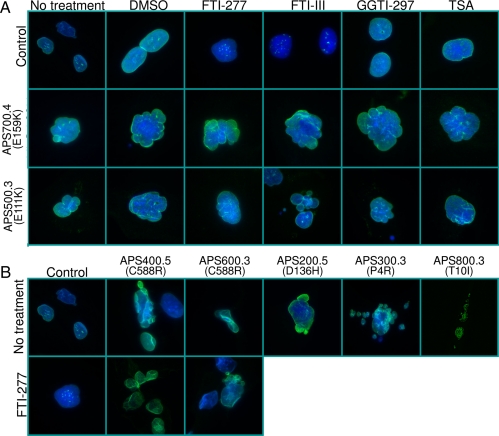
Nuclear morphology of skin fibroblasts from APS patients and controls and response to various pharmacological agents. A, Indirect immunofluorescence microscopy was conducted using lamin A/C antibody (H-110). The top row shows control cells, the middle row shows cells from patient APS 700.4 (E159K), and the bottom row shows cells from patient 500.3 (E111K). The first column shows cell morphology without any treatment, the second column with dimethylsulfoxide, and the next columns after incubation of the cells for 48 h with 10 μm farnesyl transferase inhibitors, FTI-277 or FTI-III, geranylgeranyl transferase inhibitor, GGTI-297, or a histone deacetylase inhibitor, trichostatin A (TSA), respectively. Fibroblasts from both patients showed misshapen, bilobed, or multilobed nuclei or nuclear blebs. The localization of lamin A to the periphery of the nucleus was not affected. Incubation of the cells with various pharmacological agents failed to correct the nuclear abnormalities. B, Indirect immunofluorescence microscopy lamin A/C antibody (H-110) in control fibroblast cells and those from patients APS 400.5 (C588R), 600.3 (C588R), 200.5 (D136H), 300.3 (P4R), and 800.3 (T10I). The bottom row shows control cells and those from patients APS 400.5 (C588R) and 600.3 (C588R) after incubation for 48 h with 10 μm FTI-277.
Immunoblotting
Western blots from the fibroblasts of our patients with APS and the normal control showed presence of lamins A and C without any abnormal species (Fig. 8).
Figure 8.
Immunoblot analysis of cell lysates from skin fibroblasts of seven patients with APS. Shown is a representative immunoblot of cell lysates obtained from the fibroblasts of patients with APS probed with amino terminal-specific antilamin A/C antibody, N-18, showing lamin C and mature lamin A. None of the APS patients showed accumulation of any abnormal species of lamins A or C. Included were also cell lysates from a patient with HGPS (AG06917), demonstrating accumulation of progerin, a patient with MAD due to ZMPSTE24 deficiency (MAD 3300.3) showing accumulation of prelamin A (40), and a normal healthy subject (control).
Discussion
In-depth phenotyping of 11 APS patients reveals marked phenotypic heterogeneity but also identifies many distinguishing features from those with HGPS and MAD (Table 4). Similar to HGPS patients (1), many of the mutations in our patients were de novo, but at least one mutation, P4R, was transmitted in an autosomal dominant fashion affecting two generations. There was no evidence of nonpenetrance, although there was some phenotypic heterogeneity observed within the APS 300 pedigree. Whereas two affected subjects had hearing loss and cardiac valvular involvement, the 22-yr-old daughter of the proband has not had these manifestations yet. Previously, phenotypic heterogeneity was noted among two subjects with the same R133L heterozygous mutation (17).
Table 4.
Comparison of clinical features of allelic disorders, HGPS, MAD, and APS due to LMNA mutations
| Transmission | HGPS Sporadic, autosomal dominant (de novo mutations) | MAD Autosomal recessive | APS Sporadic, autosomal dominant (de novo mutations) |
|---|---|---|---|
| Onset | 1 yr | 2–4 yr | 4–17 yr |
| Age at death (number of patients) | 12.6 (51) | 10, 16 (2) | 15 (1) |
| Hair loss | |||
| Scalp | 2++ (early) | + (late)−/+ | |
| Eyebrows | 2++ | − | + |
| Eyelids | 2++ | − | − |
| Mandibular hypoplasia | + | 2++ | −/+ |
| Clavicular resorption | + | 2++ | −/+ |
| Acroosteolysis | + | 2++ | −/+ |
| Lipodystrophy | Generalized | Partial | Normal/partial/generalized |
| Atherosclerosis | 2++ | − | −/+ |
| Valvular anomalies | −/+ | − | −/2++ |
In contrast to patients with MAD and HGPS (7,23,24,25), APS patients have either no acroosteolysis or only mild changes of the terminal phalanges. Only mild clavicular hypoplasia was noted in one patient and mandibular hypoplasia was not severe. APS patients had only slight evidence of loss of scalp hair. The onset of clinical manifestations in APS patients also seems to be slightly delayed compared with HGPS and MAD patients (7,23,24,25). Finally, even though the data related to the age of death are scant, APS patients seem to live longer than HGPS patients, who have a median age of death about 13 yr (23,24).
Previous reports (14) and the current data demonstrate the pattern of body fat distribution in APS patients with evidence of both generalized and partial lipodystrophy. Although three of them had normal fat distribution, two of these were prepubertal and may develop lipodystrophy after puberty as seen in familial partial lipodystrophy of Dunnigan variety (26) and MAD (25).
Our study reveals marked metabolic abnormalities, such as severe insulin-resistant diabetes, hypertriglyceridemia, and hepatic steatosis in APS patients compared with HGPS and MAD patients. Gordon et al. (27) reported near-normal serum lipids in patients with HGPS. Patients with MAD do develop mild glucose intolerance, hyperinsulinemia, and diabetes mellitus (25,28). Some APS patients showed only mild glucose intolerance and did not have hyperinsulinemia. Interestingly, none of our patients except one had acanthosis nigricans. However, they had mottling, pigmentation, and sclerosis of the skin.
Our study also revealed other novel features associated with laminopathies. Valvulopathy has been reported in only a few patients with HGPS or APS, with aortic and mitral valve calcifications being the common abnormalities (16,23,29). In our cohort, six of the 11 patients had evidence of cardiac valve involvement resulting in valvular replacement in three of them. In fact, one of them died soon after valve replacement surgery. Aortic and mitral valves were the most commonly affected, with calcifications leading to decreased leaflet excursion and valvular incompetence. In one of the pedigrees (APS 300), two affected patients with P4R mutation had sensorineural hearing loss. Previously, clinically significant deafness has not been reported in any patient with LMNA mutation. Only recently, all 11 patients with HGPS who underwent a hearing test were reported to have conductive hearing loss, and two subjects also had high-frequency sensorineural loss (24). Other peculiar clinical findings include osteoporosis in the 50-yr-old patient with P4R mutation, which is a novel finding in patients with laminopathies. Previously we reported progeroid features with arthropathy and tendinous calcifications in a 44-yr-old male with homozygous S573L LMNA mutation (30). We were also impressed with the lack of breast tissue in many female patients with APS despite lack of evidence of hypogonadism with normal or irregular menstrual periods. This suggests either the lack of response of mammary glands to estrogens and progesterone or the atrophy of breast tissue. Others, however, reported lack of breast development with premature ovarian failure in five patients (15,19).
It is postulated that the cellular toxicity in patients with HGPS may be related to accumulation of farnesylated progerin. Previously, in vitro studies on skin fibroblasts or lymphoblasts from HGPS patients reveal marked reduction in nuclear morphological abnormalities on incubation with FTIs responsible for farnesylation of cysteine in the CAAX motif of prelamin A (31) or by inhibitors of farnesyl synthesis (32). Toth et al. (33) reported a modest reduction in the frequency of dysmorphic nuclei (23 to 17% and 21 to 12%, respectively) in fibroblasts from APS patients with heterozygous LMNA mutations R644C and E578V on incubation with FTI PB-43. In contrast, we did not demonstrate any significant change in nuclear morphological abnormalities in four APS patients with different LMNA mutations on incubation with two different types of FTIs, GGTI, or trichostatin-A. Consistent with our observations, recent data suggest that cellular toxicity with HGPS mutation may occur whether or not progerin is farnesylated (34).
Thus, our data will argue that there are probably multiple mechanisms causing cellular toxicity in patients with LMNA mutations. We recently reported that accumulation of farnesylated prelamin A mutant causes toxicity by affecting the nuclear pore complexes, which can be reversed by FTIs (35). However, many lamin A mutations are proximal to the carboxy-terminal region to affect the posttranslational processing of prelamin A. In support of this contention, cell lysates from our APS patients do not show presence of prelamin A on immunoblots. Capanni et al. (36) did report slight prelamin A accumulation in fibroblast cell lysates of an APS patient with S143F LMNA mutation using prelamin A-specific antibody but not lamin A/C antibody. Therefore, we believe that manifestations in these patients may be due to impairment of interaction of mutant lamin A with other nuclear lamina proteins, transcription factors, or chromatin. Interestingly, lamins A and C form homo- and heterodimers, which involve interaction between the amino and carboxy terminal residues. Whether the extreme amino terminal mutations such as P4R and T10I cause the progeroid phenotype due to disruption of dimerization is a possibility to be explored further.
Acknowledgments
We are thankful to Drs. Sara S. Cathey, John Betteridge, Robert Wallerstein, Laura Mazzanti, and Phillip Gorden for referring their patients for evaluation to us. We also thank Sarah Gilmore, Sarah Masood, and Ruth Giselle Huet for help with illustrations, mutational screening, and immunoblotting.
Footnotes
This work was supported by National Institutes of Health Grants R01-DK54387 and M01-RR00633 and Southwest Medical Foundation.
Disclosure Summary: All authors have nothing to declare.
First Published Online October 29, 2009
Abbreviations: APS, Atypical progeroid syndrome; DEXA, dual-energy x-ray absorptiometry; HGPS, Hutchinson-Gilford progeria syndrome; MAD, mandibuloacral dysplasia; MRI, magnetic resonance imaging.
References
- Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, Collins FS 2003 Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423:293–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, Lyonnet S, Stewart CL, Munnich A, Le Merrer M, Lévy N 2003 Lamin a truncation in Hutchinson-Gilford progeria. Science 300:2055 [DOI] [PubMed] [Google Scholar]
- Fukuchi K, Katsuya T, Sugimoto K, Kuremura M, Kim HD, Li L, Ogihara T 2004 LMNA mutation in a 45 year old Japanese subject with Hutchinson-Gilford progeria syndrome. J Med Genet 41:e67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev SA, De Sandre-Giovannoli A, Shani AA, Levy N 2007 An association of Hutchinson-Gilford progeria and malignancy. Am J Med Genet A 143A:1821–1826 [DOI] [PubMed] [Google Scholar]
- Moulson CL, Fong LG, Gardner JM, Farber EA, Go G, Passariello A, Grange DK, Young SG, Miner JH 2007 Increased progerin expression associated with unusual LMNA mutations causes severe progeroid syndromes. Hum Mutat 28:882–889 [DOI] [PubMed] [Google Scholar]
- Novelli G, Muchir A, Sangiuolo F, Helbling-Leclerc A, D'Apice MR, Massart C, Capon F, Sbraccia P, Federici M, Lauro R, Tudisco C, Pallotta R, Scarano G, Dallapiccola B, Merlini L, Bonne G 2002 Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding lamin A/C. Am J Hum Genet 71:426–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simha V, Agarwal AK, Oral EA, Fryns JP, Garg A 2003 Genetic and phenotypic heterogeneity in patients with mandibuloacral dysplasia-associated lipodystrophy. J Clin Endocrinol Metab 88:2821–2824 [DOI] [PubMed] [Google Scholar]
- Garg A, Cogulu O, Ozkinay F, Onay H, Agarwal AK 2005 A novel homozygous Ala529Val LMNA mutation in Turkish patients with mandibuloacral dysplasia. J Clin Endocrinol Metab 90:5259–5264 [DOI] [PubMed] [Google Scholar]
- Plasilova M, Chattopadhyay C, Pal P, Schaub NA, Buechner SA, Mueller H, Miny P, Ghosh A, Heinimann K 2004 Homozygous missense mutation in the lamin A/C gene causes autosomal recessive Hutchinson-Gilford progeria syndrome. J Med Genet 41:609–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal AK, Kazachkova I, Ten S, Garg A 2008 Severe mandibuloacral dysplasia-associated lipodystrophy and progeria in a young girl with a novel homozygous Arg527Cys LMNA mutation. J Clin Endocrinol Metab 93:4617–4623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi F, Gullotta F, Columbaro M, Filareto A, D'Adamo M, Vielle A, Guglielmi V, Nardone AM, Azzolini V, Grosso E, Lattanzi G, D'Apice MR, Masala S, Maraldi NM, Sbraccia P, Novelli G 2007 Compound heterozygosity for mutations in LMNA in a patient with a myopathic and lipodystrophic mandibuloacral dysplasia type A phenotype. J Clin Endocrinol Metab 92:4467–4471 [DOI] [PubMed] [Google Scholar]
- Verstraeten VL, Broers JL, van Steensel MA, Zinn-Justin S, Ramaekers FC, Steijlen PM, Kamps M, Kuijpers HJ, Merckx D, Smeets HJ, Hennekam RC, Marcelis CL, van den Wijngaard A 2006 Compound heterozygosity for mutations in LMNA causes a progeria syndrome without prelamin A accumulation. Hum Mol Genet 15:2509–2522 [DOI] [PubMed] [Google Scholar]
- Kirschner J, Brune T, Wehnert M, Denecke J, Wasner C, Feuer A, Marquardt T, Ketelsen UP, Wieacker P, Bonnemann CG, Korinthenberg R 2005 p.S143F mutation in lamin A/C: a new phenotype combining myopathy and progeria. Ann Neurol 57:148–151 [DOI] [PubMed] [Google Scholar]
- Csoka AB, Cao H, Sammak PJ, Constantinescu D, Schatten GP, Hegele RA 2004 Novel lamin A/C gene (LMNA) mutations in atypical progeroid syndromes. J Med Genet 41:304–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Lee L, Kudlow BA, Dos Santos HG, Sletvold O, Shafeghati Y, Botha EG, Garg A, Hanson NB, Martin GM, Mian IS, Kennedy BK, Oshima J 2003 LMNA mutations in atypical Werner’s syndrome. Lancet 362:440–445 [DOI] [PubMed] [Google Scholar]
- Caux F, Dubosclard E, Lascols O, Buendia B, Chazouillères O, Cohen A, Courvalin JC, Laroche L, Capeau J, Vigouroux C, Christin-Maitre S 2003 A new clinical condition linked to a novel mutation in lamins A and C with generalized lipoatrophy, insulin-resistant diabetes, disseminated leukomelanodermic papules, liver steatosis, and cardiomyopathy. J Clin Endocrinol Metab 88:1006–1013 [DOI] [PubMed] [Google Scholar]
- Jacob KN, Baptista F, dos Santos HG, Oshima J, Agarwal AK, Garg A 2005 Phenotypic heterogeneity in body fat distribution in patients with atypical Werner’s syndrome due to heterozygous Arg133Leu lamin A/C mutation. J Clin Endocrinol Metab 90:6699–6706 [DOI] [PubMed] [Google Scholar]
- Mory PB, Crispim F, Kasamatsu T, Gabbay MA, Dib SA, Moisés RS 2008 Atypical generalized lipoatrophy and severe insulin resistance due to a heterozygous LMNA p.T10I mutation. Arq Bras Endocrinol Metab 52:1252–1256 [DOI] [PubMed] [Google Scholar]
- McPherson E, Turner L, Zador I, Reynolds K, Macgregor D, Giampietro PF 2009 Ovarian failure and dilated cardiomyopathy due to a novel lamin mutation. Am J Med Genet A 149A:567–572 [DOI] [PubMed] [Google Scholar]
- Doh YJ, Kim HK, Jung ED, Choi SH, Kim JG, Kim BW, Lee IK 2009 Novel LMNA gene mutation in a patient with atypical Werner’s syndrome. Korean J Intern Med 24:68–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard D, Fourcade G, Milhaud D, Bessis D, Esteves-Vieira V, Boyer A, Roll P, Bourgeois P, Levy N, De Sandre-Giovannoli A 2009 Novel LMNA mutation in atypical Werner syndrome presenting with ischemic disease. Stroke 40:e11–e14 [DOI] [PubMed] [Google Scholar]
- Misra A, Garg A 2003 Clinical features and metabolic derangements in acquired generalized lipodystrophy: case reports and review of the literature. Medicine (Baltimore) 82:129–146 [DOI] [PubMed] [Google Scholar]
- Hennekam RC 2006 Hutchinson-Gilford progeria syndrome: review of the phenotype. Am J Med Genet A 140:2603–2624 [DOI] [PubMed] [Google Scholar]
- Merideth MA, Gordon LB, Clauss S, Sachdev V, Smith AC, Perry MB, Brewer CC, Zalewski C, Kim HJ, Solomon B, Brooks BP, Gerber LH, Turner ML, Domingo DL, Hart TC, Graf J, Reynolds JC, Gropman A, Yanovski JA, Gerhard-Herman M, Collins FS, Nabel EG, Cannon 3rd RO, Gahl WA, Introne WJ 2008 Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med 358:592–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simha V, Garg A 2002 Body fat distribution and metabolic derangements in patients with familial partial lipodystrophy associated with mandibuloacral dysplasia. J Clin Endocrinol Metab 87:776–785 [DOI] [PubMed] [Google Scholar]
- Garg A, Peshock RM, Fleckenstein JL 1999 Adipose tissue distribution pattern in patients with familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab 84:170–174 [DOI] [PubMed] [Google Scholar]
- Gordon LB, Harten IA, Patti ME, Lichtenstein AH 2005 Reduced adiponectin and HDL cholesterol without elevated C-reactive protein: clues to the biology of premature atherosclerosis in Hutchinson-Gilford progeria syndrome. J Pediatr 146:336–341 [DOI] [PubMed] [Google Scholar]
- Freidenberg GR, Cutler DL, Jones MC, Hall B, Mier RJ, Culler F, Jones KL, Lozzio C, Kaufmann S 1992 Severe insulin resistance and diabetes mellitus in mandibuloacral dysplasia. Am J Dis Child 146:93–99 [DOI] [PubMed] [Google Scholar]
- Stehbens WE, Wakefield SJ, Gilbert-Barness E, Olson RE, Ackerman J 1999 Histological and ultrastructural features of atherosclerosis in progeria. Cardiovasc Pathol 8:29–39 [DOI] [PubMed] [Google Scholar]
- Van Esch H, Agarwal AK, Debeer P, Fryns JP, Garg A 2006 A homozygous mutation in the lamin A/C gene associated with a novel syndrome of arthropathy, tendinous calcinosis and progeroid features. J Clin Endocrinol Metab 91:517–521 [DOI] [PubMed] [Google Scholar]
- Glynn MW, Glover TW 2005 Incomplete processing of mutant lamin A in Hutchinson-Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase inhibition. Hum Mol Genet 14:2959–2969 [DOI] [PubMed] [Google Scholar]
- Varela I, Pereira S, Ugalde AP, Navarro CL, Suárez MF, Cau P, Cadiñanos J, Osorio FG, Foray N, Cobo J, de Carlos F, Lévy N, Freije JM, López-Otin C 2008 Combined treatment with statins and aminobisphosphonates extends longevity in a mouse model of human premature aging. Nat Med 14:767–772 [DOI] [PubMed] [Google Scholar]
- Toth JI, Yang SH, Qiao X, Beigneux AP, Gelb MH, Moulson CL, Miner JH, Young SG, Fong LG 2005 Blocking protein farnesyltransferase improves nuclear shape in fibroblasts from humans with progeroid syndromes. Proc Natl Acad Sci USA 102:12873–12878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Andres DA, Spielmann HP, Young SG, Fong LG 2008 Progerin elicits disease phenotypes of progeria in mice whether or not it is farnesylated. J Clin Invest 118:3291–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Garg A, Agarwal AK 2007 Mislocalization of prelamin A Tyr646Phe mutant to the nuclear pore complex in human embryonic kidney 293 cells. Biochem Biophys Res Commun 355:78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capanni C, Mattioli E, Columbaro M, Lucarelli E, Parnaik VK, Novelli G, Wehnert M, Cenni V, Maraldi NM, Squarzoni S, Lattanzi G 2005 Altered pre-lamin A processing is a common mechanism leading to lipodystrophy. Hum Mol Genet 14:1489–1502 [DOI] [PubMed] [Google Scholar]
- Jackson AS, Pollock ML, Ward A 1980 Generalized equations for predicting body density of women. Med Sci Sports Exerc 12:175–181 [PubMed] [Google Scholar]
- Jackson AS, Pollock ML 1978 Generalized equations for predicting body density of men. Br J Nutr 40:497–504 [DOI] [PubMed] [Google Scholar]
- Dezenberg CV, Nagy TR, Gower BA, Johnson R, Goran MI 1999 Predicting body composition from anthropometry in pre-adolescent children. Int J Obes Relat Metab Disord 23:253–259 [DOI] [PubMed] [Google Scholar]
- Miyoshi Y, Akagi M, Agarwal AK, Namba N, Kato-Nishimura K, Mohri I, Yamagata M, Nakajima S, Mushiake S, Shima M, Auchus RJ, Taniike M, Garg A, Ozono K 2008 Severe mandibuloacral dysplasia caused by novel compound heterozygous ZMPSTE24 mutations in two Japanese siblings. Clin Genet 73:535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazess RB, Barden HS, Bisek JP, Hanson J 1990 Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr 51:1106–1112 [DOI] [PubMed] [Google Scholar]