Abstract
Objective: Large waist circumference (WC) is associated with cardiovascular disease and type 2 diabetes. The present study determined differences in lipoprotein particle size and subclass concentration and markers of vascular smooth muscle function in children using WC percentile cutoffs.
Research Design and Methods: Participants were 182 children (87 black, 92 female) aged 8–<18 yr. Each participant had a measurement of WC and a fasting blood draw for the measurement of lipoprotein particle concentration and size and circulating biomarkers of endothelial function. Participants were divided into age-, sex-, and ethnicity-specific WC percentiles of below 75th, 75th to 90th, and at least 90th percentiles, and differences in lipoproteins and vascular smooth muscle markers were compared among groups.
Results: Children in the 90th percentile or higher for WC had significantly smaller low-density lipoprotein and high-density lipoprotein size than children with WC below this percentile. For lipoprotein concentration, small low-density lipoprotein and large very low density lipoprotein and chylomicrons were lower, and large high density lipoprotein concentrations were higher in children whose WC was below the 75th percentile compared with those with WC in the 90th percentile or higher. Concentrations of the vascular smooth muscle biomarkers, intercellular adhesion molecule-1, and E-selectin were significantly higher in children with WC in the 90th percentile or higher than in children below the 75th percentile.
Conclusion: Youths with WC in the 90th percentile or higher have an atherogenic lipoprotein profile with increased concentrations of biomarkers of vascular smooth muscle dysfunction. Given that atherosclerosis begins in childhood, such evidence suggests that these children should be targeted for interventions to reduce adiposity at an early age.
Youths with waist circumference ≥ 90th percentile have increased cardiometabolic risk manifested in an atherogenic lipoprotein profile with increased concentrations of biomarkers of vascular smooth muscle dysfunction.
In children, waist circumference (WC) and visceral fat distribution have been associated with risk factors for cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM) including insulin resistance (1), hypertension (2,3), and adverse lipid profiles (3,4). A central fat distribution pattern may influence CVD and T2DM risk more than overall adiposity. We previously demonstrated that in obese youth with the same BMI and total body adiposity, insulin resistance and cardiometabolic risk markers are higher in those with high visceral adipose tissue (5) and/or high WC (1,3).
Given the association of central fat distribution with CVD and T2DM disease risk and evidence that WCs are increasing at a faster rate in children than body mass index (BMI) (6), several researchers have attempted to establish representative age-, race-, and sex-specific percentile cutoff criteria for WC in children (7,8,9). In addition, a number of studies have attempted to either determine differences in CVD and T2DM risk factors based on the degree of visceral adiposity (4,10) or examine the relationship between WC and CVD and T2DM risk factors (11,12,13,14,15,16). These studies help to confirm the importance of central obesity in disease risk. Nonetheless, direct measurement of visceral adiposity is expensive and problematic, and although investigators have established that WC is important in predicting disease risk, only a few studies have attempted to establish differences in disease risk based on representative cutoff criteria that are simple to apply clinically (1,2,3,17). These investigations have found that when children are separated into representative WC centiles, those children with higher WC have decreased insulin sensitivity (1), increased fasting insulin (1), higher blood pressure (2,3), higher total and low-density lipoprotein (LDL) cholesterol (17), lower high-density lipoprotein (HDL) cholesterol (2,17), and increased triglycerides (TG) (3).
In adults, visceral adiposity is associated with small, dense LDL particles, elevated apolipoprotein B, elevated fasting and postprandial TG, and low HDL cholesterol (18,19). Indeed, a combination of a high WC along with high fasting TG referred to as the “hypertriglyceridemic waist” is predictive of an atherogenic metabolic triad where individuals are characterized by the simultaneous occurrence of hyperinsulinemia, hyperapolipoprotein B, and small LDL particles (20). In children and adolescents, central obesity is associated with dyslipidemia assessed by the traditional lipid profile (3,4,21), and several studies show that WC predicts standard lipid concentrations in youth (11,13,15,16,21). However, traditional lipid measures only partially predict disease risk (22). Although the presence of small, dense LDL particles has been shown in obese children (12,14), it remains to be determined whether atherogenic lipoproteins segregate with WC percentiles.
Although atherosclerosis is a disease of lipid accumulation, the earliest lesions, fatty streaks, consist of macrophages and T-lymphocytes that precede cholesterol deposition. Several molecules that are responsible for macrophage adhesion accumulate at arterial branches. These circulating biomarkers of vascular smooth muscle function are increased in response to inflammation and play an important role in the formation of atherosclerotic plaque (23). In adults, with (24) and without T2DM (25), these molecules correlate positively with waist girth. It is possible that WC is an underpinning factor in both the inflammatory and lipoprotein response that occurs with atherosclerosis. Whether both changes can be observed in youth in different WC percentiles requires examination.
Thus, in the present study we aimed to: 1) examine differences in lipoprotein particle size and concentration in children and adolescents categorized according to nationally representative WC centiles; and 2) examine differences in biomarkers of vascular smooth muscle function using the same WC criteria. We hypothesized that youth within the highest WC percentiles will have a significantly worse atherogenic lipoprotein profile and elevated biomarkers of vascular smooth muscle dysfunction.
Subjects and Methods
Subjects
Participants consisted of 182 black and white normal-weight and overweight otherwise healthy children and adolescents, except for seven who had impaired glucose tolerance (IGT), aged 8–<18 yr. Some of the participants were reported before as part of an ongoing grant investigating childhood insulin resistance (26,27). Study participants were recruited through newspaper and bulletin board advertisements. All studies were approved by the Institutional Review Board of the University of Pittsburgh. All participants and their parents gave written informed assent and consent after a thorough explanation of the proposed study. Exclusion criteria included diagnosed diabetes and the use of medications that influence glucose, lipid metabolism, or blood pressure. Participants’ health was assessed by medical history, physical examination, and routine hematological and biochemical tests. Pubertal development was assessed by physical examination according to Tanner criteria.
Methods and procedures
The participants and the data reported here are part of an ongoing National Institutes of Health (NIH)-funded RO1 grant investigating race-related differences in childhood insulin sensitivity and secretion, in which all participants were admitted to the Children’s Hospital of Pittsburgh NIH-funded Pediatric Clinical and Translational Research Center for a hyperinsulinemic-euglycemic clamp (n = 36), a hyperglycemic clamp (n = 34), or both (n = 112). Herein we report the fasting lipoprotein data. Body weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, using standardized equipment. WC was obtained at the midpoint between the lowest rib and the iliac crest as reported by us before (1).
Body composition
Total body fat was assessed using dual energy x-ray absorptiometry. Abdominal sc and visceral adipose tissues were determined from a single axial image (10-mm thickness) of the abdomen at the level of the L4–L5 intervertebral disc using computed tomography. Both methods have been described previously (26).
Lipoprotein particle size and concentration
Fasting blood samples were collected on all children for analysis of lipoprotein particle size and concentration.
Biochemical measurements
Plasma lipid concentrations [total, HDL, and LDL cholesterol and total and very low-density lipoprotein (VLDL) TG] were determined using the standards of the Centers for Disease Control and Prevention as described previously (28). Concentrations of lipoprotein subclasses and particle size were determined using nuclear magnetic resonance spectroscopy (LipoScience Inc., Raleigh, NC) (29). Using this method, the quantity of each subclass is reported in particle concentration units (nanomoles of particles per liter for VLDL and LDL and micromoles per liter for HDL). VLDL, LDL, and HDL were separated into 10 subclass categories: large VLDL (including chylomicrons) (>60 nm); medium VLDL (35–60 nm); small VLDL (27–35 nm); intermediate-density lipoprotein (IDL; 23–27 nm); large LDL (21.2–23 nm); medium-small LDL (19.8–21.2 nm); very small LDL (18–19.8 nm); large HDL (8.8–13 nm); medium HDL (8.2–8.8nm); and small HDL (7.3–8.2 nm). Average lipoprotein particle sizes were computed as the sum of the diameter of each subclass multiplied by its relative mass percentage as estimated from the amplitude of its methyl nuclear magnetic resonance signal. Markers of vascular smooth muscle function, intercellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM-1), and E-selectin were quantified using commercially available double-sandwich enzyme-linked immunoassays (R&D Systems, Minneapolis, MN).
Statistical analysis
Statistical procedures were performed using SPSS 15.0 for Windows (SPSS, Chicago, IL). To investigate the relationship between WC and lipoprotein particle size and concentration and markers of vascular smooth muscle function, participants were divided into age-, sex-, and ethnicity-specific nationally representative WC percentiles of low (<75th percentile), moderate (75th to 90th percentiles), and high (≥90th percentile). These WC percentiles were based on cross-sectional data from the Third National Health and Nutrition Examination Survey (NHANES III) (8). Differences in categorical variables were tested among groups using the χ2 test. A one-way ANOVA or the nonparametric Kruskall-Wallis test, based on the nonviolation of statistical assumptions, was used to compare differences in lipoprotein particle size and concentration and vascular smooth muscle markers among WC percentiles. Race, gender, and pubertal development (Tanner stage) were added as covariates in an analysis of covariance (ANCOVA) to control for their influence on lipoprotein profiles and vascular markers among WC percentiles. Because seven of the participants were diagnosed with IGT, we also analyzed our data set excluding these subjects to control for any influence this condition may have had. To further investigate effects of race and gender on lipoprotein particle size and concentration and endothelial markers among WC percentiles, 2 × 3 factorial ANOVA were employed. Where appropriate, Tukey’s post hoc comparisons were used to identify differences among groups. Pearson’s correlation coefficient was used to examine the relationship of WC to lipoproteins and vascular smooth muscle markers as continuous variables. Data are presented as mean ± se. Significance was set at P < 0.05.
Results
Differences in physical characteristics
Physical characteristics of the participants by WC percentile are presented in Table 1. Children in the highest WC percentile were older and more advanced through puberty than children with a WC below the 75th percentile. The groups differed significantly in body weight, BMI, BMI percentile, fat mass, percentage body fat, WC, and abdominal visceral and sc fat area.
Table 1.
Physical characteristics and fasting lipids of the participants by WC percentiles
| <75th percentile | 75th–90th percentiles | ≥90th percentile | P ANOVA | |
|---|---|---|---|---|
| n | 67 | 30 | 85 | |
| Race (black/white) | 33/34 | 16/14 | 38/47 | ns |
| Gender (male/female) | 28/39 | 12/18 | 50/35 | ns |
| IGT | 0 | 0 | 7 | <0.001 |
| Age (yr) | 12.2 ± 0.3b | 13.0 ± 0.4 | 13.8 ± 0.2 | <0.001 |
| Tanner stage | ||||
| I | 22 | 3 | 1 | <0.001 |
| II–III | 19 | 4 | 36 | <0.001 |
| IV–V | 26 | 23 | 48 | <0.001 |
| Height (cm) | 150.7 ± 1.6a,b | 158.4 ± 2.0b | 165.0 ± 1.2 | <0.001 |
| Weight (kg) | 44.8 ± 1.6a,b | 63.3 ± 2.9b | 96.1 ± 2.4 | <0.001 |
| BMI (kg/m2) | 19.3 ± 0.4a,b | 24.9 ± 0.8b | 34.9 ± 0.6 | <0.001 |
| BMI percentile | 56.7 ± 3.2a,b | 88.3 ± 2.1b | 98.3 ± 0.1 | <0.001 |
| Fat mass (kg) | 9.3 ± 0.8a,b | 18.7 ± 1.6b | 38.5 ± 1.1 | <0.001 |
| Body fat (%) | 20.0 ± 1.1a,b | 29.5 ± 1.6b | 42.6 ± 0.6 | <0.001 |
| Waist circumference (cm) | 65.2 ± 1.0a,b | 79.3 ± 1.3b | 103.8 ± 1.4 | <0.001 |
| Visceral adipose tissue (cm2) | 14.9 ± 1.0a,b | 31.8 ± 2.9b | 72.3 ± 3.9 | <0.001 |
| Subcutaneous adipose tissue (cm2) | 89.9 ± 9.1a,b | 194.1 ± 20.2b | 507.8 ± 18.8 | <0.001 |
| Total cholesterol (mmol/liter) | 4.01 ± 0.08b | 3.88 ± 0.16b | 4.29 ± 0.11 | 0.039 |
| LDL cholesterol (mmol/liter) | 2.32 ± 0.07b | 2.28 ± 0.14 | 2.61 ± 0.09 | 0.020 |
| HDL cholesterol (mmol/liter) | 1.29 ± 0.04a,b | 1.11 ± 0.04 | 1.07 ± 0.03 | <0.001 |
| Total TG (mmol/liter) | 0.93 ± 0.05b | 1.11 ± 0.10 | 1.37 ± 0.09 | <0.001 |
| VLDL TG (mmol/liter) | 0.18 ± 0.01b | 0.22 ± 0.02 | 0.27 ± 0.02 | <0.001 |
Race, gender, IGT, and Tanner stages were compared using χ2. All other variables were compared using one-way ANOVA with post hoc Tukey test. ns, Not significant.
Significantly different from 75th–90th percentiles, P < 0.05.
Significantly different from 90th percentile or higher, P < 0.05.
Lipid concentrations
Fasting traditional lipid profiles are presented in Table 1. Children in the highest WC percentile group had significantly higher total cholesterol than children in the other two groups and higher LDL cholesterol, TG, and VLDL TG than children with a WC below the 75th percentile. Concentrations of HDL cholesterol were higher in children with a WC below the 75th percentile than above the 75th percentile. Correcting for race, gender, and pubertal development did not change these findings. Excluding participants with IGT changed the significance for total cholesterol from P = 0.039 to P = 0.090 and for LDL cholesterol from P = 0.020 to P = 0.066. Total cholesterol (P = 0.005), TG (P = 0.001), and VLDL TG (P < 0.001) concentrations were lower in black than white participants. Girls had lower total (P = 0.004) and LDL (P = 0.023) cholesterol, TG (P = 0.008), and VLDL (P = 0.008) than boys.
Significant positive correlations (P < 0.05) existed between WC and total cholesterol (r = 0.185), LDL cholesterol (r = 0.253), total TG (r = 0.281), and VLDL TG (r = 0.282), and a negative correlation existed between WC and HDL cholesterol (r = −0.416).
Lipoprotein particle size
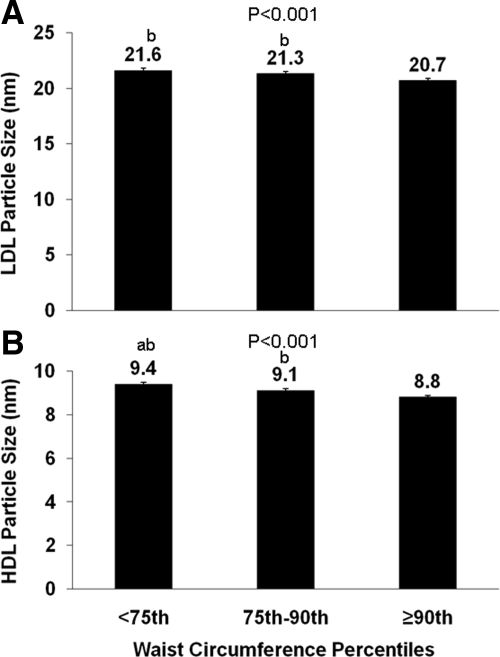
Figure 1 depicts LDL (Fig. 1A) and HDL (Fig. 1B) lipoprotein particle size among WC percentile groups. Children above the 90th percentile for WC had smaller LDL and HDL than children with WC below this percentile. The HDL particle size also differed between children with WC above and below the 75th percentile. No difference was seen in VLDL particle size among groups (P = 0.172). After correcting for race, gender, and pubertal development using ANCOVA, these findings did not change. Excluding participants with IGT did not change these results.
Figure 1.
LDL (A) and HDL (B) particle size by WC percentiles (<75th, 75th–90th, ≥90th). Differences were compared using one-way ANOVA with P values provided and with post hoc Tukey correction. a, Significantly different from 75th–90th percentile, P < 0.05; b, significantly different from ≥90th percentile, P < 0.05.
Factorial ANOVA revealed that HDL differed between race, with blacks having overall larger HDL than whites (P = 0.024). A race × WC interaction was observed for VLDL size (P = 0.026), with blacks having little difference in size among WC groups, whereas in whites VLDL size was larger as the WC percentiles increased. Lipoprotein particle sizes were similar between males and females. However, a gender × WC interaction was observed for VLDL size (P = 0.018), with girls having little difference in size among WC groups, whereas in boys VLDL size increased with waist percentiles.
WC correlated significantly with VLDL particle size (r = 0.168; P = 0.024) and negatively with HDL (r = −0.583; P < 0.001) and LDL (r = −0.477; P < 0.001) particle size.
Lipoprotein particle concentrations
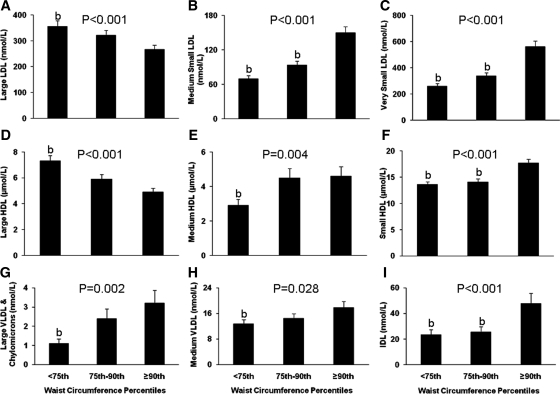
Figure 2 depicts lipoprotein particle concentration among the groups. Total LDL particle concentrations were significantly higher in children with a WC in the 90th percentile or higher, compared with the other two groups (<75th percentile, 708 ± 26 nmol/liter; 75th–90th percentile, 777 ± 40 nmol/liter; ≥90th percentile, 1025 ± 43 nmol/liter; P < 0.001 for ANOVA). Large LDL (Fig. 2A) particle concentrations were lower in children in the 90th percentile of WC compared with those below the 75th percentile. Conversely, medium (Fig. 2B) and very small (Fig. 2C) LDL concentrations were higher in those children above than below the 90th percentile of WC. Total HDL particle concentrations were significantly higher in children with a WC in the 90th percentile or higher compared with the other two groups (<75th percentile, 23.8 ± 0.5 μmol/liter; 75th–90th percentiles, 24.4 ± 0.6 μmol/liter; ≥90th percentile, 27.3 ± 0.5 μmol/liter; P < 0.001 for ANOVA). Large HDL concentrations (Fig. 2D) were lower, whereas medium (Fig. 2E) and small (Fig. 2F) HDL concentrations were higher in children with WC above the 90th percentile compared with those below the 75th percentile. Concentrations of large VLDL and chylomicron (Fig. 2G), medium VLDL (Fig. 2H), and IDL (Fig. 2I) were higher in children with WC above the 90th percentile. After correcting for race, gender, and pubertal development, all differences in lipoprotein particle concentrations remained except for medium VLDL (Fig. 2H), which was no longer different among groups (P = 0.227). Excluding participants with IGT changed the significance for medium VLDL from P = 0.028 to P = 0.146.
Figure 2.
Concentrations of large (A), medium small (B), and very small (C) LDL particles; large (D), medium (E), and small (F) HDL particles; and large VLDL and chylomicron (G), medium VLDL (H), and IDL (I) particles by WC percentiles (<75th, 75th–90th, ≥90th). Differences were compared using one-way ANOVA with P values provided, and with post hoc Tukey correction. a, Significantly different from 75th–90th percentile, P < 0.05; b, significantly different from ≥90th percentile, P < 0.05.
Large VLDL and chylomicrons (P = 0.001) and medium (P < 0.001) and small (P = 0.024) VLDL were lower in blacks than whites in terms of particle concentrations. For gender, large VLDL and chylomicrons (P = 0.003) and medium HDL (P = 0.034) were lower in females than males. A gender × WC interaction was observed for medium HDL concentrations (P = 0.001), with girls having little difference in concentration among WC groups, whereas in boys concentration increased with WC centiles.
Significant positive correlations (P < 0.05) existed between WC and total (r = 0.173), large (r = 0.246), and medium (r = 0.204) VLDL; total (r = 0.480), small (r = 0.515), medium small (r = 0.511), and very small (r = 0.513) LDL; IDL (r = 0.246); and total (r = 0.257), medium (r = 0.116), and small (r = 0.434) HDL. There were significant negative correlations (P < 0.05) between WC and large LDL (r = −0.211) and large HDL (r = −0.401) particles.
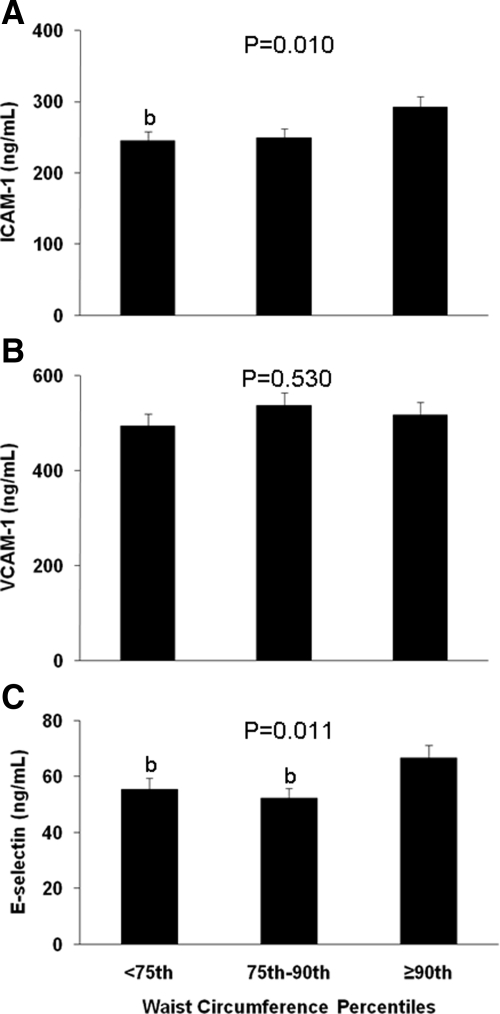
Vascular biomarkers
Concentrations of the vascular smooth muscle biomarkers ICAM-1, VCAM-1, and E-selectin are presented in Fig. 3. Both ICAM-1 and E-selectin were significantly higher in children with WC above the 90th percentile than those below the 75th percentile. No difference was observed in VCAM-1 among groups. After correcting for race, gender, and pubertal development using ANCOVA, the above observations did not change. For vascular biomarkers, there were significant positive correlations between WC and ICAM-1 (r = 0.195; P = 0.009) and E-selectin (r = 0.180; P = 0.015).
Figure 3.
Plasma concentrations of circulating vascular smooth muscle biomarkers by WC percentiles (<75th, 75th–90th, ≥90th): ICAM-1 (A), VCAM-1 (B), and E-selectin (C). Differences were compared using one-way ANOVA with P values provided, and with post hoc Tukey correction. a, Significantly different from 75th–90th percentile, P < 0.05; b, significantly different from ≥90th percentile, P < 0.05.
Discussion
The present study demonstrates significant differences in lipoprotein particle size and concentration and markers of vascular smooth muscle function in children and adolescents based on nationally representative WC percentile cutoffs. Youths with large WC, in the 90th percentile or higher based on NHANES data, have an atherogenic lipoprotein profile of increased concentrations of small dense LDL, small HDL and large VLDL particles along with significantly smaller LDL and HDL particle sizes. In addition, ICAM-1 and E-selectin, early markers of atherosclerotic CVD, are significantly higher in those children with WC in the 90th percentile or higher.
The current data are the first to establish differences in lipoprotein particle size and particle concentration in youth using nationally representative cutoff criteria for WC. However, several other studies have examined relationships between central obesity and standard lipid profiles in youth. The Boglausa Heart Study found that when 360 children aged 6–18 yr were divided into quintiles of obesity, truncal fat was strongly associated with adverse lipid concentrations, including total cholesterol, TG, and VLDL-TG, in the most obese children (11). Our own group (3) demonstrated that WC was significantly associated with TG, HDL cholesterol, and TG/HDL cholesterol ratio, and VLDL TG even after controlling for BMI percentile, suggesting that a high WC influences lipid profiles in excess of overall body adiposity. Other investigations support these observations and have shown that WC independently explains variations in total, HDL, and LDL cholesterol and TG (13,15,16,21). Moreover, differences in concentrations of these blood lipids have been found when children are separated into representative percentiles of WC (2,3,17). Collectively, such observations suggest that representative percentiles of WC can be used to aid in the assessment of CVD and T2DM risk. One question that remains to be determined is whether a threshold effect exists with respect to WC percentiles. We found significant correlations between many of our variables and WC when data were analyzed as continuous variables. In addition, our data demonstrate that the differences in variables occurred between participants with a WC in the 90th percentile or higher compared with the other two groups. Possibly, changes in the parameters we examined only manifest themselves at the 90th percentile or higher. Evidence also suggests that other changes related to CVD and T2DM become prominent at the same level (1,3).
Small, dense LDL particles are predictive of coronary heart disease (22,30,31). We found that both the size of LDL particles and concentrations of small LDL particles differed significantly between children above and below the 90th percentile for WC. Increased small, dense LDL concentrations have previously been related to WC and visceral fat in obese children (14). When Australian children were divided into tertiles based on LDL peak particle diameter, those children with the smallest LDL diameter were found to have higher WC than children in the other tertiles; however, this difference was not statistically significant (12). Finally, a recent study demonstrated that when 48 obese adolescents were divided into two groups based on hepatic fat fraction, those with a high hepatic fat fraction, who also had significantly higher visceral fat, had significantly smaller LDL size and higher concentrations of small LDL particles (4).
HDL particle size decreased with increasing WC percentiles, with concentrations of large HDL lower and small HDL higher in children in the 90th percentile of WC of higher. Large HDL particles have an inverse relationship with coronary heart disease, whereas smaller HDL particles are positively associated with the disease (32,33). A previous investigation found that children with a WC above the 90th percentile had a significantly greater risk of having low HDL cholesterol (2). Moreover, the previously cited study (4) examining hepatic fat fraction and lipoprotein profiles in adolescents found smaller HDL particle size and lower large HDL particle concentrations in children with high hepatic fat. These observations are supported by a recent study in adults with T2DM which found that HDL particle size was significantly related to visceral adiposity and WC (34). Collectively, these data suggest that WC and visceral adiposity are important determinants of an atherogenic HDL particle profile in youth.
It is likely that VLDL particles differ in atherogenicity, with some investigators suggesting that the large particles are most strongly related to arterial disease and obesity (33,35). We found that concentrations of large VLDL and chylomicrons and medium VLDL were higher in children with WC above the 90th percentile than those below the 75th percentile. Data from the Bogalusa Heart Study (13) previously found that concentrations of large VLDL were significantly related to WC. Interestingly, the Bogalusa data (13) demonstrated that concentrations of large VLDL increased rapidly with WC in white children, whereas WC was only weakly associated with concentrations of large VLDL in black children. This observation is similar to findings in the present study of a greater increase in VLDL size among waist percentiles in white than black youth and lower concentrations of VLDL particles in black children. We recently reported that black/white differences in VLDL, but not HDL or LDL, particle size existed after adjusting for visceral adiposity, which supports this notion further (36). Increased TG clearance in blacks (37) is a potential explanation for this difference.
During the early stages of atherosclerosis, biomarkers of vascular smooth muscle dysfunction are increased in response to inflammation and play an important role in plaque formation and lipid deposition (23). Our findings demonstrate a combination of endothelial dysfunction with an atherogenic lipoprotein profile in youth underpinned by central obesity. Our observation that ICAM-1 and E-selectin concentrations were higher in those children with a WC in the 90th percentile or above compared with those below the 75th percentile is similar to findings in adults with (24) and without T2DM (25) where both molecules were positively correlated with waist girth. Moreover, in adults the relationship between insulin sensitivity and ICAM-1 and E-selectin is lost after controlling for visceral adiposity (25), and reductions in E-selectin are significantly correlated to changes in WC (38), demonstrating the importance of central fat distribution in determining the concentrations of these molecules.
Given that atherogenesis is a disease of inflammation and lipid accumulation and autopsy evidence that atherosclerotic lesions start early in childhood (39), our data provide clinical relevance of the heightened cardiometabolic risk with large WC in childhood. With respect to the size of the differences in lipoprotein and vascular biomarkers among our WC groups, evidence from the adult literature suggests that they are important. Recent data (40) from the European Prospective Investigation into Cancer and Nutrition (EPIC-Norfolk study) found a mean LDL diameter of 21.0 nm in subjects who developed coronary artery disease (CAD) compared with 21.1 nm in subjects matched for gender, age, and time of enrollment into the study, who remained free of CAD during follow-up. LDL particle size was also inversely related to CAD. This difference is much smaller than the difference in LDL size of 21.6 and 20.7 nm, respectively, in the below 75th percentile and the 90th percentile or higher WC groups we report here. Such data support the premise that WC plays an important role in CVD.
Our participants ranged in age from 8–18 yr and included black and white and male and female children. Thus, growth, race, and gender were all potential confounders. Participants, however, were placed into WC percentiles based on age-, sex-, and ethnicity-specific data (8). Moreover, we corrected for these variables in our ANOVA analyses (race, gender, and pubertal development), and significance values showed little change, with only medium VLDL concentrations no longer significant. Additionally, in many instances these covariates did not significantly predict the variable being analyzed. Collectively, this suggests that WC exerts an independent effect on the lipoproteins and vascular markers measured. This supposition is supported by other studies in the pediatric literature demonstrating an effect of WC on CVD and T2DM risk factors independently of other confounding factors (1,2,3,17).
In conclusion, we demonstrate that children and adolescents with WC in the 90th percentile or higher, based on NHANES data, demonstrate an atherogenic lipoprotein profile and increased concentrations of biomarkers of vascular smooth muscle dysfunction consistent with heightened cardiometabolic risk. Given autopsy evidence of atherosclerotic lesions starting early in childhood (39), combined with our present findings, we suggest that children with abdominal obesity should be targeted for interventions at an early age to reduce cardiovascular risk.
Acknowledgments
We express our gratitude to all the children and their parents who volunteered to participate in this study, without whom science could not be advanced. We are grateful to the nursing staff of the Pediatric Clinical and Translational Research Center for their outstanding care of the participants and meticulous attention to the research, and to Resa Stauffer for all the laboratory analyses.
Footnotes
This work was supported by National Institutes of Health Grants R01 HD-27503 (to S.A.A.), K24 HD-01357 (to S.A.A.) and UL1 RR024153 CTSA (previously M01_RR-00084).
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 21, 2009
Abbreviations: ANCOVA, Analysis of covariance; BMI, body mass index; CAD, coronary artery disease; CVD, cardiovascular disease; HDL, high-density lipoprotein; ICAM-1, intercellular adhesion molecule-1; IDL, intermediate-density lipoprotein; IGT, impaired glucose tolerance; LDL, low-density lipoprotein; T2DM, type 2 diabetes mellitus; TG, triglyceride(s); VCAM-1, vascular adhesion molecule-1; VLDL, very low-density lipoprotein; WC, waist circumference.
References
- Lee S, Bacha F, Gungor N, Arslanian SA 2006 Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatr 148:188–194 [DOI] [PubMed] [Google Scholar]
- Maffeis C, Pietrobelli A, Grezzani A, Provera S, Tatò L 2001 Waist circumference and cardiovascular risk factors in prepubertal children. Obes Res 9:179–187 [DOI] [PubMed] [Google Scholar]
- Lee S, Bacha F, Arslanian SA 2006 Waist circumference, blood pressure, and lipid components of the metabolic syndrome. J Pediatr 149:809–816 [DOI] [PubMed] [Google Scholar]
- Cali AM, Zern TL, Taksali SE, de Oliveira AM, Dufour S, Otvos JD, Caprio S 2007 Intrahepatic fat accumulation and alterations in lipoprotein composition in obese adolescents. Diabetes Care 30:3093–3098 [DOI] [PubMed] [Google Scholar]
- Bacha F, Saad R, Gungor N, Arslanian SA 2006 Are obesity-related metabolic risk factors modulated by the degree of insulin resistance in adolescents? Diabetes Care 29:1599–1604 [DOI] [PubMed] [Google Scholar]
- McCarthy HD, Ellis SM, Cole TJ 2003 Central overweight and obesity in British youth aged 11–16 years: cross sectional surveys of waist circumference. BMJ 326:624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy HD, Jarrett KV, Crawley HF 2001 The development of waist circumference percentiles in British children aged 5.0–16.9 y. Eur J Clin Nutr 55:902–907 [DOI] [PubMed] [Google Scholar]
- Fernández JR, Redden DT, Pietrobelli A, Allison DB 2004 Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr 145:439–444 [DOI] [PubMed] [Google Scholar]
- Eisenmann JC 2005 Waist circumference percentiles for 7- to 15-year-old Australian children. Acta Paediatrica 94:1182–1185 [DOI] [PubMed] [Google Scholar]
- Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA 2003 Obesity, regional fat distribution, and syndrome X in obese black versus white adolescents: race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab 88:2534–2540 [DOI] [PubMed] [Google Scholar]
- Freedman DS, Srinivasan SR, Harsha DW, Webber LS, Berenson GS 1989 Relation of body fat patterning to lipid and lipoprotein concentrations in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr 50:930–939 [DOI] [PubMed] [Google Scholar]
- Steinbeck KS, Bermingham MA, Mahajan D, Baur LA 2001 Low-density lipoprotein subclasses in children under 10 years of age. J Paediatr Child Health 37:550–553 [DOI] [PubMed] [Google Scholar]
- Freedman DS, Bowman BA, Otvos JD, Srinivasan SR, Berenson GS 2002 Differences in the relation of obesity to serum triacylglycerol and VLDL subclass concentrations between black and white children: the Bogalusa Heart Study. Am J Clin Nutr 75:827–833 [DOI] [PubMed] [Google Scholar]
- Kang HS, Gutin B, Barbeau P, Litaker MS, Allison J, Le NA 2002 Low-density lipoprotein particle size, central obesity, cardiovascular fitness, and insulin resistance syndrome markers in obese youths. Int J Obes Relat Metab Disord 26:1030–1035 [DOI] [PubMed] [Google Scholar]
- Srinivasan SR, Myers L, Berenson GS 2002 Distribution and correlates of non-high-density lipoprotein cholesterol in children: the Bogalusa Heart Study. Pediatrics 110:e29 [DOI] [PubMed] [Google Scholar]
- Hirschler V, Aranda C, Calcagno Mde L, Maccalini G, Jadzinsky M 2005 Can waist circumference identify children with the metabolic syndrome? Arch Pediatr Adolesc Med 159:740–744 [DOI] [PubMed] [Google Scholar]
- Savva SC, Tornaritis M, Savva ME, Kourides Y, Panagi A, Silikiotou N, Georgiou C, Kafatos A 2000 Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes Relat Metab Disord 24:1453–1458 [DOI] [PubMed] [Google Scholar]
- Tchernof A, Lamarche B, Prud'Homme D, Nadeau A, Moorjani S, Labrie F, Lupien PJ, Després JP 1996 The dense LDL phenotype: association with plasma lipoprotein levels, visceral obesity, and hyperinsulinemia in men. Diabetes Care 19:629–637 [DOI] [PubMed] [Google Scholar]
- Couillard C, Bergeron N, Prud'homme D, Bergeron J, Tremblay A, Bouchard C, Mauriège P, Després JP 1998 Postprandial triglyceride response in visceral obesity in men. Diabetes 47:953–960 [DOI] [PubMed] [Google Scholar]
- Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Alméras N, Bergeron J, Gaudet D, Tremblay G, Prud'homme D, Nadeau A, Després JP 2000 Hypertriglyceridemic waist: a marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation 102:179–184 [DOI] [PubMed] [Google Scholar]
- Freedman DS, Serdula MK, Srinivasan SR, Berenson GS 1999 Relation of circumferences and skinfold thicknesses to lipid and insulin concentrations in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr 69:308–317 [DOI] [PubMed] [Google Scholar]
- Packard CJ 1996 LDL subfractions and atherogenicity: an hypothesis from the University of Glasgow. Curr Med Res Opin 13:379–390 [DOI] [PubMed] [Google Scholar]
- Ross R 1999 Atherosclerosis—an inflammatory disease. N Engl J Med 340:115–126 [DOI] [PubMed] [Google Scholar]
- Kent Jr JW, Comuzzie AG, Mahaney MC, Almasy L, Rainwater DL, VandeBerg JL, MacCluer JW, Blangero J 2004 Intercellular adhesion molecule-1 concentration is genetically correlated with insulin resistance, obesity, and HDL concentration in Mexican Americans. Diabetes 53:2691–2695 [DOI] [PubMed] [Google Scholar]
- Targher G, Bonadonna RC, Alberiche M, Zenere MB, Muggeo M, Bonora E 2001 Relation between soluble adhesion molecules and insulin sensitivity in type 2 diabetic individuals: role of adipose tissue. Diabetes Care 24:1961–1966 [DOI] [PubMed] [Google Scholar]
- Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J 2002 Hyperinsulinemia in African-American children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes 51:3014–3019 [DOI] [PubMed] [Google Scholar]
- Lee S, Gungor N, Bacha F, Arslanian S 2007 Insulin resistance: link to the components of the metabolic syndrome and biomarkers of endothelial dysfunction in youth. Diabetes Care 30:2091–2097 [DOI] [PubMed] [Google Scholar]
- Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P 1996 Prior to use of estrogen replacement therapy are users healthier than nonusers. Am J Epidemiol 143:971–978 [DOI] [PubMed] [Google Scholar]
- Otvos JD, Jeyarajah EJ, Bennett DW, Krauss RM 1992 Development of a proton nuclear magnetic resonance spectroscopic method for determining plasma lipoprotein concentrations and subspecies distributions from a single, rapid measurement. Clin Chem 38: 1632–1638 [PubMed] [Google Scholar]
- Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH 1996 A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA 276:882–888 [PubMed] [Google Scholar]
- Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Després JP 1997 Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Québec Cardiovascular Study. Circulation 95:69–75 [DOI] [PubMed] [Google Scholar]
- Miller NE 1987 Associations of high density lipoprotein subclasses and apolipoproteins with ischemic heart disease and coronary atherosclerosis. Am Heart J 113:589–597 [DOI] [PubMed] [Google Scholar]
- Freedman DS, Otvos JD, Jeyarajah EJ, Barboriak JJ, Anderson AJ, Walker JA 1998 The measurement of lipoprotein subclasses with proton nuclear magnetic resonance spectroscopy: associations with the extent of documented coronary artery disease. Arterioscler Thromb Vasc Biol 18:1046–1053 [DOI] [PubMed] [Google Scholar]
- Sam S, Haffner S, Davidson MH, D'Agostino Sr RB, Feinstein S, Kondos G, Perez A, Mazzone T 2008 Relationship of abdominal visceral and subcutaneous adipose tissue with lipoprotein particle number and size in type 2 diabetes. Diabetes 57:2022–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar N, Tan CE, Han TS, Forster L, Lean ME, Shepherd J, Packard CJ 1998 Associations of indices of adiposity with atherogenic lipoprotein subfractions. Int J Obes Relat Metab Disord 22:432–439 [DOI] [PubMed] [Google Scholar]
- Burns SF, Lee S, Arslanian SA 12 August 2009 In vivo insulin sensitivity and lipoprotein particle size and concentration in black and white children. Diabetes Care doi: 10.2337/dc09-0380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friday KE, Srinivasan SR, Elkasabany A, Dong C, Wattigney WA, Dalferes Jr E, Berenson GS 1999 Black-white differences in postprandial triglyceride response and postheparin lipoprotein lipase and hepatic triglyceride lipase among young men. Metabolism 48:749–754 [DOI] [PubMed] [Google Scholar]
- Trøseid M, Lappegård KT, Mollnes TE, Arnesen H, Seljeflot I 2005 Changes in serum levels of E-selectin correlate to improved glycaemic control and reduced obesity in subjects with the metabolic syndrome. Scand J Clin Lab Invest 65:283–290 [DOI] [PubMed] [Google Scholar]
- Strong JP, Malcom GT, McMahan CA, Tracy RE, Newman 3rd WP, Herderick EE, Cornhill JF 1999 Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA 281:727–735 [DOI] [PubMed] [Google Scholar]
- El Harchaoui K, van der Steeg WA, Stroes ES, Kuivenhoven JA, Otvos JD, Wareham NJ, Hutten BA, Kastelein JJ, Khaw KT, Boekholdt SM 2007 Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women. The EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol 49:547–553 [DOI] [PubMed] [Google Scholar]