Abstract
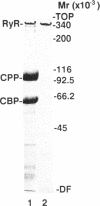
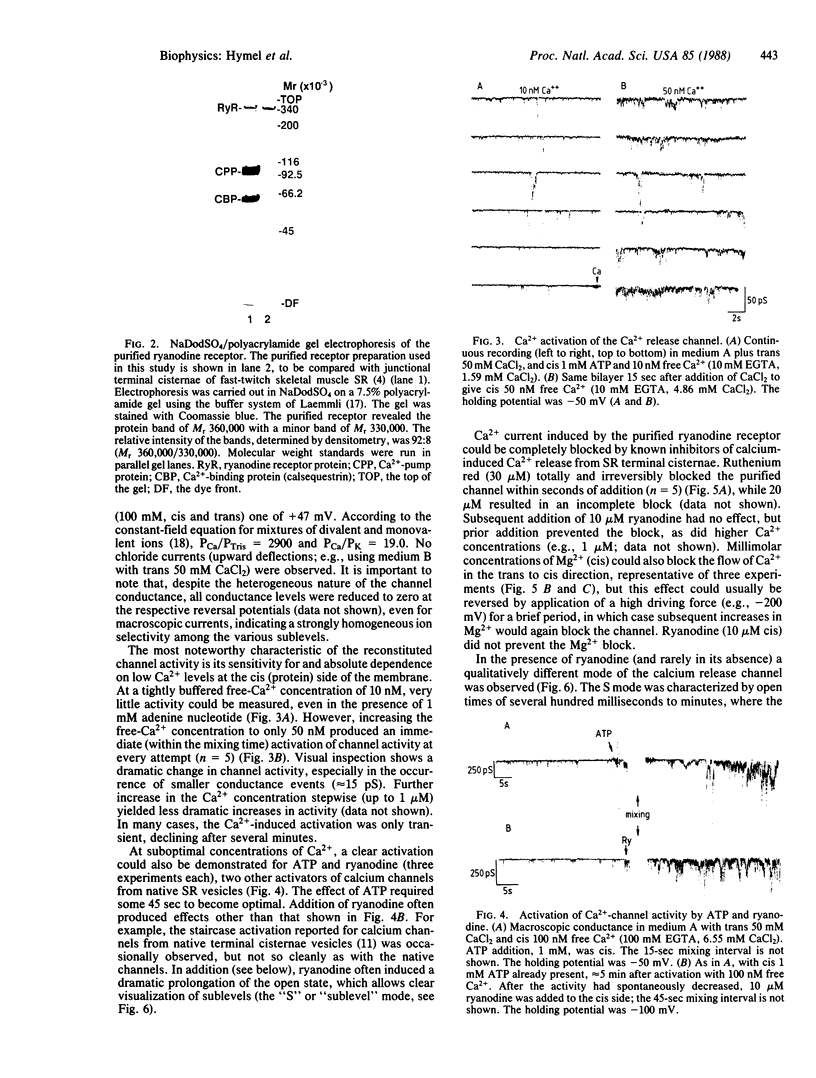
The ryanodine receptor of sarcoplasmic reticulum (SR) from fast-twitch skeletal muscle has been purified and found by electron microscopy to be equivalent to the feet structures that are involved in situ in the junctional association of transverse tubules with terminal cisternae of SR. We now find that when the purified receptor is incorporated into vesicle-derived planar bilayers, it forms Ca2+-specific channels, which are dependent on submicromolar Ca2+ for activity. In the presence of 1 mM ATP, the channel shows essentially no activity at 10 nM Ca2+ but becomes highly activated at 50 nM Ca2+. At suboptimal Ca2+ levels (100 nM), the channel is strongly activated by 1 mM ATP and can be blocked by ruthenium red, both effects being prevented by higher Ca2+ levels (1 microM). Mg2+, added from the cis side at millimolar concentrations, blocks Ca2+ flux through the channel from trans to cis (equivalent to flux from luminal to myoplasmic compartment). Ryanodine stabilizes the open state of the channel and blocks the action of ruthenium red to close the channel. Thus, the purified ryanodine receptor incorporated into a bilayer has the Ca2+-channel characteristics consistent with the calcium release observed in isolated terminal cisternae vesicles. Furthermore, ryanodine induced the appearance of a sublevel gating mode characterized by long open conductance states, which were integral multiples of the smallest observed conductance, 3.8 pS in 50 mM Ca2+. The purified receptor consists essentially of a single-sized high molecular weight polypeptide (Mr. approximately equal to 360,000), which on reconstitution forms the square rectangles diagnostic of the feet structures. We conclude that the identity of the Ca2+-release channel of SR is the foot structure, which consists of an oligomer of the high molecular weight polypeptide.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell K. P., Knudson C. M., Imagawa T., Leung A. T., Sutko J. L., Kahl S. D., Raab C. R., Madson L. Identification and characterization of the high affinity [3H]ryanodine receptor of the junctional sarcoplasmic reticulum Ca2+ release channel. J Biol Chem. 1987 May 15;262(14):6460–6463. [PubMed] [Google Scholar]
- Chu A., Volpe P., Costello B., Fleischer S. Functional characterization of junctional terminal cisternae from mammalian fast skeletal muscle sarcoplasmic reticulum. Biochemistry. 1986 Dec 16;25(25):8315–8324. doi: 10.1021/bi00373a028. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Engel A., Massalski A., Schindler H., Dorset D. L., Rosenbusch J. P. Porin channel triplets merge into single outlets in Escherichia coli outer membranes. Nature. 1985 Oct 17;317(6038):643–645. doi: 10.1038/317643a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Ogunbunmi E. M., Dixon M. C., Fleer E. A. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flockerzi V., Oeken H. J., Hofmann F., Pelzer D., Cavalié A., Trautwein W. Purified dihydropyridine-binding site from skeletal muscle t-tubules is a functional calcium channel. Nature. 1986 Sep 4;323(6083):66–68. doi: 10.1038/323066a0. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Nunzi G. Junctional feet and particles in the triads of a fast-twitch muscle fibre. J Muscle Res Cell Motil. 1983 Apr;4(2):233–252. doi: 10.1007/BF00712033. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J Biol Chem. 1987 Nov 15;262(32):15637–15642. [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem. 1987 Feb 5;262(4):1740–1747. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léoty C., Léauté M. Membrane potential and contractures in segments cut from rat fast and slow twitch muscles. Pflugers Arch. 1982 Oct;395(1):42–48. doi: 10.1007/BF00584966. [DOI] [PubMed] [Google Scholar]
- Martonosi A. N. Mechanisms of Ca2+ release from sarcoplasmic reticulum of skeletal muscle. Physiol Rev. 1984 Oct;64(4):1240–1320. doi: 10.1152/physrev.1984.64.4.1240. [DOI] [PubMed] [Google Scholar]
- Meissner G., Darling E., Eveleth J. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry. 1986 Jan 14;25(1):236–244. doi: 10.1021/bi00349a033. [DOI] [PubMed] [Google Scholar]
- Owen J. D. The determination of the stability constant for calcium-EGTA. Biochim Biophys Acta. 1976 Nov 18;451(1):321–325. doi: 10.1016/0304-4165(76)90282-8. [DOI] [PubMed] [Google Scholar]
- Rios E., Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987 Feb 19;325(6106):717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Saito A., Seiler S., Chu A., Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol. 1984 Sep;99(3):875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler H. Formation of planar bilayers from artificial or native membrane vesicles. FEBS Lett. 1980 Dec 15;122(1):77–79. doi: 10.1016/0014-5793(80)80405-4. [DOI] [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein from Escherichia coli outer membranes forms voltage-controlled channels in lipid bilayers. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3751–3755. doi: 10.1073/pnas.75.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein in planar membranes: clusters of channels in a native environment and their functional reassembly. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2302–2306. doi: 10.1073/pnas.78.4.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler H., Spillecke F., Neumann E. Different channel properties of Torpedo acetylcholine receptor monomers and dimers reconstituted in planar membranes. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6222–6226. doi: 10.1073/pnas.81.19.6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Schwartz L. M., McCleskey E. W., Almers W. Dihydropyridine receptors in muscle are voltage-dependent but most are not functional calcium channels. 1985 Apr 25-May 1Nature. 314(6013):747–751. doi: 10.1038/314747a0. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Sarcoplasmic reticulum contains adenine nucleotide-activated calcium channels. Nature. 1985 Aug 1;316(6027):446–449. doi: 10.1038/316446a0. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Single channel measurements of the calcium release channel from skeletal muscle sarcoplasmic reticulum. Activation by Ca2+ and ATP and modulation by Mg2+. J Gen Physiol. 1986 Nov;88(5):573–588. doi: 10.1085/jgp.88.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Single-channel calcium and barium currents of large and small conductance from sarcoplasmic reticulum. Biophys J. 1986 Nov;50(5):921–928. doi: 10.1016/S0006-3495(86)83533-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]