Abstract
Background: Occult ovarian insufficiency is associated with infertility, impaired response to ovarian stimulation, and reduced live birth rates in women treated with assisted reproductive technologies. Although a decline in ovarian follicle number is expected with age, the proximate causes of occult ovarian insufficiency in young women remain poorly understood. Abnormalities in telomere length and telomerase activity in human granulosa cells may serve as molecular markers for this condition.
Methods: A cross-sectional study was performed. Subjects (37 yr old or less) undergoing in vitro fertilization were classified as cases of occult ovarian insufficiency or controls with mechanical infertility (male or tubal factor). Granulosa cells were acquired at the time of oocyte retrieval to quantify telomere length and telomerase activity.
Results: Fifty-four women were enrolled. Human granulosa cell telomerase activity was demonstrated, and lack of granulosa cell telomerase activity was associated with occult ovarian insufficiency (odds ratio, 11.0; 95% confidence interval, 1.3–495.6; P = 0.02). Telomeres were shorter in women with occult ovarian insufficiency than in controls (relative telomere/single copy gene ratio, 1.88 vs. 3.15; P = 0.039).
Conclusions: Aberrant telomere homeostasis is associated with occult ovarian insufficiency in young women. This finding is consistent with the presence of telomeric attenuation that has been shown in multiple age-related conditions.
Occult ovarian insufficiency is associated with shortened telomeres and diminished telomerase activity in human granulosa cells.
Primary ovarian insufficiency describes a spectrum of declining ovarian function and reduced fecundity due to a decrease in initial follicle number, an increase in follicle destruction, or poor follicular response to gonadotropin stimulation (1,2). Three categories of ovarian insufficiency have been described that span a continuum from least to most severe dysfunction. Reduced fecundity, regular cycles, and premenopausal FSH levels characterize occult ovarian insufficiency. The development of elevated FSH levels indicates the presence of biochemical ovarian insufficiency. Women with overt ovarian insufficiency have both elevated FSH levels and amenorrhea (1,3). Diminished ovarian reserve (DOR) is a term that has been used to characterize women at risk for poor performance with assisted reproductive technologies (4,5,6,7,8,9). The term is used to predict prognosis as a function growing follicle number and the reproductive potential of harvested oocytes (4,5,6,7,8,9). Compared with other infertility diagnoses, DOR is associated with the lowest odds of pregnancy with assisted reproductive technologies because affected women develop few oocytes with hyperstimulation and have low implantation rates after embryo transfer (3,4,7,10). Poor responsiveness to gonadotropin stimulation as seen in DOR patients may also have serious consequences beyond refractory infertility and has been shown to predict early menopause in several series (11,12,13). Primary ovarian insufficiency has been suggested as the preferred term to describe DOR because it allows for an accurate characterization of the severity of ovarian dysfunction regardless of the underlying etiology. Accordingly, we have chosen to use occult ovarian insufficiency throughout this report to capture the fundamental clinical features of DOR: regular menstrual cycles, reduced fecundity, and elevated FSH levels below the menopausal range.
Only a small number of genetic and molecular pathways have been described that cause derangements in human follicle number and function severe enough to result in primary ovarian insufficiency (1,2,14,15,16,17,18). Fewer still are the number of specific environmental and chemical exposures with evidence of toxicity to ovarian follicles sufficient to cause early menopause (1,19,20,21,22). The establishment of the initial follicular cohort—a critical element in the determination of ovarian function and reproductive life span—appears to be governed by two key factors: the efficiency of germ cell proliferation in utero and the trajectory of follicular depletion thereafter (1,2,14). It is universally accepted that the peak number of approximately 6 million germ cells is achieved at 20 wk gestation and that this number can never be replenished (1,23,24,25). Follicle number decreases continually from this point forward due to programmed cell death of oocytes and granulosa cells (14,26,27). Any individual who achieves less than the peak number of follicles (as in blepharophimosis, ptosis, epicanthus inversus syndrome) (5,14,15,16,17) and/or experiences extremely rapid follicular atresia (as in Monosomy X, chemotherapy exposure, or Fragile-X mental retardation-1 premutation carriers) (18,19,20,21,22,23,24,25,26,27,28,29) is at risk for premature ovarian insufficiency. Unfortunately, the majority of young women with ovarian insufficiency do not have a discrete cause that can be identified.
We have chosen to consider the association of granulosa cell telomere homeostasis with occult ovarian insufficiency in women 37 yr of age and younger. Telomeres consist of repetitive arrays of (5′-TTAGGG-3′)n that cap the ends of all eukaryotic chromosomes (30,31,32). Telomeres shorten with successive rounds of DNA replication because most somatic cells lack the proper machinery to replicate the ends of lagging strand DNA and replenish telomere repeats (30,31,32,33,34,35). Over successive cell divisions, this results in critically short, dysfunctional telomeres that trigger replicative senescence through p53/RB intracellular pathways (36,37). Telomere attrition has been postulated to play a role in the development of age-related disease by mediating the onset of cellular senescence and imposing a limit on the proliferative life span of certain cell types (31,33,34,38,39,40).
One of the critical components of telomere homeostasis is the activity of telomerase, a ribonucleoprotein enzyme capable of extending telomeres in cell populations such as embryonic, germline, and stem cells (34,35,41,42). Moreover, telomerase has been shown to enhance the proliferation of certain stem cell populations independent of its role in maintaining telomere length (42,43,44).
In the human, normal ovaries express telomerase (45), and human oocytes and embryos have been found to exhibit telomerase activity (46,47). Lavranos et al. (48) demonstrated telomerase activity in the granulosa cells of bovine follicles and suggested that active telomerase permits the proliferation of granulosa cells required for proper follicle formation.
We hypothesized that telomere length homeostasis in human granulosa cells is associated with the presence of occult ovarian insufficiency. To test this hypothesis, granulosa cell telomere length and telomerase activity were compared between cases of ovarian insufficiency and controls with mechanical infertility (male factor or tubal factor).
Subjects and Methods
Subjects and clinical procedures
Cases of occult ovarian insufficiency were defined as women age 37 yr or less with infertility and a history of FSH elevation (in the premenopausal range) before initiation of in vitro fertilization (IVF). An early follicular phase (d 2–4 of the menstrual cycle) FSH of 11.4 IU/liter or greater in conjunction with an estradiol level of less than 293.6 pmol/liter was used to diagnose occult ovarian insufficiency. This cutoff was based on data from our population of IVF patients demonstrating very few pregnancies in women with FSH values above this level (49). Controls were women undergoing IVF with normal FSH testing and either male factor infertility (abnormal semen analysis on more than one occasion) or tubal factor infertility (history of ectopic pregnancy, bilateral tubal ligation, bilateral salpingectomy, or tubal obstruction on hysterosalpingogram). Immunoassays to measure FSH and estradiol were performed on the Siemens Immulite 2500 (Siemens Healthcare Diagnostics, Deerfield, IL). The sensitivity for FSH using this assay is 0.5 IU/liter, and the total coefficient of variation is 4.8%. For the estradiol assay, the sensitivity is 110 pmol/liter, with a coefficient of variation of 6.2%.
This study was approved by the Institutional Review Board of the Hospital of the University of Pennsylvania. Women undergoing IVF treatment at Penn Fertility Care were approached at cycle initiation to provide informed consent to participate in the study. The stimulation regimen for subjects involved the administration of recombinant FSH (Gonal F; Serono Pharmaceuticals, Rockland, MA) in combination with pituitary down-regulation using a GnRH agonist (Luprolide Acetate; TAP Pharmaceuticals, Lake Forest, IL) starting either in the midluteal phase or early follicular phase (minidose flare). Follicular fluid was aspirated from follicles at the time of ultrasound-guided transvaginal oocyte retrieval. Granulosa cells were isolated from the follicular fluid.
Granulosa cell isolation
Total fluid from all follicular puncture sites at the time of oocyte retrieval was pooled for each subject. The follicular fluid was centrifuged for 15 min at 300 × g and a temperature of 4 C to recover cellular material. The cells were layered over of a 50% Ficoll gradient (Ficoll-Paque; Amersham Biosciences, Buckinghamshire, UK) and centrifuged at 300 × g for 15 min at 4 C. Granulosa cells were isolated from the interface layer, washed once with PBS, and then resuspended in PBS. This process resulted in a population of cells with minimal lymphocyte contamination (less than 5%). The cells were stored at −80 C until use.
Assessment of telomere length
DNA was extracted from granulosa cells using the DNeasy Tissue Kit (QIAGEN Inc., Mississauga, Ontario, Canada). Thereafter, telomere length was assessed using a modification of the quantitative PCR method described by Cawthon (50,51,52,53,54).This technique measures the factor by which the ratio of telomere repeat copy number to single-gene copy number differs between an unknown sample and a reference DNA sample. As a result, this method generates a relative Telomere/Single Copy Gene (T/S) ratio that is proportional to average telomere length. For each specimen, two PCRs were carried out—one to amplify telomere repeats (Tel PCR), and the other to amplify the β-globin gene (S PCR). The SYBR Green Jumpstart Taq Ready Mix (Sigma Aldrich, St. Louis, MO) was used for all quantitative PCRs. Each reaction was carried out in triplicate using the Opticon 2 detector system on the MJ Research PTC200 DNA Engine thermal cycler (Waltham, MA). Before starting PCR, a mixture of sample DNA (5 ng), Escherichia coli DNA (40 ng), 10X Taq polymerase buffer (magnesium free), and diethylpyrocarbonate water was made and heated at 94 C for 30 min to fully denature the DNA.
For telomere PCR, the following conditions and primers were used; 100 nm of primer Tel 1b (5′-CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT-3′) and 900 nm of primer Tel 2b (5′-GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT-3′) were used to amplify sample DNA under the following cycling conditions: an initial polymerase activation step at 94 C for 1 min followed by 30 cycles of 95 C for 15 sec and 56 C for 1 min. For β-globin gene PCR, 300 nm of primer hgb 1 (5′-CTTCTGACACAACTGTGTTCACTAGC-3′) and 700 nm of primer hgb 2 (5′-CACCAACTTCATCCACGTTCACC-3′) were used to amplify sample DNA with the following cycling conditions: an initial polymerase activation step at 94 C for 1 min, followed by 33 cycles of 95 C for 15 sec, 58 C for 20 sec, and 72 C for 20 sec. Negative controls using water in lieu of sample DNA were processed during each PCR run. Serial dilutions (seven concentrations from 25 ng to 1 ng in water) of reference DNA were used to construct standard curves in both Tel PCR and β-globin gene PCRs, allowing for relative quantification of samples.
Method of telomerase activity assessment
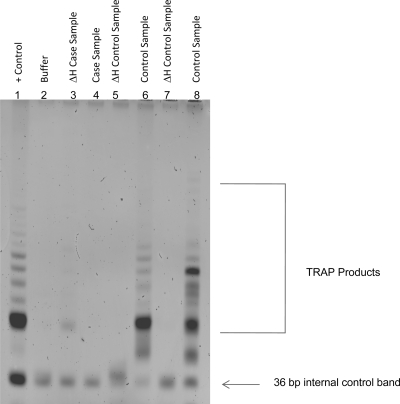
Telomerase activity in samples was measured using a PCR-based assay (TRAPeze Telomerase Detection Kit; Millipore, Billerica, MA). Granulosa cells were first exposed to 1X CHAPS lysis buffer to extract protein. Protein concentration of each extract was determined using the Bradford method after establishing a standard curve of protein concentration. Extracts with a concentration range of 10–750 μg were suitable for assay. Each extract was then combined with TRAPeze reagents to perform the two-step PCR-based assay. Because telomerase is a heat-sensitive enzyme, each sample extract was heat treated before PCR to generate a sample-specific negative control. Polyacrylamide gel electrophoresis was used to distinguish telomerase extension products amplified by PCR. Telomerase activity (positive or negative) was determined based on the presence or absence of a characteristic laddering pattern of telomerase products compared with positive and negative controls (Fig. 1). The sensitivity of this assay under optimal conditions is 50 telomerase-positive cells.
Figure 1.
TRAP assay results in three test samples using polyacrylamide gel electrophoresis. Telomerase activity was demonstrated in two control samples (and absent in heat-inactivated negative controls) and absent in a sample from a case with occult ovarian insufficiency. Lane 1, Telomerase-positive control cells; lane 2, buffer, negative control; lanes 3 and 4, case subject: heat-inactivated extract and untreated extract; lanes 5 and 6, control subject: heat-inactivated extract and untreated extract; lanes 7 and 8, control subject: heat-inactivated extract and untreated extract.
Statistical analysis
Continuous variables were described as means (± sd or ± sem) if they were normally distributed or medians if they were not. Graphical methods were employed to evaluate the normality of all continuous variables. Because the relative T/S ratios denoting telomere length were not normally distributed in this population, these values were log-transformed to use parametric statistical tests to interpret these data.
Associations between categorical variables were tested using the Fisher’s exact test. Comparisons of continuous variables between groups were made using the Student’s t test or the Wilcoxon rank sum test where appropriate. Spearman’s coefficient was used to determine correlations between continuous variables. A P value of less than 0.05 was considered statistically significant for nominal comparisons of variables, and a Bonferroni correction was applied to minimize the chance of a type I error in the multiple comparisons made in IVF cycle outcomes. Odds ratios (ORs) were calculated to determine the association between telomerase activity (present or absent) and occult ovarian insufficiency. Logistic regression was used to create a model for occult ovarian insufficiency and control for multiple confounders. All statistical tests were performed using STATA 9 software (College Station, TX).
The following definitions were used to categorize pregnancy outcomes. Positive pregnancy was defined as a serum B-human chorionic gonadotropin level greater than 5 mIU/ml measured 2 wk after oocyte retrieval, and clinical pregnancy was defined as the presence of a gestational sac on ultrasound after a positive pregnancy test. A live birth was defined as delivery of a viable fetus at or after 24 wk gestation.
Our sample size of 54 patients was based on the following assumptions: a difference of 45% in the proportion of cases lacking telomerase compared with controls, 80% power, α error of 0.05, and a 3:1 ratio of controls to cases.
Results
A total of 54 patients consisting of 12 cases of occult ovarian insufficiency and 42 controls undergoing IVF for the first time at Penn Fertility Care were enrolled for study participation. Baseline characteristics of the study population are listed in Table 1.
Table 1.
Baseline characteristics of study participants
| Occult ovarian insufficiency | Controls | |
|---|---|---|
| n | 12 | 42 |
| Median age (range) | 34.5 (30–37) | 33 (23–37)a |
| White | 10 (83%) | 28 (67%)a |
| Non-white | 2 (17%) | 14 (33%)a |
| Median gravity (range) | 0 (0–2) | 0 (0–5)a |
| Median parity (range) | 0 (0–1) | 0 (0–3)a |
P = Not significant.
IVF cycle outcomes between cases and controls were compared. Numbers of follicles, numbers of oocytes, maximum estradiol concentration, and total amount of gonadotropin administered were significantly different between the groups (Table 2). When the adjusted P value for the 13 comparisons made in IVF cycle outcomes was applied (new P value = 0.004 or 0.05/13), all comparisons remained statistically significant except for the number of mature oocytes, number of atretic oocytes, and number of eight-cell embryos.
Table 2.
IVF cycle outcomes in cases compared to controls
| Occult ovarian insufficiency | Controls | |
|---|---|---|
| n | 12 | 42 |
| Median maximum pre-IVF FSH (range) | 14.3 IU/liter (11.4–21.6) | 6.05 IU/liter (3.9–9.9) (P = 0.0001) |
| Median total units of total gonadotropin administered (range) | 6,487.5 (2,587.5–6,900) | 2,887.5 (1,537.5–5,775) (P < 0.0001) |
| Mean maximum estradiol ± sd | 8,006.8 pmol/liter ± 3,786 | 13,853.1 pmol/liter ± 5,627.2 (P = 0.002) |
| Mean no. of follicles ± sd | 11.4 ± 5.2 | 24 ± 9.7 (P = 0.0001) |
| Median no. of oocytes retrieved (range) | 8.5 (2–17) | 16.5 (3–29) (P = 0.0004) |
| Mean no. of mature oocytes ± sd | 6.8 ± 4.2 | 11.4 ± 5.8 (P = 0.01) |
| Mean percentage fertilization | 70% | 69% (P = 0.37) |
| Median atretic eggs (range) | 0 (0–2) | 1 (0–9) (P = 0.03) |
| Median no. of eight-cell embryos (range) | 0 (0–4) | 1 (0–6) (P = 0.04) |
| Pregnancy rate per retrieval | 33.3% | 45.2% (P = 0.53) |
| Clinical pregnancy rate per retrieval | 25% | 40.5% (P = 0.5) |
| Miscarriage rate | 33.3% | 17.6% (P = 0.51) |
| Live birth rate per retrieval | 16.7% | 33.3% (P = 0.47) |
The IVF response data for the 12 cases of occult ovarian insufficiency are elaborated in detail in Table 3. These figures highlight two key features of ovarian insufficiency: 1) the challenge of consistently achieving significant numbers of mature oocytes even when considerable gonadotropin stimulation is used; and 2) the poor pregnancy outcomes in this population.
Table 3.
Demographics and IVF cycle characteristics of subjects with occult ovarian insufficiency
| Subject no. | Age | Race | Peak FSH (IU/liter) | Pretreatment FSH (IU/liter) | Total units of gonadotropin administered | Peak estradiol (pmol/liter) | Oocytes | Mature oocytes | Atretic oocytes | Embryos | Live birth |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | White | 15 | 5.9 | 6900 | 7,735 | 5 | 3 | 0 | 0 | No |
| 2 | 36 | White | 15 | 8.1 | 6300 | 9,306 | 10 | 10 | 0 | 9 | No |
| 3 | 30 | White | 14.9 | 5.8 | 6900 | 13,531 | 17 | 16 | 0 | 13 | No |
| 4 | 37 | White | 21.6 | 13.3 | 6675 | 14,269 | 12 | 10 | 1 | 9 | No |
| 5 | 35 | White | 11.6 | 6.2 | 7500 | 6,178 | 11 | 11 | 2 | 7 | No |
| 6 | 36 | White | 14.3 | 8.9 | 5550 | 6,578 | 3 | 3 | 0 | 2 | No |
| 7 | 33 | White | 14.3 | 5.1 | 6900 | 3,616 | 8 | 6 | 2 | 5 | No |
| 8 | 34 | Asian | 26.1 | 7.7 | 6300 | 4,064 | 2 | 2 | 0 | 1 | No |
| 9 | 30 | White | 11.4 | 6.1 | 2587.5 | 10,712 | 7 | 7 | 0 | 6 | Yes |
| 10 | 33 | White | 12.7 | 12.7 | 6900 | 4,460 | 9 | 6 | 0 | 5 | No |
| 11 | 36 | Asian | 12.4 | 10.3 | 5775 | 8,946 | 11 | 9 | 1 | 4 | Yes |
| 12 | 34 | White | 12.6 | 10.5 | 5700 | 3,106 | 2 | 1 | 0 | 1 | No |
There was a significant relationship between telomerase activity and the odds of occult ovarian insufficiency (Table 4). Women lacking granulosa cell telomerase activity were 11 times more likely to have occult ovarian insufficiency than those who exhibited telomerase activity [OR, 11; 95% confidence interval (CI), 1.3–495.6]. Granulosa cell telomeric shortening was also associated with occult ovarian insufficiency. Average telomere length (expressed as the log-transformed relative T/S ratio) was 1.88 in cases and 3.15 in controls (P = 0.03). Logistic regression was used to further characterize the influence of telomerase inactivity on the odds of occult ovarian insufficiency while controlling for telomere length, race (white vs. non-white), and categorical age (<35, 35–37). After adjusting for these factors, women lacking telomerase activity were 14.5 times more likely to have occult ovarian insufficiency than with telomerase activity (OR, 14.5; 95% CI, 1.4–149.5). There were no significant interactions between variables in the explanatory model.
Table 4.
Telomere length, telomerase activity, and OR for occult ovarian insufficiency based on absent telomerase activity
| Telomere length ± sem | No telomerase activity detected (%) | OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|---|
| Occult ovarian insufficiency (n = 12) | 1.88 ± 0.69a | 11 of 12 (92%) | 11 (1.3–495.6)b | 14.5 (1.4–149.5)c |
| Controls (n = 42) | 3.15 ± 0.25 | 21 of 42 (50%) | Reference | Reference |
P = 0.039, for difference in mean telomere length compared to controls.
P = 0.02, OR for occult ovarian insufficiency based on absent telomerase activity.
P = 0.024, OR for occult ovarian insufficiency based on absent telomerase activity adjusted for age, race, and telomere length.
IVF cycle outcomes based on the presence or absence of telomerase are described in Table 5. There was a statistically significant association between lack of telomerase and mean number of follicles produced as well as median pretreatment FSH concentration. However, when the adjusted P value for the 13 comparisons made in IVF cycle outcomes was applied (new P value = 0.004), these differences were no longer statistically significant. Several other relevant outcomes, including total dose of gonadotropin administered, number of oocytes retrieved, number of atretic oocytes, percentage fertilization, and pregnancy rate per retrieval demonstrated a trend toward a significant relationship with absent telomerase activity.
Table 5.
IVF cycle outcomes in subjects with and without telomerase activity
| Telomerase activity present | Telomerase activity absent | |
|---|---|---|
| n | 22 | 32 |
| Median maximum pre-IVF FSH (range) | 5.7 IU/liter (3–14.3) | 7.7 IU/liter (3.5–26.1) (P = 0.04) |
| Median total amount of total gonadotropin administered (units) (range) | 2,887.5 (1,387.5–6,900) | 4031.3 (1,537.5–7,500) (P = 0.08) |
| Mean maximum estradiol | 13,157 pmol/liter ± 6,102.1 | 12,140 pmol/liter ± 5,614.4 (P = 0.55) |
| Mean follicles± sd | 24.8 ± 8.9 | 19.1 ± 10.6 (P = 0.05) |
| Median oocytes (range) | 19.5 (6–33) | 12 (2–41) (P = 0.06) |
| Mean no. of mature oocytes ± sd | 11.9 ± 5.3 | 9.3 ± 5.9 (P = 0.12) |
| Median atretic oocytes (range) | 2.4 (0–9) | 1 (0–7) (P = 0.07) |
| Mean percentage fertilization | 78% | 66% (P = 0.058) |
| Median no. of eight-cell embryos (range) | 1.5 (0–5) | 0.5 (0–6) (P = 0.1536) |
| Pregnancy rate per retrieval | 59.1% | 31.3% (P = 0.054) |
| Clinical pregnancy rate per retrieval | 50% | 28.1% (P = 0.15) |
| Miscarriage rate | 20% | 15.4% (P = 1.0) |
| Live birth rate per retrieval | 40.9% | 21.2% (P = 0.23) |
Conversely, there were no statistically significant associations between mean granulosa cell telomere length and any of the IVF cycle outcomes described above (data not shown).
The correlation between total amount of gonadotropins administered during IVF and telomere length was assessed using the Spearman correlation coefficient. The Spearman’s rho was −0.0747 (P = 0.59).
Discussion
Although multiple investigations have examined the role of telomere-induced cellular dysfunction in age-related disease, this is the first in the English literature to evaluate the relationship between telomere length, telomerase activity, and occult ovarian insufficiency using human granulosa cells (55). Our finding of shortened telomeres in women with occult ovarian insufficiency is consistent with several studies that have demonstrated an association between telomere attrition and reproductive senescence (56,57). Compared with these investigations in which telomeres were measured in peripheral blood leukocytes, we investigated telomere length in the human follicle.
The association between occult ovarian insufficiency and granulosa cell telomeric shortening is likely multifactorial. It is possible that intrinsic telomere length differences exist in women with ovarian insufficiency, making their granulosa cells more likely to begin proliferation with shorter telomeres than women with normal ovarian function. Alternatively, telomeric attrition in young women with ovarian insufficiency could be a function of the kinetics of granulosa cell division in human follicles. Limited granulosa cell proliferative capacity has been demonstrated as a feature distinguishing women with ovarian insufficiency from those without it (58,59). In the follicles of women with ovarian insufficiency, more divisions may be required per competent granulosa cell to achieve a total population doubling. A greater number of replications per cell would naturally result in accelerated telomere erosion in the cells capable of dividing. The significantly higher amount of gonadotropin administered to women with occult ovarian insufficiency does not appear to explain the telomeric attrition in this group. The Spearman coefficient for correlation between total amount of gonadotropin and telomere length was very weak and not statistically significant.
The association between lack of granulosa cell telomerase activity and occult ovarian insufficiency could represent a mechanism by which telomere erosion is accelerated and replicative capacity is impaired. Telomerase activity is present in regenerative tissues with significant proliferative requirements where telomere loss would be poorly tolerated (34,41,48). Ovarian follicular development in humans requires an explosive increase in granulosa cell number from as few as three progenitors per follicle to tens of thousands of cells before ovulation (60). In the bovine model, telomerase activity is absent in pregranulosa cells of primordial follicles, activated when follicles begin to grow, and highest in preantral and small antral follicles with the most robust cell proliferation; telomerase activity declines in later stages of follicular development (46). Insufficient activation of telomerase in the early stages of follicular development could hasten telomere attrition and severely compromise cellular functions required for proper follicular maturation. Furthermore, because telomerase expression appears to have a role in supporting stem cell proliferation independent of its role in replenishing telomeres (42,43,44), its absence in association with occult ovarian insufficiency further supports its strength as a marker of granulosa cell dysfunction.
IVF outcomes as a function case status were also investigated. As expected, subjects with occult ovarian insufficiency achieved significantly lower estradiol levels, fewer numbers of follicles, and fewer oocytes than controls, despite receiving more gonadotropin stimulation during IVF (Table 2). Cases also produced fewer eight-cell embryos than controls (median of 0 and 1, respectively). Conversely, women with ovarian insufficiency had slightly fewer atretic oocytes than controls (median of 0 and 1, respectively). Given the relatively modest size of our sample, it is possible that chance played a role in the comparisons of atretic oocytes and eight-cell embryos between groups.
Clinical pregnancy rates, miscarriage rates, and live birth rates per retrieval were all lower in women with ovarian insufficiency than controls. Although these differences did not reach statistical significance, each outcome was more favorable in controls and was consistent with the published literature showing poor pregnancy rates in women with ovarian insufficiency (3,4,5,8,9). We cannot definitively conclude from the analysis of our data that women with ovarian insufficiency have diminished oocyte quality compared with unaffected women of comparable age. Whether or not young women with occult ovarian insufficiency have poor oocyte quality and greater odds of generating aneuploid embryos is an area of debate with some investigators suggesting a relationship (61,62,63,64) and others refuting this (65,66,67).
The association between telomerase activity and IVF outcomes was also tested. All of the outcomes measured (Table 5) were more favorable in subjects with telomerase activity than in those without it. Moreover, FSH values in women with telomerase activity were lower than in those lacking enzyme activity (5.7 and 7.7 IU/liter, respectively; P = 0.04). This finding suggests that subtle differences in biomarkers can have significant molecular correlates.
A strength of this investigation is the restriction of subjects to women age 37 or younger. The choice of this age criterion was based on the desire to investigate a population of cases who we believed were affected with occult ovarian insufficiency prematurely rather than those with physiological ovarian aging. We felt that using 37 as an upper age limit generates a population of cases in which the definition of occult ovarian insufficiency is unambiguous and the interaction of age is minimized.
The cross-sectional nature of this study imparts a limit on our ability to draw conclusions about the timing of changes in telomere homeostasis relative to the onset of ovarian insufficiency. Furthermore, we obtained our molecular exposures of interest from luteinized, terminally differentiated granulosa cells which are morphologically distinct from proliferating granulosa cells in early stage follicles. However, justification for our approach resides in the fact that occult ovarian insufficiency is a rare condition and that using granulosa cells obtained at the time of IVF is an established approach to investigating molecular pathways in infertile women (58,59,60,68,69). In our center, the prevalence of occult ovarian insufficiency in women pursuing IVF has ranged between 8 and 9% in women 37 yr old or less in recent years. Data from the Society for Assisted Reproductive Technology also provide reliable national estimates for the prevalence of occult ovarian insufficiency in infertile women pursuing IVF (categorized as DOR in the statistics compiled by the Centers for Disease Control and Prevention). The prevalence of occult ovarian insufficiency across all age categories has ranged from 3–9% over the past 10 yr (10,70,71,72,73,74,75,76).
In summary, granulosa cell telomeric shortening and diminished telomerase activity are associated with occult ovarian insufficiency in young women. The results of this investigation may have implications for women’s health beyond the realm of fertility outcomes. The follicular atresia that drives ovarian dysfunction is irreversible; only the rate is variable. If young women affected with occult ovarian insufficiency develop and maintain an accelerated rate of follicular loss, they may be at risk for frank ovarian failure and its clinical sequelae at earlier than expected ages. Ultimately, it is hoped that in studying granulosa cell function further, a better understanding of both oocyte quality and molecular reproductive dysfunction can be achieved. Further research aimed at the discovery of exposures that target important molecular pathways in human granulosa cells and that negatively influence granulosa cell competence could be an important step in predicting women at risk for poor reproductive outcomes, refractory infertility, and overt primary ovarian insufficiency.
Acknowledgments
We would like to thank Dr. Jun Wei and Sheila Paul for their contributions to the assays for telomere length and telomere activity. We would like to thank Samantha Bunso, Jennifer Bucci, and Kathy Moosbrugger for their support and assistance with data abstraction and granulosa cell processing. We would also like to thank Dr. Carmen Williams and Dr. Caleb Kallen for their helpful comments on the manuscript.
Footnotes
This work was funded by National Institutes of Health Grants K12 HD043459-04 (to S.B.) and T32 HD040135 (to S.B.), the University of Pennsylvania Research Foundation (to S.B.), and a McCabe Fund Pilot Award (to S.B.).
Disclosure Summary: H.R., S.R., A.S., C.C., and K.B. have no disclosures. S.B. was previously an educational consultant for Serono pharmaceuticals.
First Published Online October 28, 2009
Abbreviations: CI, Confidence interval; DOR, diminished ovarian reserve; IVF, in vitro fertilization; OR, odds ratio; T/S, telomere/single copy gene ratio.
References
- Welt CK 2008 Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 68:499–509 [DOI] [PubMed] [Google Scholar]
- Nelson LM 2009 Primary ovarian insufficiency. N Engl J Med 360:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi AJ, Raynault MF, Bergh PA, Drews MR, Miller BT, Scott Jr RT 2001 Reproductive outcome in patients with diminished ovarian reserve. Fertil Steril 76:666–669 [DOI] [PubMed] [Google Scholar]
- The Practice Committee of the American Society for Reproductive Medicine 2006 Age and infertility in women. Fertil Steril 86:S248–S252 [DOI] [PubMed] [Google Scholar]
- Barnhart K, Osheroff J 1998 FSH as a predictor of fertility potential. Curr Opin Obstet Gynecol 10:227–232 [DOI] [PubMed] [Google Scholar]
- Butts SF, Seifer DB 2009 6 office tests to assess ovarian reserve, and what they tell you. OBG Management 20:29–40 [Google Scholar]
- Bukulmez O, Arici A 2004 Assessment of ovarian reserve. Curr Opin Obstet Gynecol 16:231–237 [DOI] [PubMed] [Google Scholar]
- Toner JP, Philput CB, Jones GS, Muasher SJ 1991 Basal follicle stimulating hormone level is a better predictor of in vitro fertilization outcome than age. Fertil Steril 55:784–791 [DOI] [PubMed] [Google Scholar]
- Muasher SJ, Oehninger S, Simonetti S, Matta J, Ellis LM, Liu HC, Jones GS, Rosenwaks Z 1988 The value of basal and/or stimulated serum gonadotropin levels in prediction of stimulation response and in vitro fertilization outcome. Fertil Steril 50:298–307 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention 2006 Assisted Reproductive Technology (ART) Report. Atlanta: CDC [Google Scholar]
- de Boer EJ, den Tonkelaar I, te Velde ER, Burger CW, Klip H, van Leeuwen FE 2002 Low number of retrieved oocytes at IVF treatment is predictive of early menopause. Fertil Steril 77:978–985 [DOI] [PubMed] [Google Scholar]
- de Boer EJ, den Tonkelaar I, te Velde ER, Burger CW, van Leeuwen FE 2003 Increased risk of early menopausal transition and natural menopause after poor response at first IVF treatment. Hum Reprod 18:1544–1552 [DOI] [PubMed] [Google Scholar]
- Nikolaou D, Templeton A 2004 Early ovarian ageing. Eur J Obstet Gynecol Reprod Biol 113:126–133 [DOI] [PubMed] [Google Scholar]
- Speroff L, Fritz M 2004 Clinical gynecologic endocrinology and infertility. 7th ed. Baltimore: Lippincott, Williams and Wilkins [Google Scholar]
- Laml T, Preyer O, Umek W, Hengstschlager M, Hanzal H 2002 Genetic disorders in premature ovarian failure. Hum Reprod Update 8:483–491 [DOI] [PubMed] [Google Scholar]
- Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, Ristaldi MS, Marzella R, Rocchi M, Nicolino M, Lienhardt-Roussie A, Nivelon A, Verloes A, Schlessinger D, Gasparini P, Bonneau D, Cao A, Pilia G 2001 The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet 27:159–166 [DOI] [PubMed] [Google Scholar]
- Fraser IS, Shearman RP, Smith A, Russell P 1988 An association between blepharophimosis, resistant ovary syndrome and true menopause. Fertil Steril 50:747–751 [DOI] [PubMed] [Google Scholar]
- Sherman SL 2000 Premature ovarian failure in the fragile X syndrome. Am J Med Genet 97:189–194 [DOI] [PubMed] [Google Scholar]
- Lobo RA 2005 Potential options for preservation of fertility in women. N Engl J Med 353:64–73 [DOI] [PubMed] [Google Scholar]
- Tulandi T, Huang JY, Tan SL 2008 Preservation of female fertility. Obstet Gynecol 112:1160–1172 [DOI] [PubMed] [Google Scholar]
- Cramer DW, Harlow BL, Xu H, Fraer C, Barbieri R 1995 Cross-sectional and case controlled analysis of the association between smoking and early menopause. Maturitas 22:79–87 [DOI] [PubMed] [Google Scholar]
- Paszkowski T, Clarke RN, Hornstein MD 2002 Smoking induces oxidative stress inside the graafian follicle. Hum Reprod 17: 921–925 [DOI] [PubMed] [Google Scholar]
- Baker TG 1963 A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B Biol Sci 158:417–433 [DOI] [PubMed] [Google Scholar]
- Gondos B, Bhiraleus P, Hobel CJ 1971 Ultrastructural observations on germ cells in human fetal ovaries. Am J Obstet Gynecol 110:644–652 [DOI] [PubMed] [Google Scholar]
- The Committee on Gynecologic Practice of the American College of Obstetricians and Gynecologists and the Practice Committee of the American Society for Reproductive Medicine 2008 Age-related fertility decline: a committee opinion. Fertil Steril 90:S154–S155 [DOI] [PubMed] [Google Scholar]
- Rolaki A, Drakakis P, Millingos S, Loutradis D, Makrigiannakis A 2005 Novel trends in follicular development, atresia and corpus luteum regression: a role for apoptosis. Reprod Biomed Online 11:93–103 [DOI] [PubMed] [Google Scholar]
- Vaskivuo TE, Tapanainen JS 2003 Apoptosis in the human ovary. Reprod Biomed Online 6:24–35 [DOI] [PubMed] [Google Scholar]
- ACOG Committee on Genetics 2006 Screening for fragile X syndrome. ACOG Committee Opinion No. 338. Obstet Gynecol 107:1483–1485 [DOI] [PubMed] [Google Scholar]
- Sybert VP, McCauley E 2004 Turner’s syndrome. N Engl J Med 351:1227–1238 [DOI] [PubMed] [Google Scholar]
- de Lange T 2002 Protection of mammalian telomeres. Oncogene 21:532–540 [DOI] [PubMed] [Google Scholar]
- Saldanha SN, Andrews LG, Tollefsbol TO 2003 Assessment of telomere length and factors that contribute to its stability. Eur J Biochem 270:389–403 [DOI] [PubMed] [Google Scholar]
- Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, Meyne J, Ratliff RL, Wu JR 1988 A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA 85:6622–6626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird DM, Kipling D 2004 The extent and significance of telomere loss with age. Ann NY Acad Sci 1019:265–268 [DOI] [PubMed] [Google Scholar]
- Fossel M 1998 Telomerase and the aging cell: implications for human health. JAMA 279:1732–1735 [DOI] [PubMed] [Google Scholar]
- Buys CH 2000 Telomeres, telomerase and cancer. N Engl J Med 342:1282–1283 [DOI] [PubMed] [Google Scholar]
- Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA 1999 p53 deficiency rescues the adverse effects of telomerase loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97:527–538 [DOI] [PubMed] [Google Scholar]
- Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T 1999 p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 283:1321–1325 [DOI] [PubMed] [Google Scholar]
- Zhang X, Mar V, Zhou W, Harrington L, Robinson MO 1999 Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev 13:2388–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, Greider CW 1990 Telomeres shorten during ageing of human fibroblasts. Nature 354:458–460 [DOI] [PubMed] [Google Scholar]
- Shawi M, Autexier C 2008 Telomerase, senescence and aging. Mech Ageing Dev 129:3–10 [DOI] [PubMed] [Google Scholar]
- Hahn WC 2003 Role of telomeres and telomerase in the pathogenesis of human cancer. J Clin Oncol 21:2034–2043 [DOI] [PubMed] [Google Scholar]
- Flores I, Cayuela ML, Blasco MA 2005 Effects of telomerase and telomere length on epidermal stem cell behavior. Science 309:1253–1256 [DOI] [PubMed] [Google Scholar]
- Sarin KY, Cheung P, Gilison D, Lee E, Tennen RI, Wang E, Artandi MK, Oro AE, Artandi SE 2005 Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature 436:1048–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW 1994 Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011–2015 [DOI] [PubMed] [Google Scholar]
- Wright DL, Jones EL, Mayer JF, Oehninger S, Gibbons WE, Lanzendorf SE 2001 Characterization of telomerase activity in the human oocyte and preimplantation embryo. Mol Hum Reprod 7:947–955 [DOI] [PubMed] [Google Scholar]
- Liu L, Bailey SM, Okuka M, Muñoz P, Li C, Zhou L, Wu C, Czerwiec E, Sandler L, Seyfang A, Blasco MA, Keefe DL 2007 Telomere lengthening early in development. Nat Cell Biol 9:1436–1441 [DOI] [PubMed] [Google Scholar]
- Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW 1996 Telomerase activity in human germline and embryonic tissues and cells. Dev Genet 18:173–179 [DOI] [PubMed] [Google Scholar]
- Lavranos TC, Mathis JM, Latham SE, Kalionis B, Shay JW, Rodgers RJ 1999 Evidence for ovarian granulosa stem cells: telomerase activity and localization of the telomerase RNA component in bovine ovarian follicles. Biol Reprod 61:358–366 [DOI] [PubMed] [Google Scholar]
- Esposito MA, Coutifaris C, Barnhart KT 2002 A moderately elevated day 3 FSH concentration has limited predictive value, especially in younger women. Hum Reprod 17:118–123 [DOI] [PubMed] [Google Scholar]
- Cawthon RM 2002 Telomere measurement by quantitative PCR. Nucleic Acids Res 30:e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getliffe KM, Al Dulaimi D, Martin-Ruiz C, Holder RL, von Zglinicki T, Morris A, Nwokolo CU 2005 Lymphocyte telomere dynamics and telomerase activity in inflammatory bowel disease: effect of drugs and smoking. Aliment Pharmacol Ther 21:121–131 [DOI] [PubMed] [Google Scholar]
- Broberg K, Björk J, Paulsson K, Höglund M, Albin M 2005 Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. Carcinogenesis 26:1263–1271 [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz C, Saretzki G, Petrie J, Ladhoff J, Jeyapalan J, Wei W, Sedivy J, von Zglinicki T 2004 Stochastic variation in telomere shortening rate causes heterogeneity of human fibroblast replicative life span. J Biol Chem 279:17826–17833 [DOI] [PubMed] [Google Scholar]
- Gil ME, Coetzer TL 2004 Real-time quantitative PCR of telomere length. Mol Biotechnol 27:169–172 [DOI] [PubMed] [Google Scholar]
- Liu W, Zhu GJ 2003 Expression of telomerase in human ovarian luteinized granulosa cells and its relationship to ovarian function. Zhonghua Fu Chan Ke Za Zhi 38:402–404 [PubMed] [Google Scholar]
- Hanna CW, Bretherick KL, Gair JL, Fluker MR, Stephenson MD, Robinson WP 2009 Telomere length and reproductive aging. Hum Reprod 24:1206–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydos SE, Elhan AH, Tükün A 2005 Is telomere length one of the determinants of reproductive life span? Arch Gynecol Obstet 272:113–116 [DOI] [PubMed] [Google Scholar]
- Seifer DB, Charland C, Berlinsky D, Penzias AS, Haning Jr RV, Naftolin F, Barker BE 1993 Proliferative index of human lutenized granulosa cells varies as a function of ovarian reserve. Am J Obstet Gynecol 169:1531–1535 [DOI] [PubMed] [Google Scholar]
- Seifer DB, Gardiner AC, Ferreira KA, Peluso JJ 1996 Apoptosis as a function of ovarian reserve in women undergoing in vitro fertilization. Fertil Steril 66:593–598 [DOI] [PubMed] [Google Scholar]
- Van Deerlin PG, Cekleniak N, Coutifaris C, Boyd J, Strauss 3rd JF 1997 Evidence for the oligoclonal origin of the granulosa cell population of the mature human follicle. J Clin Endocrinol Metab 82:3019–3024 [DOI] [PubMed] [Google Scholar]
- Hofmann GE, Khoury J, Thie J 2000 Recurrent pregnancy loss and diminished ovarian reserve. Fertil Steril 74:1192–1195 [DOI] [PubMed] [Google Scholar]
- Gürbüz B, Yalti S, Ozden S, Ficicioglu C 2004 High basal estradiol level and FSH/LH ratio in unexplained recurrent pregnancy loss. Arch Gynecol Obstet 270:37–39 [DOI] [PubMed] [Google Scholar]
- Trout SW, Seifer DB 2000 Do women with unexplained recurrent pregnancy loss have higher day 3 serum FSH and estradiol values? Fertil Steril 74:335–337 [DOI] [PubMed] [Google Scholar]
- Nasseri A, Mukherjee T, Grifo JA, Noyes N, Krey L, Copperman AB 1999 Elevated day 3 serum follicle stimulating hormone and/orestradiol may predict fetal aneuploidy. Fertil Steril 71:715–718 [DOI] [PubMed] [Google Scholar]
- Thum MY, Abdalla HI, Taylor D 2008 Relationship between women’s age and basal follicle-stimulating hormone levels with aneuploidy risk in in vitro fertilization treatment. Fertil Steril 90:315–321 [DOI] [PubMed] [Google Scholar]
- Weghofer A, Barad D, Li J, Gleicher N 2007 Aneuploidy rates in embryos from women with prematurely declining ovarian function: a pilot study. Fertil Steril 88:90–94 [DOI] [PubMed] [Google Scholar]
- Massie JA, Burney RO, Milki AA, Westphal LM, Lathi RB 2008 Basal follicle stimulating hormone as a predictor of fetal aneuploidy. Fertil Steril 90:2351–2355 [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Christenson LK, McAllister JM, Strauss 3rd JF 2002 Growth differentiation factor-9 inhibits 3′ 5′-adenosine monophosphate-stimulated steroidogenesis in human granulosa and theca cells. J Clin Endocrinol Metab 87:2849–2856 [DOI] [PubMed] [Google Scholar]
- Christenson LK, Stouffer RL, Strauss 3rd JF 2001 Quantitative analysis of the hormone-induced hyperacetylation of histone H3 associated with the steroidogenic acute regulatory protein gene promoter. J Biol Chem 276:27392–27399 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention 1999 Assisted Reproductive Technology (ART) Report. Atlanta: CDC [Google Scholar]
- Centers for Disease Control and Prevention 2000 Assisted Reproductive Technology (ART) Report. Atlanta: CDC [Google Scholar]
- Centers for Disease Control and Prevention 2001 Assisted Reproductive Technology (ART) Report. Atlanta: CDC [Google Scholar]
- Centers for Disease Control and Prevention 2002 Assisted Reproductive Technology (ART) Report. Atlanta: CDC [Google Scholar]
- Centers for Disease Control and Prevention 2003 Assisted Reproductive Technology (ART) Report. Atlanta: CDC [Google Scholar]
- Centers for Disease Control and Prevention 2004 Assisted Reproductive Technology (ART) Report. Atlanta: CDC [Google Scholar]
- Centers for Disease Control and Prevention 2005 Assisted Reproductive Technology (ART) Report. Atlanta: CDC [Google Scholar]