Abstract
Context: Anorexia nervosa (AN) and functional hypothalamic amenorrhea (HA) are associated with low bone density, anxiety, and depression. Women with AN and HA have elevated cortisol levels. Significant hypercortisolemia, as in Cushing’s disease, causes bone loss. It is unknown whether anxiety and depression and/or cortisol dysregulation contribute to low bone density in AN or HA.
Objective: Our objective was to investigate whether hypercortisolemia is associated with bone loss and mood disturbance in women with HA and AN.
Design and Setting: We conducted a cross-sectional study in a clinical research center.
Participants: We studied 52 women [21 healthy controls (HC), 13 normal-weight women with functional HA, and 18 amenorrheic women with AN].
Outcome Measures: Serum samples were measured every 20 min for 12 h overnight and pooled for average cortisol levels. Bone mineral density (BMD) was assessed by dual-energy x-ray absorptiometry (DXA) at anteroposterior and lateral spine and hip. Hamilton Rating Scales for Anxiety (HAM-A) and Depression (HAM-D) were administered.
Results: BMD was lower in AN and HA than HC at all sites and lower in AN than HA at the spine. On the HAM-D and HAM-A, AN scored higher than HA, and HA scored higher than HC. Cortisol levels were highest in AN, intermediate in HA, and lowest in HC. HAM-A and HAM-D scores were associated with decreased BMD. Cortisol levels were positively associated with HAM-A and HAM-D scores and negatively associated with BMD.
Conclusions: Hypercortisolemia is a potential mediator of bone loss and mood disturbance in these disorders.
In a study of healthy women, normal-weight women with functional hypothalamic amenorrhea, and amenorrheic women with anorexia nervosa, higher 12-hour overnight pooled cortisol levels were associated with lower bone mineral density and increased scores of anxiety and depressive symptoms.
Anorexia nervosa (AN), a psychiatric disorder predominantly affecting young women, is characterized by restrictive eating resulting in extremely low body weight, amenorrhea, and bone loss (1). Functional hypothalamic amenorrhea (HA) in normal-weight women, prevalent in the same age group, leads to less severe decreases in bone mineral density (BMD) (2,3). Both groups of patients are hypoestrogenemic; the greater bone loss in AN is thought to result from nutritional deficiencies and additional endocrine abnormalities (1). Altered hypothalamic-pituitary-adrenal (HPA) dynamics and hypercortisolemia have been reported in AN (4,5,6,7) and HA (8,9,10) and may play an important role. Significant elevations in cortisol, for example in Cushing’s syndrome or after administration of high-dose glucocorticoids, are known to cause bone loss through multiple mechanisms including promotion of osteoclastic bone resorption over osteoblastic bone formation. Although there is some evidence linking cortisol to decreased markers of bone formation in HA (3) and AN (3,4), and to BMD in AN (11), a clear relationship between cortisol dysregulation and bone loss in these populations has not been established.
HPA dysregulation is also a feature of major depression (12,13) and anxiety disorders (14,15), and there is evidence that it may underlie symptomatology (16,17). There is increasing data establishing major depression as a risk factor for osteoporosis (18,19,20,21,22). It is not known whether patients with anxiety are more susceptible to osteoporosis. Anxiety and depression are more common in HA (23) and AN (24,25,26,27), but whether relative hypercortisolemia is a mechanism underlying bone loss is unclear.
We therefore investigated the link between hypercortisolemia, psychiatric symptoms, and bone loss in women with HA and AN. We hypothesized that relatively higher levels of cortisol in AN compared with HA could be a contributing factor for increased bone loss and symptoms of anxiety and depression in this group.
Subjects and Methods
Subjects
We studied 52 women [21 healthy controls (HC), 13 normal-weight women with functional HA, and 18 amenorrheic women with AN]. All subjects were recruited from the community through advertisements and referrals from healthcare providers.
Subjects with AN met DSM-IV criteria (28) including intense fear of gaining weight, emphasis on body shape, weight less than 85% of ideal body weight (IBW) as determined by the 1983 Metropolitan Life tables (29), and lack of menses for at least three consecutive months.
Subjects with HA were 90–110% of IBW and reported amenorrhea for at least three consecutive months. Exclusion criteria included polycystic ovarian syndrome, hyperprolactinemia, premature ovarian failure, and history of AN.
Healthy female volunteers were 90–110% of IBW and reported regular menstrual periods. HC had no history of amenorrhea, disordered eating, or significant anxiety or depression.
All subjects had normal thyroid function tests. Subjects were excluded if they had an alanine aminotransferase greater than 90 U/liter, a serum creatinine greater than 4.5 mg/dl, any condition known to affect bone metabolism other than HA or AN, or history of diabetes mellitus. Additional exclusion criteria included active abuse of drugs or alcohol, use of medication known to affect bone metabolism, or cortisol levels within 3 months (including estrogen), use of depot medroxyprogesterone within 6 months, use of a bisphosphonate within a year, and pregnancy or breastfeeding within 6 months of the study.
Methods
This study was approved by the Institutional Review Boards of Partners Health Care, Inc., and Massachusetts Institute of Technology. Written informed consent was obtained from all subjects before any procedures. All subjects were admitted to the General Clinical Research Center of Massachusetts General Hospital for an outpatient screening visit and an inpatient overnight visit.
At the screening visit, height, weight, and elbow breadth were measured by research dietitians, blood was drawn for screening laboratory tests, and a comprehensive history and physical exam were performed. Exercise patterns and alcohol intake were assessed. Percent IBW was calculated as described above. Body mass index (BMI) was obtained by dividing the weight in kilograms by the square of height in meters. Frame size was determined by comparing elbow breadth to race-specific norms derived from the United States Health and Nutritional Examination Survey I (30).
During the inpatient overnight visit, percent IBW and BMI were reevaluated. Medical history and physical exam were performed. BMD and body composition were assessed by dual-energy x-ray absorptiometry (Hologic 4500; Hologic, Inc., Waltham, MA). This technique has a precision of 0.01 g/cm2 at the lumbar spine and 3% for fat mass (31). Study staff administered the Hamilton Rating Scale for Depression (HAM-D) (32) and Hamilton Rating Scale for Anxiety (HAM-A) (33). An iv catheter was placed, and subjects were allowed to acclimate to their rooms for at least 2 h, followed by frequent sampling of blood every 20 min from 2000–0800 h. Blood draws were performed by experienced research nurses at the Clinical Research Center with identical procedures between patients and controls. Subjects were asked to fast starting at 2000 h. Subjects were allowed to sleep through the night and were not woken up by the nurses. All healthy controls presented for their overnight visit during the follicular phase of their menstrual cycle.
Biochemical analysis
Serum was stored at −80 C until analysis. Overnight serum samples were pooled for average cortisol levels. Serum cortisol was measured by chemiluminescent microparticle immunoassay (Architect System; Abbot Diagnostics, Abbott Park, IL). The intraassay coefficient of variation (CV) was reported for three levels ranging from 2.1–4.8%, and the total CV was reported for three levels, ranging from 3.9–7.7%. The sensitivity was 0.8 μg/dl. Fasting serum IGF-I levels were measured by chemiluminescent immunometric assay (Immulite 2000; Diagnostics Products Corp., Los Angeles, CA). The intraassay CV was reported for six levels ranging from 2.3–3.9%, and the total CV was reported for six levels, ranging from 3.7–8.1%. The sensitivity was 20 ng/ml. Fasting leptin levels were measured by radioimmunoassay (LINCO Research, a division of Millipore, Inc., St. Charles, MO). The intraassay CV ranged from 3.4–8.3% and the interassay CV ranged from 3.6–6.3%. The sensitivity was 0.5 ng/ml.
Data analysis
JMP Statistical Discoveries (version 5.01; SAS Institute, Inc., Cary, NC) was used for statistical analyses. Clinical characteristics, hormone levels, BMD and psychiatric measures were compared using ANOVA. Multiple comparisons were controlled for using the Tukey-Kramer test. Univariate regression analysis was used to investigate the associations between cortisol levels and BMD as well as psychiatric measures. Stepwise regression analysis was performed to further investigate determinants of BMD and psychiatric scores. Statistical significance was defined as a two-tailed P value < 0.05. Data are reported as mean ± sem.
Results
Patient characteristics
Patient characteristics are presented in Table 1. Subjects in each group did not differ in age. As expected, AN had lower mean BMI, percent IBW, and percent fat compared with HC. BMI and percent IBW in HA were higher than AN but lower than HC. There was a trend toward lower percent fat in HA compared with HC and significantly higher percent fat in HA compared with AN. Age of menarche was not significantly different between groups. AN reported a mean of 58.9 ± 14.9 months of amenorrhea; this was not statistically different from the mean of 33.2 ± 9.0 months of amenorrhea reported by HA. By design, HC had no history of amenorrhea. Identified etiological factors for HA included exercise (eight subjects), weight loss (three subjects), and stress (eight subjects). Cortisol levels in those who had HA attributable to exercise did not significantly differ from those who had HA due to other causes. IGF-I levels were lower in AN than HA. Leptin levels were higher in HC than HA and lowest in AN. However, there were no significant differences in leptin levels after controlling for BMI or body fat. Groups did not differ in mean hours of sleep during the frequent sampling procedure.
Table 1.
Baseline characteristics
| HC, n = 21 | HA, n = 13 | AN, n = 18 |
P values
|
|||
|---|---|---|---|---|---|---|
| HC vs. HA | HC vs. AN | HA vs. AN | ||||
| Age (yr) | 27.1 ± 1.5 | 27.3 ± 1.6 | 25.5 ± 1.4 | 0.93 | 0.45 | 0.41 |
| BMI (kg/m2) | 22.6 ± 0.4 | 21.0 ± 0.4 | 18.2 ± 0.2 | 0.01a | <0.0001a | <0.0001a |
| % IBW | 99.3 ± 1.3 | 93.4 ± 1.4 | 79.8 ± 0.9 | 0.02a | <0.0001a | <0.0001a |
| % Fat (DXA) | 26.7 ± 1.0 | 24.1 ± 0.9 | 17.9 ± 0.8 | 0.08 | <0.0001a | <0.0001a |
| Age of menarche (yr) | 12.8 ± 0.3 | 12.7 ± 0.4 | 13.2 ± 0.3 | 0.69 | 0.41 | 0.29 |
| Months amenorrhea | 0 | 33.2 ± 9.0 | 58.9 ± 14.9 | <0.0001 | <0.0001a | 0.19 |
| Hours exercise/wk | 3.8 ± 0.5 | 5.7 ± 0.9 | 7.7 ± 1.5 | 0.06 | 0.01a | 0.28 |
| No. alcoholic drinks/wk | 1.8 ± 0.5 | 3.2 ± 0.7 | 1.5 ± 0.5 | 0.08 | 0.66 | 0.04 |
| IGF-I (ng/ml) | 228.7 ± 18.1 | 249.7 ± 22.5 | 187.5 ± 17.5 | 0.48 | 0.12 | 0.04 |
| Leptin (ng/ml) | 9.1 ± 0.8 | 6.3 ± 0.8 | 2.8 ± 0.5 | 0.03a | <0.0001a | 0.0006a |
Results are means ± sem.
P < 0.05 after correcting for multiple comparisons using Tukey-Kramer.
Mean overnight serum cortisol levels
Pooled 12-h overnight blood samples for cortisol are reported in Table 2. HA and AN had higher mean cortisol levels than HC. There was a trend toward higher mean levels of cortisol in AN compared with HA.
Table 2.
Cortisol, BMD, and psychiatric measures
| HC | HA | AN |
P values
|
|||
|---|---|---|---|---|---|---|
| HC vs. HA | HC vs. AN | HA vs. AN | ||||
| Cortisol | ||||||
| Mean 12-h pooled cortisol (μg/dl) | 7.6 ± 0.4 | 9.4 ± 0.9 | 11.2 ± 0.9 | 0.04 | <0.0001a | 0.09 |
| Bone | ||||||
| BMD (g/cm2) | ||||||
| AP spine | 1.04 ± 0.02 | 0.92 ± 0.03 | 0.83 ± 0.03 | 0.005a | <0.0001a | 0.05 |
| Lateral spine | 0.83 ± 0.02 | 0.72 ± 0.02 | 0.63 ± 0.03 | 0.001a | <0.0001a | 0.02a |
| Total hip | 0.99 ± 0.03 | 0.85 ± 0.02 | 0.80 ± 0.03 | 0.002a | <0.0001a | 0.19 |
| Z-scores | ||||||
| AP spine | −0.12 ± 0.19 | −1.04 ± 0.29 | −1.86 ± 0.28 | 0.01a | <0.0001a | 0.06 |
| Lateral spine | 0.38 ± 0.23 | −1.02 ± 0.27 | −2.18 ± 0.33 | 0.0006a | <0.0001a | 0.02 |
| Total hip | 0.31 ± 0.23 | −0.72 ± 0.19 | −1.15 ± 0.22 | 0.004a | <0.0001a | 0.17 |
| Psychiatric measures | ||||||
| HAM-D | 1.1 ± 0.4 | 6.6 ± 1.2 | 13.3 ± 1.2 | <0.0001a | <0.0001a | 0.0007a |
| HAM-A | 1.5 ± 0.5 | 7.5 ± 1.1 | 11.3 ± 1.3 | <0.0001a | <0.0001a | 0.05a |
Results are means ± sem.
P < 0.05 after correcting for multiple comparisons using Tukey-Kramer.
BMD
BMD and Z-scores are reported in Table 2 and Fig. 1. HA and AN had lower mean BMD and Z-scores than HC at all sites. Mean BMD and Z-scores were lower in AN than HA at the anteroposterior (AP) and lateral spine but not the hip. Fourteen percent of HC had BMD at one site or more that was greater than 1 sd below the norm based on age, sex, and ethnicity (Z-score less than −1.0), and 0% of HC had Z-scores less than −2.0. Sixty-nine percent of HA had Z-scores of less than −1.0, and 31% had Z-scores less than −2.0 at one site or more. Eighty-nine percent of AN had Z-scores of less than −1.0, and 44% of AN had Z-scores less than −2.0 at one site or more.
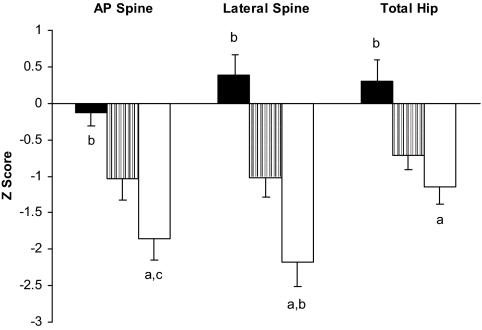
Figure 1.
BMD. AN (white) and HA (gray) had lower mean Z-scores than HC (black) at the AP spine (−1.86 ± 0.28 vs. −1.04 ± 0.29 vs. −0.12 ± 0.19, P < 0.01), lateral spine (−2.18 ± 0.33 vs. −1.02 ± 0.27 vs. 0.38 ± 0.23, P ≤ 0.0006) and total hip (−1.15 ± 0.22 vs. −0.72 ± 0.19 vs. 0.31 ± 0.23, P < 0.004). Mean Z-scores were significantly lower in AN than HA at the lateral spine (P = 0.02), and there was a trend toward lower mean Z-scores in AN compared with HA at the AP spine (P = 0.06). a, P < 0.0001 vs. HC; b, P ≤ 0.02 vs. HA; c, P = 0.06 vs. HA.
Psychiatric measures
Results of psychiatric measures are reported in Table 2. Women with AN had higher mean scores than HA on the HAM-D and HAM-A, and women with HA had higher mean scores on these measures than HC. A score of 7 or below on the HAM-D was considered normal and above 7 abnormal (34). On that basis, the mean HAM-D score of 13 for AN was above the normal range; the mean HAM-D score of 7 for HA was at the high end of the normal range; and the mean HAM-D score of 1 for HC was normal. On the HAM-D, 83% of AN had abnormal scores; only three subjects had normal scores. In contrast, 31% of those with HA had abnormal scores, and 69% were normal. All HC scored in the normal range on the HAM-D. A HAM-A score below 18 was considered normal. All HC, all HA, and all but two AN had HAM-A scores in the normal range.
Correlations between cortisol, BMD, and psychiatric measures
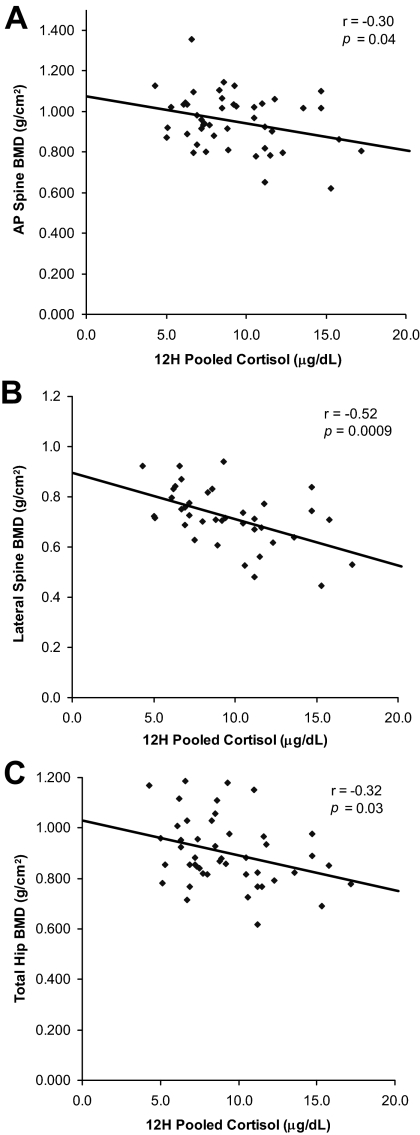
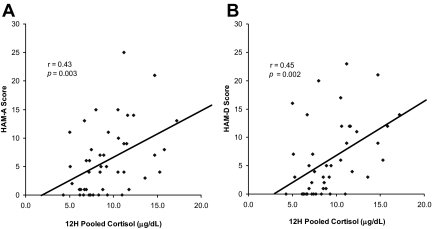
As shown in Table 3, HAM-D and HAM-A scores were negatively associated with BMD at all sites. Figure 2 presents univariate correlations between 12-h overnight serum cortisol levels and BMD. Cortisol levels were negatively associated with BMD at the AP and lateral spine as well as the hip. We entered 12-h overnight cortisol, months of HA, age, and IGF-I levels into stepwise regression models. At the AP spine, 9% of the variance of BMD was attributable to cortisol (P = 0.05). At the lateral spine, 27% of the variance of BMD was attributable to cortisol and an additional 5% to months of HA, explaining 32% of the total variance. At the hip, 18% of the variance of BMD was attributable to months of HA and an additional 5% to cortisol, explaining 23% of the total variance. Figure 3 shows the relationship between 12-h overnight serum cortisol levels and psychiatric measures. There were strong associations between cortisol levels and scores on the HAM-D and HAM-A.
Table 3.
Relationship between psychiatric measures and BMD
| r | P value | |
|---|---|---|
| HAM-D | ||
| AP spine | −0.50 | 0.0002 |
| Lat spine | −0.47 | 0.002a |
| Hip | −0.56 | <0.0001 |
| HAM-A | ||
| AP spine | −0.38 | 0.005 |
| Lat spine | −0.38 | 0.01a |
| Hip | −0.46 | 0.0007 |
After controlling for 12-h cortisol levels, no longer significant.
Figure 2.
Univariate correlations between 12-h pooled cortisol levels and BMD. Cortisol levels negatively predicted BMD at the AP spine (A) (r = −0.30; P = 0.04), lateral spine (B) (r = −0.52; P = 0.0009), and total hip (C) (r = −0.32; P = 0.03).
Figure 3.
Univariate correlations between 12-h pooled cortisol levels and psychiatric measures. Cortisol levels showed strong associations with HAM-A (A) (r = 0.43; P = 0.003) and HAM-D (B) (r = 0.45; P = 0.002) scores.
Discussion
This study identifies normal-weight women with HA as an intermediate phenotype between healthy menstruating women and those with AN in terms of severity of hypercortisolemia, mood disturbance, and bone loss. Importantly, cortisol levels were strongly associated with scores of anxiety and depressive symptoms and negatively correlated with BMD. This implicates cortisol as a possible mediator of dysregulated mood and bone loss in these disorders. Alternatively, psychiatric symptomatology in AN and HA may increase cortisol levels, precipitating bone loss.
In a study of 49 amenorrheic women (30 with AN and 19 with HA) matched for age of menarche and duration of amenorrhea, and 30 healthy controls, Grinspoon et al. (3) showed that T-scores at the AP spine and hip were lower in HA compared with healthy controls and lowest in those with AN. Our study confirms decreased BMD in these amenorrheic groups of patients, with more severe bone loss in AN. Although hypercortisolemia is considered a potential mediator of AN-induced bone loss, there are few studies documenting an association between cortisol and low bone density in this population. Biller et al. (11) reported a negative correlation between 24-h urine free cortisol corrected for creatinine clearance and BMD at the spine in females aged 14–42 yr with AN. Misra et al. (4) performed overnight frequent sampling of serum for cortisol levels in adolescent girls with AN and found that cortisol secretion was an inverse predictor of bone formation markers. In a study of women with AN and functional HA, Grinspoon et al. (3) found an inverse relationship between urine free cortisol in amenorrheic women and osteocalcin, a marker of bone formation. Our investigation showed higher levels of overnight pooled serum cortisol in women with AN and HA compared with eumenorrheic healthy controls and a trend toward higher levels in AN compared with HA. There were strong negative associations between cortisol levels and BMD at the AP spine, lateral spine, and hip. In a stepwise regression model that included total months of amenorrhea, age, and IGF-I levels, 12-h pooled cortisol was the best predictor of variability of BMD at the AP and lateral spine. Together, these data support the role of cortisol in the moderate and severe bone loss associated with HA and AN, respectively. Hypercortisolemia may explain the lack of efficacy of estrogen replacement in restoring BMD in these disorders (35,36).
Anxiety and depression are recognized features of Cushing’s and potential side effects of exogenous glucocorticoids; symptoms improve with normalization of corticosteroid levels (37,38). HPA dysregulation is well established in major depression and may mediate symptoms, in part through limbic glucocorticoid receptor and CRH receptor type I activation (16,17). Normalization of response to dexamethasone-CRH testing, a measure of cortisol dynamics, may herald clinical improvement in major depression and has been advocated as an early surrogate marker for drug efficacy (13,16). Blockade of cortisol synthesis by medications like ketoconazole and metyrapone has been shown to improve symptoms of depression (39,40). Although less robust, there is also evidence of abnormal cortisol regulation and hypercortisolemia in anxiety disorders (14,15). Patients with AN have high rates of comorbid anxiety and depression (24,25,26,27), and HA has been associated with anxiety and depressive symptoms as well as vulnerability to stress (23,41). However, it is unknown whether these psychiatric symptoms in AN and HA are due to cortisol dysregulation. Previous research shows that cognitive behavioral therapy can restore ovulatory function in women with functional HA, highlighting the important role of cognition and attitudes in driving endocrine function (42). Likely, the relationship between mood and HPA function is bidirectional. Although this study cannot determine causality, our results clearly demonstrate a link between hypercortisolemia and scores of anxiety and depressive symptoms in these populations. The more modest psychiatric symptoms in HA compared with AN may be due to less significant hypercortisolemia. It is also possible that cortisol dysregulation is the fundamental disorder underlying a disease spectrum with more severe symptomatology seen in AN compared with HA.
In recent years, depression has been identified as a potential risk factor for bone loss (20,21,22). A study of 45 females aged 13–23 yr with AN found more severe bone loss in those subjects with greater depressive symptoms (43). In premenopausal women with depression, Altindag et al. (19) reported that cortisol levels, but not HAM-D scores, negatively correlated with BMD. There is limited research investigating the relationship between anxiety and bone. In a study of 207 adolescent girls, Dorn et al. (44) showed an association between anxiety symptoms and total body bone mineral content as well as hip bone mineral content and BMD in a Caucasian subset of 125 girls. The Israeli National Health Interview Survey of more than 2000 men and women found a correlation between generalized anxiety disorder and self-reported diagnosis of osteoporosis, independent of depression (45). We showed that scores of anxiety and depressive symptoms were both negatively associated with BMD. This is consistent with previous data linking depression to bone loss and also identifies anxiety symptomatology as a marker for increased risk of decreased BMD. Notably, this study suggests clinical relevance to HAM-A scores of anxiety symptoms within the normal range, because only two subjects had abnormal scores.
The relationship between cortisol, anxiety and depressive symptoms, and bone is complex. One possibility is that hypercortisolemia drives psychiatric symptoms and bone loss in HA and AN. Alternatively, HA and AN may result in anxiety and depression, leading to elevated cortisol levels and, ultimately, bone loss. Likely, the link between HPA axis hyperactivity and disordered mood in these disorders is bidirectional. This is a cross-sectional study, and therefore causality cannot be established.
In summary, we studied the relationship between cortisol dysregulation and clinical manifestations of AN and HA, specifically low BMD and psychiatric symptoms. We demonstrated that there is an inverse correlation between cortisol levels and bone mass as well as anxiety and depressive symptom scores. Hypothalamic amenorrhea represents an intermediate phenotype between healthy women and those with AN. It is unclear whether hypercortisolemia is secondary or underlies the psychiatric pathology in these disorders. Further investigation of cortisol dysregulation and its relationship to bone loss and psychiatric comorbidities in AN and HA is warranted.
Acknowledgments
We thank the nurses and bionutritionists at Massachusetts General Hospital and the patients who participated in the study.
Footnotes
This work was supported by the following grants: Investigator-initiated grant from Bioenvision, National Institutes of Health Grants M01 RR01066 and UL1 RR025758, and the Clinical Investigator Training Program, Harvard/MIT Health Sciences and Technology-Beth Israel Deaconess Medical Center, in collaboration with Pfizer Inc. and Merck & Co.
Disclosure Summary: The authors have no conflicts to declare.
First Published Online October 16, 2009
Abbreviations: AN, Anorexia nervosa; AP, anteroposterior; BMD, bone mineral density; BMI, body mass index; CV, coefficient of variation; HA, hypothalamic amenorrhea; HAM-A, Hamilton Rating Scale for Anxiety; HAM-D, Hamilton Rating Scale for Depression; HC, healthy control; HPA, hypothalamic-pituitary-adrenal; IBW, ideal body weight.
References
- Lawson EA, Klibanski A 2008 Endocrine abnormalities in anorexia nervosa. Nat Clin Pract Endocrinol Metab 4:407–414 [DOI] [PubMed] [Google Scholar]
- Biller BM, Coughlin JF, Saxe V, Schoenfeld D, Spratt DI, Klibanski A 1991 Osteopenia in women with hypothalamic amenorrhea: a prospective study. Obstet Gynecol 78:996–1001 [PubMed] [Google Scholar]
- Grinspoon S, Miller K, Coyle C, Krempin J, Armstrong C, Pitts S, Herzog D, Klibanski A 1999 Severity of osteopenia in estrogen-deficient women with anorexia nervosa and hypothalamic amenorrhea. J Clin Endocrinol Metab 84:2049–2055 [DOI] [PubMed] [Google Scholar]
- Misra M, Miller KK, Almazan C, Ramaswamy K, Lapcharoensap W, Worley M, Neubauer G, Herzog DB, Klibanski A 2004 Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab 89:4972–4980 [DOI] [PubMed] [Google Scholar]
- Putignano P, Dubini A, Toja P, Invitti C, Bonfanti S, Redaelli G, Zappulli D, Cavagnini F 2001 Salivary cortisol measurement in normal-weight, obese and anorexic women: comparison with plasma cortisol. Eur J Endocrinol 145:165–171 [DOI] [PubMed] [Google Scholar]
- Licinio J, Wong ML, Gold PW 1996 The hypothalamic-pituitary-adrenal axis in anorexia nervosa. Psychiatry Res 62:75–83 [DOI] [PubMed] [Google Scholar]
- Lawson EA, Misra M, Meenaghan E, Rosenblum L, Donoho DA, Herzog D, Klibanski A, Miller KK 2009 Adrenal glucocorticoid and androgen precursor dissociation in anorexia nervosa. J Clin Endocrinol Metab 94:1367–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller BM, Federoff HJ, Koenig JI, Klibanski A 1990 Abnormal cortisol secretion and responses to corticotropin-releasing hormone in women with hypothalamic amenorrhea. J Clin Endocrinol Metab 70:311–317 [DOI] [PubMed] [Google Scholar]
- Berga SL, Daniels TL, Giles DE 1997 Women with functional hypothalamic amenorrhea but not other forms of anovulation display amplified cortisol concentrations. Fertil Steril 67:1024–1030 [DOI] [PubMed] [Google Scholar]
- Suh BY, Liu JH, Berga SL, Quigley ME, Laughlin GA, Yen SS 1988 Hypercortisolism in patients with functional hypothalamic-amenorrhea. J Clin Endocrinol Metab 66:733–739 [DOI] [PubMed] [Google Scholar]
- Biller BMK, Saxe V, Herzog DB, Rosenthal DI, Holzman S, Klibanski A 1989 Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinal Metab 68:548–554 [DOI] [PubMed] [Google Scholar]
- Heuser I, Yassouridis A, Holsboer F 1994 The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res 28:341–356 [DOI] [PubMed] [Google Scholar]
- Kunugi H, Ida I, Owashi T, Kimura M, Inoue Y, Nakagawa S, Yabana T, Urushibara T, Kanai R, Aihara M, Yuuki N, Otsubo T, Oshima A, Kudo K, Inoue T, Kitaichi Y, Shirakawa O, Isogawa K, Nagayama H, Kamijima K, Nanko S, Kanba S, Higuchi T, Mikuni M 2006 Assessment of the dexamethasone/CRH test as a state-dependent marker for hypothalamic-pituitary-adrenal (HPA) axis abnormalities in major depressive episode: a multicenter study. Neuropsychopharmacology 31:212–220 [DOI] [PubMed] [Google Scholar]
- Mantella RC, Butters MA, Amico JA, Mazumdar S, Rollman BL, Begley AE, Reynolds CF, Lenze EJ 2008 Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology 33:773–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudieu I, Beluche I, Norton J, Boulenger JP, Ritchie K, Ancelin ML 2008 Abnormal reactions to environmental stress in elderly persons with anxiety disorders: evidence from a population study of diurnal cortisol changes. Journal of Affective Disorders 106:307–313 [DOI] [PubMed] [Google Scholar]
- Ising M, Künzel HE, Binder EB, Nickel T, Modell S, Holsboer F 2005 The combined dexamethasone/CRH test as a potential surrogate marker in depression. Prog Neuropsychopharmacol Biol Psychiatry 29:1085–1093 [DOI] [PubMed] [Google Scholar]
- Müller MB, Wurst W 2004 Getting closer to affective disorders: the role of CRH receptor systems. Trends Mol Med 10:409–415 [DOI] [PubMed] [Google Scholar]
- Schweiger U, Deuschle M, Körner A, Lammers CH, Schmider J, Gotthardt U, Holsboer F, Heuser I 1994 Low lumbar bone mineral density in patients with major depression. Am J Psychiatry 151:1691–1693 [DOI] [PubMed] [Google Scholar]
- Altindag O, Altindag A, Asoglu M, Gunes M, Soran N, Deveci Z 2007 Relation of cortisol levels and bone mineral density among premenopausal women with major depression. Int J Clin Pract 61:416–420 [DOI] [PubMed] [Google Scholar]
- Schweiger U, Weber B, Deuschle M, Heuser I 2000 Lumbar bone mineral density in patients with major depression: evidence of increased bone loss at follow-up. Am J Psychiatry 157:118–120 [DOI] [PubMed] [Google Scholar]
- Michelson D, Stratakis C, Hill L, Reynolds J, Galliven E, Chrousos G, Gold P 1996 Bone mineral density in women with depression. N Engl J Med 335:1176–1181 [DOI] [PubMed] [Google Scholar]
- Yazici KM, Akinci A, Sütçü A, Ozçakar L 2003 Bone mineral density in premenopausal women with major depressive disorder. Psychiatry Res 117:271–275 [DOI] [PubMed] [Google Scholar]
- Fava GA, Trombini G, Grandi S, Bernardi M, Evangelisti LP, Santarsiero G, Orlandi C 1984 Depression and anxiety associated with secondary amenorrhea. Psychosomatics 25:905–908 [DOI] [PubMed] [Google Scholar]
- Herzog DB, Keller MB, Sacks NR, Yeh CJ, Lavori PW 1992 Psychiatric comorbidity in treatment-seeking anorexics and bulimics. J Am Acad Child Adolesc Psychiatry 31:810–818 [DOI] [PubMed] [Google Scholar]
- Braun DL, Sunday SR, Halmi KA 1994 Psychiatric comorbidity in patients with eating disorders. Psychol Med 24:859–867 [DOI] [PubMed] [Google Scholar]
- Pollice C, Kaye WH, Greeno CG, Weltzin TE 1997 Relationship of depression, anxiety, and obsessionality to state of illness in anorexia nervosa. Int J Eat Disord 21:367–376 [DOI] [PubMed] [Google Scholar]
- Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K 2004 Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry 161:2215–2221 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association 2000 Diagnostic and statistical manual of mental disorders. 4th ed. Washington, D.C.: American Psychiatric Association [Google Scholar]
- 1983 Metropolitan height and weight tables. Stat Bull 64:2–9 [PubMed] [Google Scholar]
- Frisancho AR, Flegel PN 1983 Elbow breadth as a measure of frame size for US males and females. Am J Clin Nutr 37:311–314 [DOI] [PubMed] [Google Scholar]
- Mazess RB, Barden HS, Bisek JP, Hanson J 1990 Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr 51:1106–1112 [DOI] [PubMed] [Google Scholar]
- Hamilton M 1960 A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M 1959 The assessment of anxiety states by rating. Br J Med Psychol 32:50–55 [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Chelminski I, Posternak M 2004 A review of studies of the Hamilton depression rating scale in healthy controls: implications for the definition of remission in treatment studies of depression. J Nerv Ment Dis 192:595–601 [DOI] [PubMed] [Google Scholar]
- Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC 1995 The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab 80:898–904 [DOI] [PubMed] [Google Scholar]
- Vescovi JD, Jamal SA, De Souza MJ 2008 Strategies to reverse bone loss in women with functional hypothalamic amenorrhea: a systematic review of the literature. Osteoporos Int 19:465–478 [DOI] [PubMed] [Google Scholar]
- Sonino N, Fava GA 2001 Psychiatric disorders associated with Cushing’s syndrome. Epidemiology, pathophysiology and treatment. CNS Drugs 15:361–373 [DOI] [PubMed] [Google Scholar]
- Warrington TP, Bostwick JM 2006 Psychiatric adverse effects of corticosteroids. Mayo Clin Proc 81:1361–1367 [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Chan T, Manfredi F, Raum W, Johnson R, Canick J 1999 Antiglucocorticoid treatment of depression: double-blind ketoconazole. Biol Psychiatry 45:1070–1074 [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI 1999 Treatment of depression with antiglucocorticoid drugs. Psychosom Med 61:698–711 [DOI] [PubMed] [Google Scholar]
- Giles DE, Berga SL 1993 Cognitive and psychiatric correlates of functional hypothalamic amenorrhea: a controlled comparison. Fertil Steril 60:486–492 [PubMed] [Google Scholar]
- Berga SL, Marcus MD, Loucks TL, Hlastala S, Ringham R, Krohn MA 2003 Recovery of ovarian activity in women with functional hypothalamic amenorrhea who were treated with cognitive behavior therapy. Fertil Steril 80:976–981 [DOI] [PubMed] [Google Scholar]
- Konstantynowicz J, Kadziela-Olech H, Kaczmarski M, Zebaze RM, Iuliano-Burns S, Piotrowska-Jastrzebska J, Seeman E 2005 Depression in Anorexia Nervosa: A Risk Factor for Osteoporosis. J Clin Endocrinol Metab 90:5382–5385 [DOI] [PubMed] [Google Scholar]
- Dorn LD, Susman EJ, Pabst S, Huang B, Kalkwarf H, Grimes S 2008 Association of depressive symptoms and anxiety with bone mass and density in ever-smoking and never-smoking adolescent girls. Arch Pediatr Adolesc Med 162:1181–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhsen K, Lipsitz J, Garty-Sandalon N, Gross R, Green MS 2008 Correlates of generalized anxiety disorder: independent of comorbidity with depression: findings from the first Israeli National Health Interview Survey (2003–2004). Soc Psychiatry Psychiatr Epidemiol 43:898–904 [DOI] [PubMed] [Google Scholar]