Abstract
Context: The association of short sleep duration with cortisol secretion has not been thoroughly examined in large community dwelling populations and the relative importance of short sleep duration and sleep disturbance is unclear.
Objective: The objective of the study was to assess the relationships between self-reported sleep duration, sleep disturbance, and salivary cortisol secretion.
Design: This was a cross-sectional analysis using data from phase 7 (2002–2004) of the Whitehall II study. Sleep disturbances were assessed using a modified version of the Jenkins Scale.
Setting: The occupational cohort was originally recruited in 1985–1989.
Participants: Analyses included 2751 participants with complete cortisol measures and who collected their first sample within 15 min of waking, were not on medication affecting cortisol secretion, and had complete information for all covariates.
Outcome Measure: Six saliva samples were taken on waking, waking + 0.5, 2.5, 8, and 12 h and bedtime for the assessment of the cortisol awakening response and the slope in cortisol secretion across the day.
Results: In mutually adjusted analyses, both sleep duration and disturbances were independently associated with a flatter diurnal slope in cortisol secretion, such that evening cortisol secretion was raised in those reporting short sleep duration and high sleep disturbance. Short sleep duration was also associated with the cortisol awakening response. These effects were independent of a number of covariates, including waking time on day of sampling and stress on the day of cortisol assessment.
Conclusion: Short sleep duration and increased sleep disturbances are independently associated with diurnal slope in cortisol secretion of a large community-based cohort of middle-aged men and women.
Subjective measures of sleep disturbance and short sleep duration are independently associated with diurnal cortisol secretion in a large-scale community-based population of middle-aged adults.
The usual amount of sleep per night has been declining among adults for more than a generation. In the United States, the median sleep time in adults was 8 h per night in 1959. By 2002, the adult median sleep time had decreased to 7 h per night (1,2). Sleep reduction is also described in European populations but data suggest more minor reductions in mean sleep duration than suggested for American populations (3).
Much of the reduction in sleep time has been attributed to voluntary sleep restriction, (2), suggesting that short sleep duration may not necessarily reflect sleep disorder. However, sleep problems and/or sleep reduction are associated with a number of morbidities including total mortality, cardiovascular disease, and type 2 diabetes (4,5,6). In the Whitehall II study, both short and long sleep duration and declines in sleep duration are associated with increased mortality. Short sleep duration is associated with cardiovascular disease-related mortality and long sleep duration with noncardiovascular-related mortality (7). The hypothalamic-pituitary-adrenal axis (HPA) has been proposed as a potential mechanism by which parameters of sleep might be associated with poor health (8). However, reports of the association of sleep parameters with cortisol secretion are mixed (9,10,11,12,13,14) and are complicated by issues concerning the nature of self-reported sleep parameters vs. objectively measured sleep parameters. For example, it has been suggested that self-reported short sleep duration reflects sleep disturbance and stress (12). The implications of this are that associations of sleep duration with health outcomes are due to sleep disturbance and stress. The HPA axis is associated with a number of adverse health behaviors including smoking (15) and intermediary markers of health including physical symptoms (16).
Sleep disturbance increases with age (17); however, the associations of short sleep duration and sleep disturbance with cortisol secretion have not been examined in large community-based studies of middle-aged and older people. Examination of the HPA axis has been hampered by a strong diurnal rhythm in activity. Thus, cortisol measures have a diurnal profile that is characterized by a substantial increase in cortisol concentration after awakening, peaking at about 30 min [the cortisol awakening response (CAR)], and a subsequent decline over the remainder of the day. Additionally, it has become apparent that these elements of cortisol secretion may have different correlates and predictors (18,19,20,21), suggesting the need to make separate assessment of these components of the diurnal rhythm. Cortisol can be assessed in saliva samples, because salivary cortisol and plasma cortisol are highly correlated (22), to capture this diurnal profile in a relatively noninvasive way.
We examined the association of self-reported sleep duration and sleep disturbance with the release of cortisol, measured from saliva samples collected throughout the day. Initial analyses will examine whether the effects of sleep duration and sleep disturbance are associated with the diurnal release of cortisol. We will examine the CAR and slope in diurnal cortisol across the day separately. Second, we examined whether the effects of sleep duration and sleep disturbance are mediated by each other or are independent of each other and additional covariates, including self-reported stress on day of cortisol assessment.
Subjects and Methods
Participants
The cohort was initially recruited between 1985 and 1988 (phase 1) from 20 London-based civil service departments, 10308 people participated (response 73%). Eight phases of the study have been completed, and details of the cohort profile have been reported elsewhere (23). This study used data from phase 7 (2002–2004) when cortisol was measured. The number of participants at phase 7 was 6941; the collection of saliva samples was instigated part way through phase 7, and of those eligible for cortisol assessment, 4609 (90.1%) returned samples. Ethical approval for the Whitehall II study was obtained from the University College London Medical School Committee on the Ethics of Human Research.
Data
The protocol for saliva collection has been described previously (15). Briefly, participants who agreed were asked to provide six saliva samples over the course of a normal weekday unrested at waking (sample 1), waking + 30 min (sample 2), waking + 2.5 h (sample 3), waking + 8 h (sample 4), waking + 12 h (sample 5), and bedtime (sample 6). Participants were instructed to record the time of sample collection in an instruction booklet (the logbook) and record information on wake time and stressful events on the day of sampling. The salivettes and logbook were then returned via mail. Salivette devices were centrifuged at 3000 rpm for 5 min, resulting in a clear supernatant of low viscosity. Salivary cortisol levels were measured using a commercial immunoassay with chemiluminescence detection (CLIA; IBL-Hamburg, Hamburg, Germany). The lower concentration limit of this assay is 0.44 nmol/liter; intra- and interassay coefficients of variance were less than 8%. Any sample greater than 50 nmol/liter was repeated.
Variables
Outcome
Cortisol values outside of 3 sd from the mean were removed (values above 75 nmol/liter, n = 27). Despite this, there was a skewed distribution of cortisol values and cortisol data were transformed by natural logarithm. For exposures, hours since awakening was based on respondents recording waking time and time of sampling in the log book.
Hours slept
These were based on calculating the difference between respondents record of time at which they fell asleep the night before sample collection and waking time on day of sample collection in the log book divided into five categories (5 h or less, 5–6 h, 6–7 h, 7–8 h, and 8 h or more).
Sleep disturbance
The four-item Jenkins sleep questionnaire (24) was used with an additional question: “how often in the past month did you have disturbed or restless sleep?” The response categories for each item are coded as follows: 1, not at all; 2, 1–3 d; 3, 4–7 d; 4, 8–14 d; 5, 15–20 d; 6, 21–31 d. A summary scale was calculated, resulting in a scale from 1 (no sleep disturbance) to 6 (sleep disturbed most nights) (Chronbach α = 0.83). For the analysis, this scale was grouped into quartiles. Initial analyses with all four quartiles of the sleep disturbance scale revealed little difference in cortisol between the first three quartiles, so for further analyses the first three quartiles grouped together (no sleep disturbance) and the fourth quartile grouped separately (sleep disturbed).
Confounders
Age, sex, current or last known employment grade, awakening time, smoking status, waist circumference, and Medical Outcome Study Short Form 36 (SF-36) Physical and Mental Component scores (25) were examined. Other confounders were also analyzed (Center for Epidemiological Studies Depression (CES-D) depression score, blood pressure, fasting blood glucose, objective functioning (walking speed, lung function), body mass index, physical activity (energy expenditure in metabolic equivalent task hours)], but because they were not associated with log cortisol in linear analyses and they had missing values, they were removed from the final models. Stress on the day of cortisol sampling was measured by questions on whether the participant had experienced a stressful event and if so how stressful this was. Responses were grouped into binary categories: no/not at all and somewhat/very stressful.
Analysis
Missing data
Some samples were not assayed due to insufficient saliva or loss of sample in transport between London and Germany, resulting in 4469 respondents with at least one cortisol sample. Of these respondents, 4155 had six cortisol samples, 3900 were not on corticosteroid medications, and 3313 took their first sample within 15 min of awakening without doing any activity first. Most participants reported saliva sampling within a short period of the allocated time. Restricting analyses to the second sample collected within 15–45 min of awakening, the third sample within 1–4 h since awakening, the fourth sample within 5–10 h since awakening, and the fifth sample within 10–14 h since awakening, and eliminating outliers resulted in 2979 respondents. Taking into account missing covariates, the analytical sample size was 2751 respondents.
Models
As the cortisol samples are clustered within individual respondents, multilevel models were analyzed. Two separate models were considered. The first analyzed the log cortisol data from the first two samples (on awakening and after 30 min) to examine the morning rise in cortisol. The second analyzed the first, third, fourth, fifth, and bedtime samples to examine the day slope in cortisol.
In each of these models, log cortisol was regressed on hours since awakening and the sleep variables (hours slept and sleep disturbance), with dummy variables for the cortisol samples. The interaction terms between the sleep variables and hours since awakening were included in the models to test whether the cortisol profiles in the morning or later in the day differed between the sleep groups as time increased. Confounders (age, sex, current or last known employment grade, awakening time, smoking status, waist circumference, SF-36 physical and mental health) were added to the final models to see whether the effects of the sleep variables on log cortisol were independent of confounding effects. Two-way interaction effects between the sleep variables and all the confounding variables were also examined.
All the analyses were carried out using MLwin software (Centre for Multilevel Modelling, University of Bristol, Bristol, UK).
Results
Table 1 displays descriptive statistics of all the variables in the analyses. We describe a typical diurnal pattern of cortisol secretion, increased depressive symptoms, more disadvantaged social position, and increased reporting of stress associated with measures of short sleep duration.
Table 1.
Participant characteristics at phase 7 (2002–2004) of the Whitehall II Study
| Sleep Disturbance scale
|
Reported sleep hours the previous night
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | n | High | n | <5 h | n | 5–6 h | n | 6–7 h | n | 7–8 h | n | 9+ h | n | |
| Sample 1 cortisol (nmol/liter) | 15.9 | 2279 | 16.0 | 673 | 12.3 | 147 | 15.2 | 381 | 16.3 | 869 | 16.1 | 1041 | 16.8 | 402 |
| Sample 2 cortisol (nmol/liter) | 24.0 | 2279 | 24.0 | 673 | 24.4 | 147 | 24.2 | 381 | 25.4 | 869 | 23.7 | 1041 | 22.2 | 402 |
| Sample 3 cortisol (nmol/liter) | 9.8 | 2279 | 9.8 | 673 | 13.2 | 147 | 11.5 | 381 | 10.2 | 869 | 9.2 | 1041 | 8.1 | 402 |
| Sample 4 cortisol (nmol/liter) | 6.2 | 2279 | 6.3 | 673 | 7.2 | 147 | 7.0 | 381 | 6.4 | 869 | 5.9 | 1041 | 5.3 | 402 |
| Sample 5 cortisol (nmol/liter) | 2.9 | 2279 | 3.1 | 673 | 4.2 | 147 | 3.4 | 381 | 3.0 | 869 | 2.8 | 1041 | 2.6 | 402 |
| Sample 6 cortisol (nmol/liter) | 2.3 | 2279 | 2.6 | 673 | 3.2 | 147 | 2.5 | 381 | 2.4 | 869 | 2.1 | 1041 | 2.4 | 402 |
| Awakening time | 06:50 | 2279 | 06:49 | 673 | 05:59 | 147 | 06:13 | 381 | 06:34 | 869 | 07:03 | 1041 | 07:44 | 402 |
| Time since awakening: sample 1 | 00:02 | 2279 | 00:02 | 673 | 00:01 | 147 | 00:01 | 381 | 00:02 | 869 | 00:02 | 1041 | 00:02 | 402 |
| Time since awakening: sample 2 | 00:32 | 2279 | 00:32 | 673 | 00:32 | 147 | 00:32 | 381 | 00:32 | 869 | 00:32 | 1041 | 00:32 | 402 |
| Time since awakening: sample 3 | 02:36 | 2279 | 02:35 | 673 | 02:37 | 147 | 02:36 | 381 | 02:36 | 869 | 02:36 | 1041 | 02:35 | 402 |
| Time since awakening: sample 4 | 08:08 | 2279 | 08:07 | 673 | 08:09 | 147 | 08:09 | 381 | 08:08 | 869 | 08:07 | 1041 | 08:08 | 402 |
| Time since awakening: sample 5 | 12:09 | 2279 | 12:10 | 673 | 12:13 | 147 | 12:10 | 381 | 12:10 | 869 | 12:09 | 1041 | 12:08 | 402 |
| Time since awakening: sample 6 | 16:28 | 2279 | 16:18 | 673 | 17:47 | 147 | 17:20 | 381 | 16:45 | 869 | 16:05 | 1041 | 15:12 | 402 |
| Sex (% men) | 77.8% | 2279 | 62.1% | 673 | 73.5% | 147 | 75.9% | 381 | 74.3% | 869 | 76.8% | 1041 | 65.2% | 402 |
| Age (yr) | 61.0 | 2279 | 61.4 | 673 | 60.7 | 147 | 59.9 | 381 | 60.4 | 869 | 61.7 | 1041 | 62.1 | 402 |
| Empl grade (percent in lowest grade) | 7.8% | 2271 | 11.6% | 670 | 14.4% | 146 | 9.5% | 379 | 8.7% | 863 | 7.3% | 1036 | 9.8% | 399 |
| SF-36 mental component score | 54.3 | 2243 | 47.1 | 664 | 50.9 | 142 | 52.2 | 372 | 52.6 | 854 | 53.1 | 1021 | 52.3 | 392 |
| SF-36 physical component score | 50.2 | 2243 | 45.4 | 664 | 47.3 | 142 | 49.3 | 372 | 49.3 | 854 | 49.8 | 1021 | 47.3 | 392 |
| Waist circumference (cm) | 91.4 | 2274 | 90.8 | 669 | 94.4 | 146 | 92.0 | 380 | 90.9 | 866 | 91.1 | 1040 | 91.1 | 400 |
| Cigarette smoker (%) | 7.8% | 2276 | 8.3% | 673 | 13.0% | 146 | 9.5% | 380 | 9.6% | 866 | 6.0% | 1037 | 5.3% | 400 |
| Fasting glucose (mmol/liter) | 5.4 | 2232 | 5.5 | 656 | 5.4 | 142 | 5.6 | 379 | 5.4 | 850 | 5.4 | 1018 | 5.5 | 386 |
| Systolic BP (mm Hg) | 128.0 | 2278 | 128.0 | 672 | 129.8 | 147 | 127.0 | 381 | 127.1 | 868 | 128.4 | 1041 | 129.1 | 401 |
| Diastolic BP (mm Hg) | 74.2 | 2278 | 74.5 | 672 | 75.5 | 147 | 73.7 | 381 | 74.1 | 868 | 74.1 | 1041 | 75.0 | 401 |
| CES-D depression (%) | 7.1% | 2168 | 32.4% | 638 | 19.7% | 137 | 15.7% | 363 | 11.8% | 830 | 11.6% | 994 | 14.4% | 376 |
| Stress (% somewhat/very stressed) | 27.6% | 2070 | 43.3% | 624 | 40.3% | 134 | 31.9% | 348 | 31.8% | 799 | 30.5% | 957 | 25.8% | 361 |
BP, Blood pressure.
Morning rise in cortisol
Table 2 displays the unadjusted and adjusted models of log cortisol on awakening and 30 min later, regressed on the sleep variables (hours slept and sleep disturbance), and hours since awakening. In model 1, the average linear slope of log cortisol on awakening was 0.51, although this increase in log cortisol reduces later on because of the negative coefficient (−0.31) for the squared term of hours since awakening. The 30-min log CAR can be estimated from the model by adding the estimates for the dummy variables for cortisol samples 1 and 2 (not shown in Table 2) to half of the sum of the estimates for the linear and squared terms for hours since awakening. This results in an estimated CAR of 5.8 nmol/liter. The nonsignificant interaction between sleep disturbance and hours since awakening suggests that sleep disturbance does not affect awakening and morning levels of cortisol.
Table 2.
Log cortisol on awakening and after 30 min (morning rise in cortisol) regressed on hours slept, sleep disturbance, hours since awakening, and potential confounders
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Hours since awakening | 0.506 (0.260) | 1.051 (0.279) | 1.050 (0.283) |
| P value | 0.54 | <0.01 | <0.01 |
| Hours since awakening squared | −0.308 (0.265) | −0.275 (0.263) | −0.288 (0.268) |
| P value | 0.25 | 0.29 | 0.28 |
| Sleep disturbance | |||
| Not sleep disturbed (first three quartiles) | 0.000 | 0.000 | |
| Sleep disturbed (top quartile) | 0.011 (0.027) | 0.019 (0.025) | |
| P value | 0.68 | 0.43 | |
| Interaction of hours since awakening and sleep disturbance | |||
| Not sleep disturbed or at awakening | 0.000 | ||
| Sleep disturbed and 1 h since awakening | −0.063 (0.058) | ||
| P value | 0.27 | ||
| Hours slept previous night | |||
| Less than 5 h | 0.000 | 0.000 | |
| 5–6 h | 0.266 (0.059) | 0.250 (0.060) | |
| 6–7 h | 0.359 (0.054) | 0.336 (0.055) | |
| 7–8 h | 0.372 (0.053) | 0.326 (0.056) | |
| 9 or more hours | 0.418 (0.058) | 0.383 (0.062) | |
| P value | <0.01 | <0.01 | |
| Interaction of hours since awakening and hours slept previous night | |||
| Less than 5 h slept or at awakening | 0.000 | 0.000 | |
| 5–6 h slept and 1 h since awakening | −0.489 (0.125) | −0.480 (0.127) | |
| 6–7 h slept and 1 h since awakening | −0.562 (0.115) | −0.554 (0.1170 | |
| 7–8 h slept and 1 h since awakening | −0.702 (0.113) | −0.666 (0.116) | |
| 9+ hours slept and 1 h since awakening | −0.905 (0.124) | −0.887 (0.127) | |
| P value | <0.01 | <0.01 | |
Models 1 and 2 adjusted for cortisol sample (dummy variables for whether the cortisol came from first or second sample). Model 3 additionally adjusted for age, sex, employment grade, waist circumference, smoking status, awakening time, SF-36 mental and physical component scores.
In model 2 (Table 2), more hours slept the previous night are associated with higher log cortisol on awakening. However, as the morning progresses, the effect of longer slept hours on cortisol is increasingly negative (indicated by the negative coefficient for the interaction between hours since awakening and hours slept). This is displayed in Fig. 1, which shows that although respondents who reported sleep of 5 h or less have the lowest log cortisol on awakening, 40 min later they have the highest log cortisol. The morning rise in cortisol is steepest for this group. Longer slept hours are associated with a progressively less steep morning rise in cortisol.
Figure 1.
Morning rise in cortisol by hours slept the previous night (estimated from model 2, Table 2).
In model 3 (Table 2), the effect of slept hours on log cortisol are adjusted for potential confounders (sleep disturbance, age, sex, employment grade, awakening time, smoking status, waist circumference, SF-36 physical and mental component scores). The interaction term between hours slept and hours since awakening remains statistically significant, suggesting that fewer hours slept (less than 5 h) is associated with a steeper morning rise in cortisol independent of potential confounders.
Diurnal slope in cortisol secretion
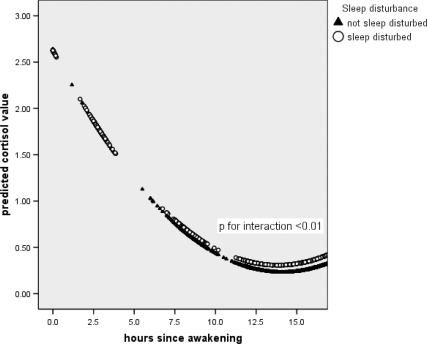
Table 3 displays the unadjusted and adjusted models of log cortisol on awakening, at 2.5 h, 8 h, 12 h later, and at bedtime, regressed on the sleep variables (hours slept and sleep disturbance) and hours since awakening. In model 1, the average linear slope of log cortisol on awakening was −0.34, indicating a decline in cortisol over the day. This decrease tails off later in the day (the squared term of hours since awakening is positive). Although sleep disturbance is associated with lower log cortisol on awakening, this is not statistically significant. However, later on in the day, sleep disturbance is associated with higher levels of cortisol (as indicated by the positive interaction between sleep disturbance and hours since awakening). This is displayed in Fig. 2, which shows that higher levels of log cortisol in those with disturbed sleep becomes apparent around 8 h since awakening, compared with those with undisturbed sleep.
Table 3.
Log cortisol on awakening and after 2.5, 8, and 12 h and bed time (diurnal slope in cortisol) regressed on hours slept, sleep disturbance, hours since awakening, and potential confounders
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Hours since awakening | −0.341 (0.050) | −0.265 (0.051) | −0.293 (0.053) |
| P value | <0.01 | <0.01 | <0.01 |
| Hours since awakening squared | 0.012 (0.002) | 0.009 (0.002) | 0.010 (0.002) |
| P value | <0.01 | <0.01 | <0.01 |
| Sleep disturbance | |||
| Not sleep disturbed (first three quartiles) | 0.000 | 0.000 | |
| Sleep disturbed (top quartile) | −0.011 (0.025) | 0.045 (0.021) | |
| P value | 0.650 | <0.05 | |
| Interaction of hours since awakening and sleep disturbance | |||
| Not sleep disturbed or at awakening | 0.000 | 0.000 | |
| Sleep disturbed and 1 h since awakening | 0.006 (0.002) | 0.006 (0.002) | |
| P value | <0.01 | <0.01 | |
| Hours slept previous night | |||
| Less than 5 h | 0.000 | 0.000 | |
| 5–6 h | 0.097 (0.055) | 0.101 (0.060) | |
| 6–7 h | 0.101 (0.050) | 0.149 (0.056) | |
| 7–8 h | 0.064 (0.050) | 0.117 (0.056) | |
| 9+ h | −0.008 (0.055) | 0.080 (0.064) | |
| P value | <0.05 | <0.01 | |
| Interaction of hours since awakening and hours slept previous night | |||
| Less than 5 h slept or at awakening | 0.000 | 0.000 | |
| 5–6 h slept and 1 h since awakening | −0.021 (0.004) | −0.020 (0.004) | |
| 6–7 h slept and 1 h since awakening | −0.027 (0.004) | −0.026 (0.004) | |
| 7–8 h slept and 1 h since awakening | −0.032 (0.004) | −0.031 (0.004) | |
| 9+ h slept and 1 h since awakening | −0.029 (0.005) | −0.028 (0.005) | |
| P value | <0.01 | <0.01 | |
Models 1 and 2 adjusted for cortisol sample (dummy variables for whether the cortisol came from first/third/fourth/fifth/sixth sample). Model 3 additionally adjusted for age, sex, employment grade, waist circumference, smoking status, awakening time, SF-36 mental and physical component scores.
Figure 2.
Day slope in cortisol by sleep disturbance (estimated from model 1, Table 3).
In model 2 (Table 3), more hours slept the previous night is marginally associated with higher log cortisol on awakening. However, as the day progresses, the effect of longer slept hours on cortisol is increasingly negative. This is displayed in Fig. 3, which shows that respondents who slept for 5 h or less have higher log cortisol later on in the day compared with those who sleep longer. This results in a flatter cortisol profile for those who slept shorter hours.
Figure 3.
Day slope in cortisol by hours slept the previous night (estimated from model 2, Table 3).
In model 3 (Table 3), the effect of sleep disturbance and slept hours on log cortisol are mutually adjusted for each other as well as adjusted for potential confounders (age, sex, employment grade, awakening time, smoking status, waist circumference, SF-36 physical and mental component scores). The statistically significant interaction terms between hours slept and hours since awakening and between sleep disturbance and hours since awakening suggest that the two sleep variables are independently associated with log cortisol. Fewer hours slept (less than 5 h) and sleep disturbance are associated with a flatter diurnal cortisol profile.
Additional potential (we use this term in the tables) confounders (blood pressure, fasting glucose, CES-D on depression and self-reported stress) were also adjusted for in additional analyses for the models reported in Tables 2 and 3. However, adding in these confounders did not change the effects of the sleep variables on cortisol, and because there were missing values for some of these confounders, they were not included in the final models (model 3) reported in Tables 2 and 3. Further analyses also examined two-way interaction effects between the sleep variables as well as between the sleep variables and all the confounding variables. There was little evidence of interaction between sleep hours and sleep disturbance. There was also little evidence that the effect of the sleep variables on cortisol differed by age, gender, social position, obesity, stress, and smoking status.
Discussion
We have shown that in a large population study of middle-aged people, self-reported short sleep duration is associated with both an increased CAR and a shallow slope in diurnal cortisol secretion. Sleep disturbance was associated with a shallow slope in cortisol secretion. The association of sleep duration and cortisol secretion was independent of sleep disturbance and of a variety of covariates, including sex, social position, health behaviors, measures of health, and stressful events on the day of sample collection. Short sleep duration and increased reporting of sleep disturbance were associated with increased evening measures of cortisol, suggesting dysregulated feedback in the HPA axis.
Associations of cortisol secretion with short sleep duration
Our findings that short sleep duration remains associated with cortisol secretion after adjustment for sleep disturbance suggests that short sleep duration per se influences cortisol secretion. This is important if one considers that recent reductions in sleep time are attributed to voluntarily reduced sleep hours rather than involuntary reductions that may be associated with health conditions (2). Sleep disturbance is reportedly associated with lower cortisol levels at awakening (26). Our data demonstrate this for sleep duration and was the main reason that short sleep duration was associated with a large CAR. Evidence suggests that both a small and large CAR is associated with poor functioning of the HPA axis (27). Cortisol secretion shows a typical diurnal pattern such that there is a nadir in cortisol at midnight, which begins to rise 2–3 h later (28). In many cases, those reporting short sleep duration collected both first and last samples early in the morning such that the bedtime collection is made when cortisol may be beginning to rise again. However, it is unlikely that shallow slopes in cortisol are due to an upturn in cortisol at the end of the day because our slopes were not calculated by the difference between waking and bedtime values and increased cortisol was not limited to the final measure of the day. Associations between self-reported sleep duration and cortisol secretion were independent of self-reported sleep disturbance or measures that may reflect chronic stress such as disadvantaged social position (29) or acute stress on the day of sampling suggesting that additional mechanisms are at play.
Association of cortisol secretion with sleep disturbance
Our findings show that increased sleep disturbance is associated with a shallow slope in the release of diurnal cortisol, which is due to raised cortisol in the evening after sleep ascertainment. This effect appears to be independent of, but smaller than, that seen for short sleep duration. In our analyses, we use sleep duration on the night before saliva sample collection, whereas we use sleep disturbance with questions on typical sleep for the previous month. Thus, sleep duration may have been more accurately assessed than sleep disturbance. However, similar effects were apparent when we examine sleep duration using a question about typical sleep duration in the previous month (data not shown). Whereas we used a well-validated scale assessing sleep disturbance, self-reported sleep duration may be a more accurate reflection of poor sleep than self-reported measures of specific sleep difficulties (12). One previous study suggests that forced night time awakenings in older groups are not associated with early morning cortisol levels (30), indicating that sleep disturbance per se may not have a large influence on the CAR. Unexpectedly, in our study, we see no association of cortisol secretion and depressive symptoms, but we do find that fatigue is associated with diminished morning cortisol secretion, no difference in the CAR, and a flat diurnal slope in cortisol (31). However, reanalysis of sleep disturbance after removal of the question on waking up tired failed to make a difference to our conclusions (data not shown).
Mechanisms
The mechanisms by which measures of sleep exert a change in cortisol secretion are not clear. We can hypothesize that there is a common confounder or mediator that underlies short sleep duration or sleep disturbance and increased cortisol secretion. Second, there may be direct association between sleep duration and disturbance and cortisol and thirdly that there is residual confounding.
Short sleep duration, increased sleep disturbance, and flat slopes in cortisol secretion may result from common mechanisms associated with chronic stress. In our analyses we examined a number of factors that may reflect or relate to chronic stress including social position, health behaviors, and measures of mental health and associations remained significant. Additional measures of chronic stress (12) may play a role but were not available in our study. Conversely, McEwen (8) conceptualized sleep debt itself as a stressor. Our findings go some way to support both or either of these notions. Participants reporting short sleep duration on the night before sampling evaluated events as more stressful compared with those not reporting short sleep duration. Despite this, the association of short sleep duration and sleep disturbance remained associated with cortisol secretion.
Evidence of an association of sleep parameters with changes in cortisol secretion is mixed, with studies supporting an association and others not (9,10,11,12,13,16). Whether short sleep duration or sleep disturbance cause changes in cortisol secretion or vice versa in our study cannot be determined from our findings. Early studies suggest that direct administration of glucocorticoids increases time awake (32). It has been suggested that increased peripheral cortisol may be a marker of central noradrenergic function. Increased central noradrenergic activity may promote wakefulness (33). It has been argued that cortisol may exert a synergistic effect in conjunction with other factors to influence sleep (13). We examined the interaction of cortisol, sleep parameters, and a number of factors but found no evidence of interactions.
We cannot discount the effects of residual confounding. We adjusted our associations with a variety of confounders and mediators and found that the association remains significant.
Whereas the effects of sleep duration on cortisol secretion in this cohort were relatively small, acute differences in cortisol concentration of this magnitude have been shown to have a detrimental effect on insulin and glucose function (34,35). The cumulative impact of raised cortisol would be substantial if experienced over a number of years and have a large impact on downstream function. There are few longitudinal studies of the association of cortisol with health; however, flat slopes in cortisol secretion are reportedly associated with increased rates of mortality in patient populations (36). Indicators point to a role for the CAR and development of poor physical health (27); however, this remains to be demonstrated empirically.
Advantages and disadvantages of the study need to be addressed. Our study is large with a high response rate in which the participants are well characterized. However, cortisol is assessed cross-sectionally at phase 7 of the study and is measured only in the day, making it impossible to evaluate total 24 h cortisol exposure. Saliva samples were collected on 1 d only because of participant burden, and this may not be ideal (37). Our population is one of white-collar workers who are healthier than the general population. We are reliant on self-report of sleep parameters, which may be problematic (38); however, our findings suggest that self-reported measures of sleep are biologically meaningful. We are reliant on self-report for time of sample collection. However, our prevalence of late reporting was similar to previously reported rates (39), and data suggest that participants are generally accurate in their recording of this information (40). Missing data on the cortisol samples, cortisol outliers, participants on corticosteroid medication, and who did not follow the cortisol sampling protocols reduced the sample size from 4469 to 2751. Additional analyses was conducted (not shown) including those who did not follow the cortisol sampling protocols with very similar results to the analyses presented in the tables and figures (n = 3558).
In summary, we conclude that short sleep duration and increased sleep disturbance are independently associated with diurnal cortisol secretion in a large community-dwelling population of middle-aged men and women. Further follow-up of this cohort will allow us to examine the role of the HPA axis in the development of morbidities associated with short sleep and sleep disturbance in older groups.
Acknowledgments
The authors thank Clemens Kirschbaum (Dresden University of Technology) for carrying out the cortisol assays. We thank all participating civil service departments and their welfare, personnel, and establishment officers; the Occupational Health and Safety Agency; the Council of Civil Service Unions; all participating civil servants in the Whitehall II study; and all members of the Whitehall II study team.
Footnotes
The Whitehall II study has been supported by grants from the Medical Research Council; Economic and Social Research Council; British Heart Foundation; Health and Safety Executive; Department of Health; Grant HL36310 from the National Heart, Lung, and Blood Institute, National Institutes of Health; Grant AG13196 from the National Institute on Aging, National Institutes of Health; Agency for Health Care Policy Research Grant HS06516; and the John D. and Catherine T. MacArthur Foundation Research Networks on Successful Midlife Development and Socioeconomic Status and Health.
Disclosure Summary: All authors have nothing to disclose.
First Published Online October 22, 2009
Abbreviations: CAR, Cortisol awakening response; CES-D, Center for Epidemiological Studies Depression; HPA, hypothalamic-pituitary-adrenal axis; SF-36, Medical Outcome Study Short Form 36.
References
- Kripke DF, Simons RN, Garfinkel L, Hammond EC 1979 Short and long sleep and sleeping pills. Is increased mortality associated? Arch Gen Psychiatry 36:103–116 [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation. Sleep in America Poll 2003 National Sleep Foundation, http://www.sleepfoundation.org [Google Scholar]
- Kronholm E, Partonen T, Laatikainen T, Peltonen M, Härmä M, Hublin C, Kaprio J, Aro AR, Partinen M, Fogelholm M, Valve R, Vahtera J, Oksanen T, Kivimäki M, Koskenvuo M, Sutela H 2008 Trends in self-reported sleep duration and insomnia-related symptoms in Finland from 1972 to 2005: a comparative review and re-analysis of Finnish population samples. J Sleep Res 17:54–62 [DOI] [PubMed] [Google Scholar]
- Newman AB, Spiekerman CF, Enright P, Lefkowitz D, Manolio T, Reynolds CF, Robbins J 2000 Cardiovascular Health Study Research Group. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. J Am Geriatr Soc 48:115–123 [DOI] [PubMed] [Google Scholar]
- Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR 2002 Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry 59:131–136 [DOI] [PubMed] [Google Scholar]
- Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, Patel S, Hu FB 2003 A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care 26:380–384 [DOI] [PubMed] [Google Scholar]
- Ferrie JE, Shipley MJ, Brunner E, Cappuccio FP, Miller MA, Kumari M, Marmot MG 2007 Sleep duration and change in sleep duration; associations with mortality in the Whitehall II cohort. Sleep 30:1659–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS 2006 Sleep deprivation as a neurobiologic and physiologic stressor: allostasis and allostatic load. Metabolism 55:S20–S23 [DOI] [PubMed] [Google Scholar]
- Leproult R, Copinschi G, Buxton O, Van Cauter E 1997 Sleep loss results in an elevation of cortisol levels the next evening. Sleep 20:865–870 [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Lin HM, Prolo P, Mastorakos G, Vela-Bueno A, Kales A, Chrousos GP 2001 Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab 86: 3787–3794 [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP 2004 Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab 89:2119–2126 [DOI] [PubMed] [Google Scholar]
- Rodenbeck A, Huether G, Rüther E, Hajak G 2002 Interactions between evening and nocturnal cortisol secretion and sleep parameters in patients with severe chronic primary insomnia. Neurosci Lett 324:159–163 [DOI] [PubMed] [Google Scholar]
- Buckley TM, Schatzberg AF 2005 On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab 90:3106–3114 [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Lin HM, Papaliaga M, Calhoun S, Vela-Bueno A, Chrousos GP, Bixler EO 2008 Short sleep duration and obesity: the role of emotional stress and sleep disturbances. Int J Obes (Lond) 32:801–809 [DOI] [PubMed] [Google Scholar]
- Badrick E, Kirschbaum C, Kumari M 2007 The relationship between smoking status and cortisol secretion. J Clin Endocrinol Metab 92:819–824 [DOI] [PubMed] [Google Scholar]
- Wrosch C, Miller GE, Lupien S, Pruessner JC 2008 Diurnal cortisol secretion and 2-year changes in older adults’ physical symptoms: the moderating roles of negative affect and sleep. Health Psychol 27:685–693 [DOI] [PubMed] [Google Scholar]
- Espiritu JR 2008 Aging-related sleep changes. Clin Geriatr Med 24:1–14, v [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C 1997 Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci 61:2539–2549 [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F 2004 The awakening cortisol response: methodological issues and significance. Stress 7:29–37 [DOI] [PubMed] [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT 2006 Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci USA 103:17058–17063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nater UM, Maloney E, Boneva RS, Gurbaxani BM, Lin JM, Jones JF, Reeves WC, Heim C 2008 Attenuated morning salivary cortisol concentrations in a population-based study of persons with chronic fatigue syndrome and well controls. J Clin Endocrinol Metab 93:703–709 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH 1989 Salivary cortisol in psychobiological research: an overview. Neuropsychobiology 22:150–169 [DOI] [PubMed] [Google Scholar]
- Marmot M, Brunner E 2005 Cohort profile: the Whitehall II study. Int J Epidemiol 34:251–256 [DOI] [PubMed] [Google Scholar]
- Jenkins CD, Stanton BA, Niemcryk SJ, Rose RM 1988 A scale for the estimation of sleep problems in clinical research. J Clin Epidemiol 41:313–321 [DOI] [PubMed] [Google Scholar]
- Ware Jr JE, Sherbourne CD 1992 The MOS 36-item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care 30:73–83 [PubMed] [Google Scholar]
- Backhaus J, Junghanns K, Hohagen F 2004 Sleep disturbances are correlated with decreased morning awakening salivary cortisol. Psychoneuroendocrinology 29:1184–1191 [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A 2009 Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol 80:265–278 [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Speigel K 1999 Circadian and sleep control of hormonal secretions. In: Turek FW, Zee P, eds. Regulation of sleep and circadian rhythms. New York: Marcel Decker; 397–425 [Google Scholar]
- Stamatakis KA, Kaplan GA, Roberts RE 2007 Short sleep duration across income, education and race/ethnic groups: population prevalence and growing disparities during 34 years of follow up. Ann Epidemiol 17:948–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettenborn L, Rosenloecher F, Kirschbaum C 2007 No effects of repeated forced wakings during three consecutive nights on morning cortisol awakening responses (CAR): a preliminary study. Psychoneuroendocrinology 32:915–921 [DOI] [PubMed] [Google Scholar]
- Kumari M, Badrick E, Chandola T, Adam EK, Stafford M, Marmot MG, Kirschbaum C, Kivimaki M 2009 Cortisol secretion and fatigue: associations in a community based cohort. Psychoneuroendocrinology (forthcoming). Doi: 10.1016/j.psyneuen.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Gillin JC, Jacobs LS, Fram DH, Snyder F 1972 Acute effect of a glucocorticoid on normal human sleep. Nature 237:398–399 [DOI] [PubMed] [Google Scholar]
- Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti Jr TD, DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW 2000 Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotrophin releasing hormone. Proc Natl Acad Sci USA 97:325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E 1999 Impact of sleep debt on metabolic and endocrine function. Lancet 354:1435–1439 [DOI] [PubMed] [Google Scholar]
- Plat L, Leproult R, L'Hermite-Baleriaux M, Fery F, Mockel J, Polonsky KS, Van Cauter E 1999 Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab 84:3082–3092 [DOI] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D 2000 Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst 92:994–1000 [DOI] [PubMed] [Google Scholar]
- Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D 2007 Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state and trait components. Psychoneuroendocrinology 32:80–86 [DOI] [PubMed] [Google Scholar]
- Bliwise DL, Young TB 2007 The parable of parabola: what the U-shaped curve can and cannot tell us about sleep. Sleep 30:1614–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C 2003 Compliance with saliva sampling protocols: electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom Med 65:313–319 [DOI] [PubMed] [Google Scholar]
- Dockray S, Bhattacharyya MR, Molloy GJ, Steptoe A 2008 The cortisol awakening response in relation to objective and subjective measures of waking in the morning. Psychoneuroendocrinology 33:77–82 [DOI] [PubMed] [Google Scholar]