Abstract
Context: Early postmenopausal women with higher testosterone (T) levels have increased insulin resistance (IR) and cardiovascular risk factors, but whether this translates into increased cardiovascular disease later in life is unknown.
Objective: The objective of the study was to determine whether higher T levels are associated with IR, the metabolic syndrome (MetSyn), and coronary heart disease (CHD) in elderly women.
Design: Total T and free T by equilibrium dialysis were measured using ultrasensitive assays in 344 women aged 65–98 yr enrolled in the Cardiovascular Health Study. Cross-sectional analyses were performed to examine the associations between total and free T and IR, MetSyn, and CHD.
Results: There was a stepwise increase in the homeostasis model assessment of insulin resistance with increasing total (P = 0.0.003) and free T (P = 0.02) level and a corresponding decrease in Quantitative Insulin Sensitivity Check Index (P < 0.001 and P = 0.002, respectively). In adjusted models, higher levels of both total and free T were strongly associated with abdominal obesity and high fasting glucose, the two MetSyn components most strongly linked to IR. After adjustment, women in the top quartile of total T levels had a 3-fold greater odds of MetSyn (odds ratio 3.15, 95% confidence interval 1.57–6.35) than those in the bottom quartile and a 3-fold greater odds of CHD (odds ratio 2.95, 95% confidence interval 1.2–7.3) than those in second quartile, whereas free T was not significantly associated with MetSyn or CHD.
Conclusions: Higher levels of T are associated with IR, MetSyn, and CHD in elderly women. Whether T is a marker or mediator of cardiovascular disease in this population merits further investigation.
Women aged 65 and older with high testosterone levels are more likely to have insulin resistance, metabolic syndrome, and coronary heart disease.
The clinical relevance of testosterone (T) in women over the age of 65 yr is unknown. Many premenopausal women with polycystic ovary syndrome (PCOS) have hyperandrogenism and insulin resistance (IR), and numerous studies have demonstrated a high prevalence of cardiovascular risk factors and the metabolic syndrome (MetSyn) in these women (1). Additional studies in peri- and early postmenopausal women without a known history of PCOS have shown that women with higher levels of T are more likely to have IR, MetSyn, and cardiovascular disease (CVD) (2,3,4,5,6,7,8,9,10,11).
The potential link between higher levels of T and IR has not been extensively examined in elderly women, the group of women with the highest prevalence of MetSyn and the greatest cardiovascular risk (12,13). Using data from the Cardiovascular Health Study (CHS), we have previously shown that levels of T are significantly lower in women aged 65 yr and older than in a reference population of younger women but that the same factors that negatively affect the production of T in younger women (bilateral oophorectomy, estrogen use, and corticosteroid use) also are associated with lower levels of T in women aged 65 yr and older (14). These data suggest that a population shift in T levels occurs with age and that the association between T and cardiovascular risk factors found in younger populations of women should track into later life. However, it is also possible that there is a threshold relationship between T and these factors and that the lower levels found in older women are biologically irrelevant. Finally, it is also possible that the association between T and IR in these older women is similar to that in older men, in whom lower T levels are associated with a greater incidence of MetSyn and CVD (15,16,17,18). Our hypothesis is consistent with the first of these scenarios, that women aged 65 yr and older with higher total and free T levels are more insulin resistant, have a greater prevalence of MetSyn, and more likely to have CVD than those with lower T levels.
Subjects and Methods
Study population
The CHS is a population-based, longitudinal study of risk factors for developing CVDs in 5888 adults aged 65 yr and older (19). Enrollment of an original cohort of 5201 adults occurred between May 1989 and June 1990 and an additional cohort of 687 predominantly African-Americans enrolled in 1992–1993. Eligible individuals were identified from an age- and gender-stratified random sample of the Medicare eligibility rosters in four U.S. communities: Washington County, MD; Pittsburgh, PA; Sacramento County, CA; and Forsyth County, NC. To be eligible, individuals had to be noninstitutionalized, expecting to remain in the area for the following 3 yr, not under active cancer treatment, not wheelchair bound in the home, not requiring a proxy respondent at entry, and capable of providing consent. Household members of the sampled individuals were recruited if eligible. The institutional review boards of all four sites and the coordinating center at the University of Washington (Seattle, WA) approved the study, and all participants gave informed consent.
These analyses are based on data from a random sample of 368 women seen at the 1992–1993 visit. This assessment included a detailed medical history, physical examination, and assessment of health status. Blood was drawn in the morning after a 12-h fast and serum was frozen in −70 C freezers for future investigations (20).
Of 368 women in the original sample, six women were excluded due to insufficient serum for analyzing free T and three extreme outliers were excluded. Nine women taking oral corticosteroids were excluded due to their extremely low T values (14), as were six other women with incomplete data on covariates, yielding 344 subjects for analysis.
Assessment of biochemical measures
Total T concentrations were measured by RIA using iodinated T as a tracer (21). This assay was developed and validated for the low range of T prevalent in HIV-infected (21,22,23) and postmenopausal women (24). The sensitivity, defined as hormone concentration corresponding to 90% percent bound in presence and absence of analyte point, was 0.22 ng/dl (0.008 nmol/liter). The intraassay coefficient of variation (CV) was 8.2%. Interassay CVs were measured in the low, medium, and high female pool and were 13.2% in the medium pool. In other pools, interassay CVs ranged from 13 to 15%. This assay was validated against liquid chromatography-tandem mass spectrometry (23). These measurements demonstrated a correlation of 0.997 between the RIA and liquid chromatography-tandem mass spectrometry measurements. Free T concentrations were measured by a sensitive equilibrium dialysis assay (21), optimized to precisely and accurately measure low concentrations. The sensitivity of this assay is 0.3 pg/ml (1.0 pmol/liter). Ten replicates of low, medium, and high female pools were used to generate intra- and interassay CVs. The respective intraassay CVs for the low, medium, and high female pools were +5.6% for the low pool, +4.6% for the medium pool, and 2.6% for the high pool (21). The interassay CVs were in the 12–15% range. Cross-reactivity of the major steroids (dehydroepiandrosterone, dehydroepiandrosterone sulfate, dihydrotestosterone, and androstenedione) was less than 1%.
Fasting glucose levels were performed on the Kodak Ektachem 700 Analyzer (Eastman Kodak Corp., Rochester, NY). Fasting serum insulin was measured by solid-phase RIA, using serum-based standards (Diagnostic Products Corp., Los Angeles, CA). As previously described (20), fasting total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides were measured directly and standardized according to Centers for Disease Control and Prevention guidelines, with low-density lipoprotein (LDL) cholesterol calculated according to the Friedewald equation (25).
Assessment of covariates
Sociodemographic characteristics included age, race, the number of ovaries removed, and estrogen use. Medication use was determined from examination of medication bottles at the time of the study visit. Blood pressure was determined by the average of duplicate measurements of sitting blood pressure evaluated in the right arm after a 5-min rest. Diabetes was defined as a fasting glucose of 126 mg/dl or greater, 2-h postload glucose of 200 mg/dl or greater, medical history of diabetes, or use of insulin or an oral hypoglycemic medication. Hypertension was defined as systolic blood pressure of 140 mm Hg or greater, diastolic blood pressure of 90 mm Hg or greater, or taking one or more antihypertensive medications.
Ascertainment of outcomes
Insulin resistance was assessed via calculation of homeostasis model assessment insulin resistance (HOMA-IR): (fasting glucose in milligrams per deciliter × insulin in microunits per milliliter)/405 (26) and insulin sensitivity by calculation of the Quantitative Insulin Sensitivity Check Index (QUICKI): 1/(log[fasting glucose in milligrams per deciliter] + log [insulin in microunits per milliliter]) (27).
As initially detailed in the National Cholesterol Education Program Adult Treatment Panel III report (28) and later modified (29), women with three or more of the following criteria were categorized as having the MetSyn: abdominal obesity (waist circumference > 88 cm); hypertriglyceridemia (fasting triglycerides ≥ 150 mg/dl); low HDL cholesterol (HDL < 50 mg/dl); high blood pressure (blood pressure ≥ 130/85 mm Hg or using antihypertensive medication); and high fasting glucose (also known as impaired fasting glucose; fasting glucose ≥ 100 mg or using antidiabetic medication).
Prevalent coronary heart disease (CHD) was defined by the occurrence of angina, myocardial infarction, coronary angioplasty, or coronary artery bypass graft surgery occurring before the blood draw from which T was measured. Provisional diagnoses of CHD were reviewed and adjudicated at periodic meetings of the study’s morbidity and mortality subcommittee, including investigators from each field center and the coordinating center. The full details of the surveillance and ascertainment of events in CHS have been published previously (30).
Statistical analysis
For analytic purposes, T levels were categorized into quartiles. Quartiles were selected for two reasons: there is no reference range for T levels in older women to define high levels, and we wanted to allow for the possibility of a nonlinear relationship. For total T, quartile (Q) 1 was 7.0 ng/dl or less, Q2 was 7.1–15.0 ng/dl, Q3 was 15.1–27.0 ng/dl, and Q4 was 27.0 ng/dl or greater. For free T, Q1 was 1.4 pg/ml or less, Q2 was 1.5–2.0 pg/ml, Q3 was 2.1–3.2 pg/ml, and Q4 was 3.3 pg/ml or greater.
Median HOMA and QUICKI measurements were calculated by quartile of total and free T level, and a trend test was performed to assess statistical significance. Women using insulin or with glucose greater than 200 mg/dl were excluded, yielding 328 women for inclusion in these analyses.
Total and free T levels were compared between women with MetSyn and women without MetSyn in the entire group of 344 women as well as between women with and without each of the individual MetSyn criteria. Student t tests were performed to assess the association between log-transformed total and free T levels and the presence or absence of MetSyn. The prevalence of MetSyn and each of its components were also examined by quartile of T and free T. Age, race, estrogen use, and bilateral ovarian removal, factors that were previously found to be important determinants of total and free T levels in women in this cohort (14), were included in multivariable logistic regression models of total and free T quartiles predicting MetSyn and each of its components. All analyses were initially performed in the total sample and were subsequently repeated excluding women with diabetes mellitus.
Multivariable logistic regression models of total and free T quartiles predicting CHD were adjusted for age, race, smoking, estrogen use, hypertension, and LDL cholesterol. Additional models including log HOMA-IR were performed.
All analyses were performed using SAS (version 8.2; SAS Institute, Cary, NC).
Results
The 344 women ranged in age from 65 to 98 yr, with a mean age of 74.4 yr (Table 1). The mean body mass index (BMI) in our study cohort was 26.9 kg/m2. Nineteen percent were current oral estrogen users. Thirteen percent had diabetes and 30% had impaired fasting glucose. Forty-five percent of our sample met the National Cholesterol Education Program criteria for the MetSyn. Mean total testosterone levels were 19.1 ng/dl (range 1–133 ng/dl) and mean free testosterone levels were 2.8 pg/ml (range 0.3–20.6 pg/ml).
Table 1.
Selected characteristics of the study sample (n = 344)
| Variable | |
|---|---|
| Age (yr) | 74.4 (5.1) |
| Education (yr) | 14.6 (4.0) |
| White (%) | 84 |
| Bilateral oophorectomy (%) | 17 |
| BMI (kg/m2) | 26.9 (5.3) |
| Current estrogen use (%) | 19 |
| Current smoking (%) | 11 |
| Total cholesterol (md/dl) | 217 (39) |
| LDL cholesterol (mg/dl) | 131 (37) |
| HDL cholesterol (mg/dl) | 59 (15) |
| Triglycerides (mg/dl) | 137 (71) |
| Total T (ng/dl) | 19.1 (17.6) |
| Free T (pg/ml) | 2.8 (2.5) |
| Fasting glucose (mg/dl) | 107 (35) |
| Fasting insulin (μIU/ml) | 13 (15) |
| Diabetes (%) | |
| Nondiabetic | 57 |
| Impaired fasting glucose | 30 |
| Diabetes | 13 |
| Systolic blood pressure (mm Hg) | 136 (21) |
| Diastolic blood pressure (mm Hg) | 71 (13) |
| MetSyn (%) | 45 |
| Abdominal obesity | 65 |
| Hypertriglyceridemia | 33 |
| Low HDL cholesterol | 29 |
| High blood pressure | 68 |
| High fasting glucose | 13 |
Data are presented as mean (sd) unless otherwise indicated.
IR
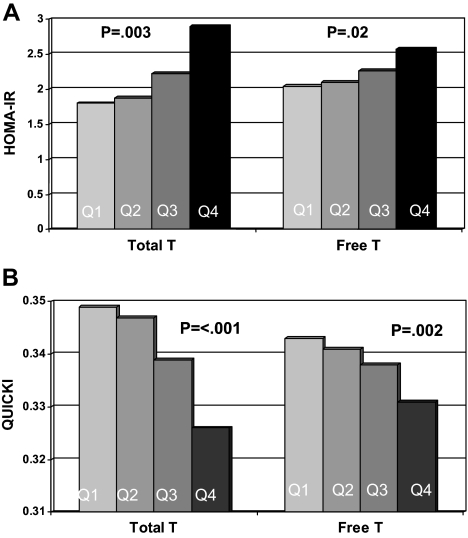
With increasing total and free T levels, there was a stepwise increase in IR, as assessed by HOMA-IR (P = 0.003 and P = 0.02, respectively; Fig. 1A). Likewise, there was a stepwise decrease in insulin sensitivity, as assessed by QUICKI, by quartile of total and free T (P ≤ 0.001 and P = 0.002, respectively; Fig. 1B).
Figure 1.
A, Median HOMA-IR by quartile of total and free T. B, Median QUICKI by total and free T quartiles. Higher HOMA-IR indicates greater insulin resistance, whereas higher QUICKI indicates greater insulin sensitivity. Women using insulin or with glucose greater than 200 mg/dl were excluded; 328 women were included in this analysis.
MetSyn
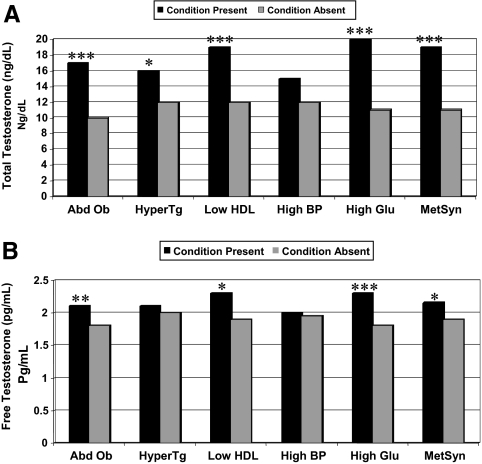
For each criterion, as well as for MetSyn as a whole, total T levels were higher in women in whom the criterion was present compared with those in whom it was absent, reaching statistical significance for all except hypertension (Fig. 2A). A similar pattern was present for free T, although the differences in free T levels between those with and without the criterion were of smaller magnitude than those seen for total T (Fig. 2B). Free T levels were significantly higher in women with MetSyn and in those with the individual components of abdominal obesity, low HDL, and high fasting glucose, but free T levels were not significantly different between those with hypertriglyceridemia or hypertension and those without these two conditions. The findings for total and free T persisted after exclusion of the 43 women with diabetes.
Figure 2.
A, Median total T levels in women with and without MetSyn. B, Median free T levels in women with and without MetSyn. Abd Ob, Abdominal obesity (waist circumference > 88 cm); HyperTG, hypertriglyceridemia (fasting triglycerides ≥ 150 mg/dl); low HDL (HDL cholesterol < 50 mg/dl); high BP, high blood pressure (blood pressure ≥ 130/85 mm Hg or using antihypertensive medication); high Glu, high fasting glucose (fasting glucose ≥ 100 mg or using antidiabetic medication). Multiply by 0.0347 to convert total T concentrations from nanograms per deciliter to nanomoles per liter. Multiply by 3.47 to convert free T concentrations from picograms per milliliter to picomoles per liter. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
When we examined the prevalence of the MetSyn by quartile of total T levels, we found a strong relationship between total T levels and MetSyn in crude (P < 0.001) and adjusted (P = 0.007) models (Table 2). The relationship was similar at lower levels (Q1 and Q2) of total T, with a stepwise effect at higher levels (Q3 and Q4). Women in the top quartile of total T levels had a 3-fold greater odds of MetSyn than those in the bottom quartile [adjusted odds ratio (OR) 3.15; confidence interval (CI) 1.57–6.35], an effect that persisted after additional adjustment for BMI (adjusted OR 2.81; CI 1.32–5.98).
Table 2.
Odds ratios of MetSyn components and MetSyn by T quartile for the entire sample (n = 344)
| Total T
|
Free T
|
|||
|---|---|---|---|---|
| Crude OR (95% CI) | Adjusted OR (95% CI) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
| Abdominal obesity | ||||
| Q1 | 1.0 | 1.0 | 1.0 | 1.0 |
| Q2 | 0.98 (0.53, 1.81) | 0.92 (0.47, 1.80) | 1.34 (0.72, 2.50) | 1.25 (0.65, 2.40) |
| Q3 | 2.11 (1.11, 3.99) | 2.01 (1.00, 4.05) | 1.81 (0.95, 3.43) | 1.56 (0.78, 3.12) |
| Q4 | 4.26 (2.08, 8.73) | 3.65 (1.68, 7.93) | 2.70 (1.38, 5.30) | 2.23 (1.08, 4.61) |
| Hypertriglyceridemia | ||||
| Q1 | 1.0 | 1.0 | 1.0 | 1.0 |
| Q2 | 1.22 (0.61, 2.43) | 1.26 (0.60, 2.63) | 0.98 (0.50, 1.91) | 0.96 (0.48, 1.92) |
| Q3 | 1.57 (0.80, 3.09) | 1.85 (0.89, 3.86) | 1.12 (0.58, 2.17) | 1.09 (0.53, 2.23) |
| Q4 | 1.95 (0.99, 3.82) | 2.19 (1.03, 4.64) | 1.30 (0.67, 2.52) | 1.52 (0.74, 3.13) |
| Low HDL cholesterol | ||||
| Q1 | 1.0 | 1.0 | 1.0 | 1.0 |
| Q2 | 1.21 (0.55, 2.67) | 0.82 (0.36, 1.90) | 1.02 (0.49, 2.14) | 0.86 (0.39, 1.89) |
| Q3 | 2.67 (1.28, 5.56) | 2.00 (0.91, 4.40) | 1.81 (0.89, 3.66) | 1.35 (0.63, 2.93) |
| Q4 | 3.46 (1.66, 7.20) | 2.29 (1.02, 5.11) | 2.10 (1.04, 4.24) | 1.82 (0.84, 3.95) |
| High blood pressure | ||||
| Q1 | 1.0 | 1.0 | 1.0 | 1.0 |
| Q2 | 0.80 (0.42, 1.53) | 0.76 (0.38, 1.53) | 0.72 (0.37, 1.38) | 0.60 (0.30, 1.20) |
| Q3 | 0.93 (0.48, 1.77) | 0.81 (0.40, 1.64) | 0.84 (0.43, 1.64) | 0.72 (0.35, 1.48) |
| Q4 | 1.41 (0.71, 2.80) | 1.28 (0.60, 2.73) | 1.04 (0.52, 2.06) | 0.80 (0.38, 1.68) |
| High fasting glucose | ||||
| Q1 | 1.0 | 1.0 | 1.0 | 1.0 |
| Q2 | 1.69 (0.83, 3.45) | 1.47 (0.70, 3.09) | 1.40 (0.72, 2.71) | 1.28 (0.64, 2.54) |
| Q3 | 3.38 (1.70, 6.72) | 2.90 (1.40, 6.01) | 2.08 (0.84, 4.64) | 1.77 (0.88, 3.56) |
| Q4 | 7.13 (3.52, 14.45) | 5.93 (2.78, 12.63) | 3.05 (1.59, 8.27) | 2.37 (1.17, 4.79) |
| Adult Treatment Panel III MetSyn | ||||
| Q1 | 1.0 | 1.0 | 1.0 | 1.0 |
| Q2 | 0.83 (0.43, 1.60) | 0.71 (0.35, 1.41) | 0.90 (0.48, 1.68) | 0.79 (0.41, 1.51) |
| Q3 | 2.09 (1.11, 3.92) | 1.81 (0.92, 3.54) | 1.33 (0.71, 2.48) | 1.09 (0.56, 2.13) |
| Q4 | 3.87 (2.02, 7.42) | 3.15 (1.57, 6.35) | 1.86 (0.99, 3.50) | 1.48 (0.75, 2.91) |
Adjusted models included age, race, estrogen use, and number of ovaries removed. Statistically significant values are indicated in bold.
Women in the top quartile of total T were also more likely to have each of the criteria of MetSyn than those in the bottom quartile, although the magnitude of these associations varied considerably and was not statistically significant for the high blood pressure criterion (Table 2). In adjusted models, the strongest associations were for high fasting glucose (OR 5.93; CI 2.78–12.6) and abdominal obesity (OR 3.65; CI 1.68–7.93), comparing Q4 with Q1, with additional associations seen for low HDL (OR 2.29; 1.02–5.11) and hypertriglyceridemia (OR 2.19; CI 1.03–4.64).
After exclusion of women with diabetes, the relationship between total T and MetSyn persisted, with a 3-fold greater odds of the metabolic syndrome in women with total T levels in Q4 vs. Q1 (adjusted OR 2.89; CI 1.36–6.16) (Table 3). Of note, the group of women with high fasting glucose in this set of analyses represents exclusively women with impaired fasting glucose. Women in the top two quartiles of total T levels were very likely to have impaired fasting glucose (OR 4.95; CI 2.18–11.26 for Q4 vs. Q1; OR 2.77; CI 1.27–6.07 for Q3 vs.Q1). Likewise, women in the top two quartiles of total T were more likely to have abdominal obesity (OR 3.46; CI 1.52–7.87 for Q4 vs. Q1; OR 2.10; CI 1.02–4.33 for Q3 vs. Q1).
Table 3.
Odds ratios of MetSyn components and MetSyn by testosterone quartile for those without diabetes (n = 301)
| Total T
|
Free T
|
|||
|---|---|---|---|---|
| Crude OR (95% CI) | Adjusted OR (95% CI) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
| Abdominal obesity | ||||
| Q1 | 1.0 | 1.0 | 1.0 | 1.0 |
| Q2 | 0.92 (0.49, 1.74) | 0.93 (0.47, 1.86) | 1.19 (0.62, 2.26) | 1.13 (0.58, 2.22) |
| Q3 | 2.06 (1.06, 3.99) | 2.10 (1.02, 4.33) | 1.81 (0.93, 3.54) | 1.63 (0.79, 3.33) |
| Q4 | 3.82 (1.78, 8.19) | 3.46 (1.52, 7.87) | 2.39 (1.17, 4.85) | 2.05 (0.96, 4.39) |
| Hypertriglyceridemia | ||||
| Q1 | 1.0 | 1.0 | 1.0 | 1.0 |
| Q2 | 1.13 (0.56, 2.30) | 1.20 (0.56, 2.55) | 0.89 (0.44, 1.77) | 0.91 (0.44, 1.86) |
| Q3 | 1.53 (0.76, 3.06) | 1.87 (0.88, 3.98) | 1.09 (0.55, 2.16) | 1.08 (0.52, 2.26) |
| Q4 | 1.74 (0.85, 3.56) | 1.96 (0.88, 4.33) | 1.09 (0.54, 2.22) | 1.27 (0.59, 2.73) |
| Low HDL cholesterol | ||||
| Q1 | 1.0 | 1.0 | 1.0 | 1.0 |
| Q2 | 1.00 (0.44, 2.27) | 0.68 (0.29, 1.65) | 0.89 (0.41, 1.91) | 0.76 (0.34, 1.72) |
| Q3 | 1.99 (0.93, 4.28) | 1.50 (0.66, 3.40) | 1.25 (0.60, 2.64) | 0.89 (0.40, 2.01) |
| Q4 | 3.01 (1.39, 6.53) | 1.97 (0.85, 4.61) | 1.70 (0.80, 3.58) | 1.40 (0.62, 3.17) |
| High blood pressure | ||||
| Q1 | 1.0 | 1.0 | 1.0 | 1.0 |
| Q2 | 0.84 (0.43, 1.63) | 0.80 (0.39, 1.65) | 0.76 (0.39, 1.51) | 0.65 (0.32, 1.33) |
| Q3 | 0.87 (0.45, 1.69) | 0.79 (0.38, 1.63) | 0.73 (0.37, 1.45) | 0.63 (0.30, 1.33) |
| Q4 | 1.29 (0.63, 2.66) | 1.26 (0.57, 2.78) | 0.98 (0.47, 2.00) | 0.80 (0.37, 1.74) |
| High fasting glucose | ||||
| Q1 | 1.0 | 1.0 | 1.0 | 1.0 |
| Q2 | 1.54 (0.70, 3.35) | 1.44 (0.64, 3.24) | 1.18 (0.58, 2.40) | 1.08 (0.52, 2.45) |
| Q3 | 3.07 (1.45, 6.48) | 2.77 (1.27, 6.07) | 1.70 (0.84, 3.44) | 1.48 (0.71, 3.11) |
| Q4 | 5.50 (2.55, 11.89) | 4.95 (2.18, 11.26) | 2.21 (1.08, 4.52) | 1.80 (0.85, 3.84) |
| Adult Treatment Panel III MetSyn | ||||
| Q1 | 1.0 | 1.0 | 1.0 | 1.0 |
| Q2 | 0.82 (0.41, 1.65 | 0.72 (0.34, 1.52) | 0.85 (0.44, 1.66) | 0.78 (0.39, 1.54) |
| Q3 | 1.87 (0.96, 3.65) | 1.74 (0.86, 3.54) | 1.10 (0.57, 2.14) | 0.93 (0.46, 1.89) |
| Q4 | 3.30 (1.64, 6.65) | 2.89 (1.36, 6.16) | 1.53 (0.78, 3.00) | 1.29 (0.63, 2.67) |
Adjusted models included age, race, estrogen use, and number of ovaries removed. Statistically significant values are indicated in bold.
In comparison, free T was not statistically significantly associated with MetSyn (adjusted OR 1.48; CI 0.75–2.91). However, free T levels were associated with the components of MetSyn that are most closely linked to IR: high fasting glucose (adjusted OR 2.37; CI 1.17–4.79) and abdominal obesity (adjusted OR 2.23; CI 1.08–4.61), comparing Q4 with Q1 (Table 2). These effects were attenuated after exclusion of women with diabetes, achieving statistical significance in the crude but not the adjusted analyses (Table 3).
CHD
A nonlinear, or J-shaped, relationship described the association of total T and CHD (Table 4). For ease of interpretation, Q2 was used as the reference category. Women in the top quartile of total T had a 3-fold greater odds of CHD compared with those in the reference category, independent of sociodemographic and traditional cardiovascular risk factors (Q4 vs. Q2 OR 2.95, CI 1.2–7.3). Free T was not associated with CHD. Adjusting for HOMA-IR decreased the magnitude of the multivariable-adjusted OR for the association between the top quartile of total T and CHD by 20% (from 2.95 to 2.35) but minimally affected the association between the top quartile of free T and CHD (from 1.41 to 1.37).
Table 4.
Odds ratios of CHD by total T and free T quartiles for the entire sample
| Total T
|
Free T
|
|||
|---|---|---|---|---|
| Crude OR (95% CI) | Adjusted OR (95% CI) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
| CHD | ||||
| Q1 | 2.12 (0.8, 5.4) | 2.12 (0.8, 5.7) | 1.23 (0.5, 2.9) | 1.19 (0.5, 2.8) |
| Q2 | 1.0 | 1.0 | 1.0 | 1.0 |
| Q3 | 1.38 (0.5, 3.6) | 1.26 (0.5, 3.4) | 0.85 (0.4, 2.0) | 0.87 (0.4, 2.1) |
| Q4 | 3.17 (1.3, 7.6) | 2.95 (1.2, 7.3) | 1.47 (0.7, 3.2) | 1.41 (0.6, 3.2) |
Adjusted models included age, race, smoking, estrogen use, hypertension, and LDL cholesterol. Statistically significant values are indicated in bold.
Discussion
In a cohort of women aged 65 yr and older, we demonstrated strong associations between total and free T levels and IR as well as components of MetSyn that are more closely tied to IR (high fasting glucose and abdominal obesity). Higher total T levels were also independently associated with hypertriglyceridemia, low HDL cholesterol, MetSyn, and prevalent CHD, although these associations were not found with free T.
Our findings suggest that even at the physiologically low levels of T found in these older women, the association between T and IR found in perimenopausal and early postmenopausal women (2,5,6,7,8,9,10,11) persists into old age. In this cross-sectional study, we could not determine the direction of causality of this relationship. Data from premenopausal women with PCOS suggest that the effect is largely a stimulatory effect of hyperinsulinemia on ovarian androgen production, although a few studies suggest that testosterone may worsen insulin resistance (31). We are aware of only two studies that examined aspects of the T-IR relationship in study populations of women with a similar mean age to ours. The Invecchiare nel CHIANTI Study reported that older women with MetSyn had higher bioavailable T, but not total T levels, and that neither measure of T was associated with MetSyn in adjusted models (32). Both T measures were assessed in linear models that did not allow for the threshold effects we saw in our study. In the Rancho Bernardo Study, bioavailable T levels were associated with fasting plasma glucose and were higher in women with impaired glucose tolerance or type 2 diabetes (33). Differences in results between these two studies and ours may in part reflect differences in assay methodology, mean BMI (lowest in the Rancho Bernardo Study and similar between inCHIANTI and CHS), and exclusion criteria (estrogen users were excluded in both inCHIANTI and the Rancho Bernardo Study, and, additionally, women with bilateral oophorectomy were excluded from the inCHIANTI analyses). The mechanisms that contribute to the T-IR relationship bear further investigation in older women, in light of the increase in visceral adiposity and resultant IR with increasing age (34) and the sustained ability of the ovary to produce testosterone after menopause (35).
Although MetSyn was highly prevalent among the participants in our study, at 45%, and has been widely studied as a risk factor for cardiovascular disease, our analyses of the individual MetSyn criteria suggest that the primary determinant of the relationship between T and MetSyn is IR, even after exclusion of women with diabetes. We do not know the menstrual histories of these women, and their premenopausal years preceded the first definition of PCOS. However, our analyses suggest that our findings are not solely confined to women with a prior history of PCOS, given a greater than 2-fold higher prevalence of abdominal obesity and high fasting glucose in women in the top 50% of T levels, compared with the bottom 25%.
Studies examining the relationship between endogenous T levels and CVD in postmenopausal women have yielded conflicting results. Several studies suggest that higher T levels are protective against CVD (36,37,38); however, these studies examined an early postmenopausal population when the prevalence of CVD is low. The Rancho Bernardo Study found no association between T and fatal CVD, although this study population had relatively low BMI values (39). On the other hand, three studies found that higher T levels are associated with increased CVD (3,10,40).
We found a J-shaped relationship between total T and CHD. Those in the bottom and top quartiles were more likely to have had a greater odds of CHD compared with those in the second quartile, although only the top quartile association was statistically significant. It is possible that those in the bottom quartile had lower T levels because of systemic underlying illness, although we did not specifically test this. Another possibility is that there is a target range of acceptable T levels and that both too little and too much have negative consequences. Interestingly, data in men have consistently shown that lower T levels are associated with increased CVD risk factors (41,42), CVD (17,18), and CVD mortality (43), suggesting a gender dimorphism.
In our study, the associations between T and MetSyn and CHD were universally stronger for total T than for free T. We do not have SHBG levels in our study population, but there are two potential explanations for our findings. The most likely explanation is that our free T assay, which was performed by equilibrium dialysis, had difficulty discriminating among the low levels found in these women, introducing additional noise into our data analyses. Alternatively, these data suggest that the increase in T had a predominant effect over the expected decrease in SHBG seen with high insulin levels in the setting of IR, and this may have contributed to the weaker association of free T levels with our outcomes.
The strengths of our study include its population-based sample of older women; the measurement of each of the Adult Treatment Panel III criteria for MetSyn; the rigorous definition of diabetes, which included an oral glucose tolerance test in those without known diabetes; the use of an adjudicated CHD outcome; and the use of highly sensitive assays. However, our analyses are cross-sectional in nature, which limits causal inference. Residual confounding is also possible despite adjustment for multiple potential confounders.
Although our study extends the finding of a T-IR relationship to a population of women of advanced age, the clinical implications of this finding are unclear. The majority of mechanistic studies performed in premenopausal women with PCOS suggest that T is simply a marker of IR (44). However, several studies support the potential for adverse effects of T on IR (31). If so, then augmented T concentrations could be an additional contributor to the age-related increase in risk of metabolic disorders in postmenopausal women. Further studies are required to determine whether a causal relationship exists between testosterone and IR and to provide more insight into the role T plays in the pathogenesis of CVD in women.
Footnotes
This work was supported by National Institute on Aging Grant K23 AG19161, an American Federation for Aging Research/Pfizer Research grant, and a career development award from the John A. Hartford Foundation (to A.R.C.); contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129, and N01-HC-15103 from the National Heart, Lung, and Blood Institute; and the Intramural Research Program of the National Institute on Aging. A full list of participating CHS investigators and institutions can be found at http://www.chs-nhlbi.org.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 21, 2009
Abbreviations: BMI, Body mass index; CHD, coronary heart disease; CHS, Cardiovascular Health Study; CI, confidence interval; CV, coefficient of variation; CVD, cardiovascular disease; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; IR, insulin resistance; LDL, low-density lipoprotein; MetSyn, metabolic syndrome; OR, odds ratio; PCOS, polycystic ovary syndrome; Q, quartile; QUICKI, Quantitative Insulin Sensitivity Check Index; T, testosterone.
References
- Hoffman LK, Ehrmann DA 2008 Cardiometabolic features of polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab 4:215–222 [DOI] [PubMed] [Google Scholar]
- Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K 2008 Menopause and the metabolic syndrome: the Study of Women’s Health Across the Nation. Arch Intern Med 168:1568–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LJ, Bairey Merz CN, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, Kelsey SF, Kip KE, Cooper-Dehoff RM, Johnson BD, Vaccarino V, Reis SE, Bittner V, Hodgson TK, Rogers W, Pepine CJ 2008 Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health-National Heart, Lung, and Blood Institute sponsored Women’s Ischemia Syndrome Evaluation. J Clin Endocrinol Metab 93:1276–1284 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Maturana MA, Breda V, Lhullier F, Spritzer PM 2008 Relationship between endogenous testosterone and cardiovascular risk in early postmenopausal women. Metabolism 57:961–965 [DOI] [PubMed] [Google Scholar]
- Golden SH, Dobs AS, Vaidya D, Szklo M, Gapstur S, Kopp P, Liu K, Ouyang P 2007 Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab 92:1289–1295 [DOI] [PubMed] [Google Scholar]
- Sutton-Tyrrell K, Wildman RP, Matthews KA, Chae C, Lasley BL, Brockwell S, Pasternak RC, Lloyd-Jones D, Sowers MF, Torréns JI 2005 Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN). Circulation 111:1242–1249 [DOI] [PubMed] [Google Scholar]
- Golden SH, Ding J, Szklo M, Schmidt MI, Duncan BB, Dobs A 2004 Glucose and insulin components of the metabolic syndrome are associated with hyperandrogenism in postmenopausal women: the atherosclerosis risk in communities study. Am J Epidemiol 160:540–548 [DOI] [PubMed] [Google Scholar]
- Weinberg ME, Manson JE, Buring JE, Cook NR, Seely EW, Ridker PM, Rexrode KM 2006 Low sex hormone-binding globulin is associated with the metabolic syndrome in postmenopausal women. Metabolism 55:1473–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalish GM, Barrett-Connor E, Laughlin GA, Gulanski BI 2003 Association of endogenous sex hormones and insulin resistance among postmenopausal women: results from the Postmenopausal Estrogen/Progestin Intervention Trial. J Clin Endocrinol Metab 88:1646–1652 [DOI] [PubMed] [Google Scholar]
- Rexrode KM, Manson JE, Lee IM, Ridker PM, Sluss PM, Cook NR, Buring JE 2003 Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation 108:1688–1693 [DOI] [PubMed] [Google Scholar]
- Maturana MA, Spritzer PM 2002 Association between hyperinsulinemia and endogenous androgen levels in peri- and postmenopausal women. Metabolism 51:238–243 [DOI] [PubMed] [Google Scholar]
- Ford ES, Giles WH, Dietz WH 2002 Prevalence of the metabolic syndrome among U.S. adults: findings from the third National Health and Nutrition Examination Survey. JAMA 287:356–359 [DOI] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y 2008 Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117:e25–146 [DOI] [PubMed] [Google Scholar]
- Cappola AR, Ratcliffe SJ, Bhasin S, Blackman MR, Cauley J, Robbins J, Zmuda JM, Harris T, Fried LP 2007 Determinants of serum total and free testosterone levels in women over the age of 65 years. J Clin Endocrinol Metab 92:509–516 [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Muller DC, Metter EJ, Maggio M, Harman SM, Blackman MR, Andres R 2007 Aging, androgens, and the metabolic syndrome in a longitudinal study of aging. J Clin Endocrinol Metab 92:3568–3572 [DOI] [PubMed] [Google Scholar]
- Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB 2006 Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab 91:843–850 [DOI] [PubMed] [Google Scholar]
- Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, Pols HA 2002 Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab 87:3632–3639 [DOI] [PubMed] [Google Scholar]
- Muller M, van den Beld AW, Bots ML, Grobbee DE, Lamberts SW, van der Schouw YT 2004 Endogenous sex hormones and progression of carotid atherosclerosis in elderly men. Circulation 109:2074–2079 [DOI] [PubMed] [Google Scholar]
- Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A 1991 The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1:263–276 [DOI] [PubMed] [Google Scholar]
- Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP 1995 Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem 41:264–270 [PubMed] [Google Scholar]
- Sinha-Hikim I, Arver S, Beall G, Shen R, Guerrero M, Sattler F, Shikuma C, Nelson JC, Landgren BM, Mazer NA, Bhasin S 1998 The use of a sensitive equilibrium dialysis method for the measurement of free testosterone levels in healthy, cycling women and in human immunodeficiency virus-infected women. J Clin Endocrinol Metab 83:1312–1318 [DOI] [PubMed] [Google Scholar]
- Javanbakht M, Singh AB, Mazer NA, Beall G, Sinha-Hikim I, Shen R, Bhasin S 2000 Pharmacokinetics of a novel testosterone matrix transdermal system in healthy, premenopausal women and women infected with the human immunodeficiency virus. J Clin Endocrinol Metab 85:2395–2401 [DOI] [PubMed] [Google Scholar]
- Choi HH, Gray PB, Storer TW, Calof OM, Woodhouse L, Singh AB, Padero C, Mac RP, Sinha-Hikim I, Shen R, Dzekov J, Dzekov C, Kushnir MM, Rockwood AL, Meikle AW, Lee ML, Hays RD, Bhasin S 2005 Effects of testosterone replacement in human immunodeficiency virus-infected women with weight loss. J Clin Endocrinol Metab 90:1531–1541 [DOI] [PubMed] [Google Scholar]
- Singh AB, Lee ML, Sinha-Hikim I, Kushnir M, Meikle W, Rockwood A, Afework S, Bhasin S 2006 Pharmacokinetics of a testosterone gel in healthy postmenopausal women. J Clin Endocrinol Metab 91:136–144 [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS 1972 Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502 [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ 2000 Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85:2402–2410 [DOI] [PubMed] [Google Scholar]
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) 2001 Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285:2486–2497 [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith Jr SC, Spertus JA, Costa F 2005 Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112:2735–2752 [DOI] [PubMed] [Google Scholar]
- Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S 1995 Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol 5:278–285 [DOI] [PubMed] [Google Scholar]
- Corbould A 2008 Effects of androgens on insulin action in women: is androgen excess a component of female metabolic syndrome? Diabetes Metab Res Rev 24:520–532 [DOI] [PubMed] [Google Scholar]
- Maggio M, Lauretani F, Ceda GP, Bandinelli S, Basaria S, Paolisso G, Ble A, Egan JM, Metter EJ, Abbatecola AM, Zuliani G, Ruggiero C, Valenti G, Guralnik JM, Ferrucci L 2007 Association of hormonal dysregulation with metabolic syndrome in older women: data from the InCHIANTI study. Am J Physiol Endocrinol Metab 292:E353–E358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman-Gruen D, Barrett-Connor E 2000 Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care 23:912–918 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, Schwartz AV, Kritchevsky S, Newman AB 2003 Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 26:372–379 [DOI] [PubMed] [Google Scholar]
- Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ 2007 Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab 92:3040–3043 [DOI] [PubMed] [Google Scholar]
- Golden SH, Maguire A, Ding J, Crouse JR, Cauley JA, Zacur H, Szklo M 2002 Endogenous postmenopausal hormones and carotid atherosclerosis: a case-control study of the atherosclerosis risk in communities cohort. Am J Epidemiol 155:437–445 [DOI] [PubMed] [Google Scholar]
- Bernini GP, Sgro' M, Moretti A, Argenio GF, Barlascini CO, Cristofani R, Salvetti A 1999 Endogenous androgens and carotid intimal-medial thickness in women. J Clin Endocrinol Metab 84:2008–2012 [DOI] [PubMed] [Google Scholar]
- Kaczmarek A, Reczuch K, Majda J, Banasiak W, Ponikowski P 2003 The association of lower testosterone level with coronary artery disease in postmenopausal women. Int J Cardiol 87:53–57 [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Goodman-Gruen D 1995 Prospective study of endogenous sex hormones and fatal cardiovascular disease in postmenopausal women. BMJ 311:1193–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips GB, Pinkernell BH, Jing TY 1997 Relationship between serum sex hormones and coronary artery disease in postmenopausal women. Arterioscler Thromb Vasc Biol 17:695–701 [DOI] [PubMed] [Google Scholar]
- Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT 2004 Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 27:1036–1041 [DOI] [PubMed] [Google Scholar]
- Haffner SM, Shaten J, Stern MP, Smith GD, Kuller L 1996 Low levels of sex hormone-binding globulin and testosterone predict the development of noninsulin-dependent diabetes mellitus in men. Am J Epidemiol 143:889–897 [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Bergstrom J 2008 Low serum testosterone and mortality in older men. J Clin Endocrinol Metab 93:68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler JE 2008 Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med 358:47–54 [DOI] [PubMed] [Google Scholar]