Abstract
Concept: Ovaries meeting criteria for polycystic ovary morphology during peak reproductive years may no longer meet the criteria with age.
Objective: Ovarian volume and follicle number decrease with age in women with polycystic ovary syndrome (PCOS), permitting age-dependent criteria for PCOM.
Design and Setting: We conducted longitudinal (7–15 year interval) and cross-sectional studies to examine polycystic ovarian morphology over time at an outpatient clinic and pathology laboratory in a tertiary care hospital.
Patients: Subjects included those with PCOS defined by the National Institutes of Health criteria (n = 11 and 483 for longitudinal and cross-sectional, respectively) and control women with regular menstrual cycles and no hyperandrogenism (n = 15 and 367), age 18–64 yr.
Interventions: Subjects underwent an ovarian ultrasound by a single observer.
Main Outcome Measures: Ovarian volume and follicle number were measured and ultrasound findings confirmed by a pathologist in a subset (n = 9).
Results: Ovarian volume (15.2 ± 7.4 vs. 7.1 ± 3.7 ml; P < 0.01) and follicle number (12.8 ± 3.2 vs. 8.1 ± 3.9; P < 0.05) decreased longitudinally in PCOS and control women (volume 11.6 ± 4.4 vs. 5.4 ± 2.2 ml and follicle number 8.3 ± 1.9 vs. 6.3 ± 1.8; both P < 0.005). Using cross-sectional data, log ovarian volume and follicle number decreased in both groups, but the decrease in log ovarian volume was less pronounced in women with PCOS than in controls (P < 0.01). A combination of age, log ovarian volume, follicle number, and testosterone distinguished PCOS subjects from controls with a receiver operator characteristic curve area of 0.90.
Conclusions: Ovarian volume and follicle number decrease with age in women with PCOS and controls necessitating age-based criteria to define polycystic ovarian morphology. It is possible to use these criteria to distinguish PCOS in women over age 40 yr.
Ovarian volume and follicle number decrease with age in both PCOS and control women, necessitating age-based criteria to define PCOS.
Polycystic ovary syndrome (PCOS) was named based on the pathological appearance of the ovary in women with menstrual irregularities and hyperandrogenism. Thus its name alludes to polycystic ovary morphology (PCOM) as a prominent feature of the disorder. Despite the apparent importance of PCOM in PCOS, the criteria used to define PCOM have only recently been standardized based on the sensitivity and specificity to identify PCOS (1,2). The Adams criteria, defined as 10 or more follicles arranged in a peripheral pattern around a dense core of stroma, have the best sensitivity for PCOS (3), whereas those of Jonard et al. (1), which define PCOM as 12 or more follicles of 2–9 mm, convey a greater specificity (1). The use of both follicle number and ovarian volume improves the power of discrimination (2). Therefore, both a follicle number of 12 or more and/or an ovarian volume of more than 10 ml have been used to create a consensus definition of PCOM (4).
PCOM has only been examined cross-sectionally in women with PCOS. In these studies, ovarian volume and follicle number correlated inversely with age (5) and were greater among premenopausal than postmenopausal women with PCOS (6). Nevertheless, the criteria defining PCOM do not take into account the changes that occur during aging. In normal ovulatory women, ovarian volume decreases with age, with measurable decreases across each decade of reproductive life starting at age 40 yr (7,8). Follicle number also decreases with age and correlates with the decrease in ovarian volume (9,10,11,12). Although PCOM is present in 16–25% of normal women with regular menstrual cycles and no PCOS (13,14,15), longitudinal studies demonstrate that the ovaries of regularly cycling women may no longer meet the criteria for PCOM after age 40 yr based on age-related changes in ovarian volume and follicle number (16). Taken together, the decline in follicle number and ovarian volume across aging in women with regular menstrual cycles suggest that similar changes may occur in the ovaries of women with PCOS.
In addition to the possibility that ovaries of women with PCOS may not meet the criteria for PCOM over time, PCOM on ultrasound has not been defined after menopause. One study suggested that women with PCOS still exhibit PCOM in menopause, as defined by eight or more follicles of 2–8 mm and an increase in ovarian stroma (6). However, pathology demonstrates absence of secondary follicles in normal postmenopausal ovaries (17). Ideally, a direct comparison between ultrasound and pathology findings is necessary to determine whether follicles on ultrasound in a postmenopausal PCOS ovary truly reflect follicles on pathology.
Retrospective, cross-sectional studies in women with PCOS reveal that menstrual cycles become more regular with age (18). This regularization along with error in the recall of menstrual cycle regularity (19), cosmetic management of hirsutism, and the decrease in ovarian volume make it difficult to diagnose a woman with PCOS after the age of 40 yr. The use of PCOM at older ages may therefore become important when determining the affected status of a mother or grandmother for PCOS genetic studies and in determining risk for insulin resistance, diabetes, and cardiovascular disease in a previously undiagnosed older woman (20,21,22). Thus, new criteria are needed to define ovarian volume and follicle number in aging women with PCOS. To formulate age-based criteria for PCOM, we first validated ultrasound findings with pathology in ovaries of women with PCOS over age 40 yr to document that echolucent structures on ultrasound represent follicles and to assess the accuracy of ovarian volume measurements. We also documented the ovarian size and follicle number in women with PCOS and controls studied longitudinally and cross-sectionally to determine parameters that define PCOM in women after the age of 40 yr. These data along with testosterone levels were then used to develop a formula to predict PCOS.
Subjects and Methods
For all subjects, PCOS was diagnosed by age 40 yr as chronic oligomenorrhea (fewer than nine menstrual periods/yr) and clinical and/or biochemical hyperandrogenism in the absence of other disorders causing the same phenotype (23). Clinical hyperandrogenism was defined by an elevated Ferriman-Gallwey score higher than 9 (24). Biochemical hyperandrogenism was defined as an androgen level greater than the 95% confidence limits in ovulatory control subjects: testosterone higher than 63 ng/ml (2.8 nmol/liter) or androstenedione levels higher than 3.8 ng/ml (13.3 nmol/liter) (24). Control subjects had menstrual cycle lengths of 25–35 d and no hyperandrogenism. All subjects had normal thyroid function and prolactin levels. Subjects were on no hormone medication. All of the studies were approved by the Institutional Review Board of the Massachusetts General Hospital. All subjects gave their written informed consent.
Pathology study
Women with PCOS (n = 9), aged 45 yr or older, had their ovaries removed for various diagnoses (Table 1). Eight of nine had been diagnosed in the Reproductive Endocrine Unit. All subjects had an ultrasound near the time of surgery performed by J.M.A. (n = 3), the radiology department (n = 3), or both (n = 3). All images were reviewed by J.M.A., M.K.M., and C.K.W.
Table 1.
Age, diagnosis, ultrasound, and pathology findings in women with PCOS undergoing oophorectomy
| Age (yr) | Menopausal status | Preoperative diagnosis | Ovary | Pathology diagnosis | Pathology of other follicles <10 mm | Follicle number ultrasound | PCOM |
|---|---|---|---|---|---|---|---|
| 62 | Menopausal | Breast cancer and ovarian cysts | R | Benign inclusion cyst | Multiple clusters of small inclusion cysts ≤2 mm | 3 | No |
| L | Benign serous cyst | Same | 4 | No | |||
| 61 | Menopausal | Elevated testosterone | R | Stromal luteoma and hyperthecosis | Few inclusion cysts, vessels in hilar area | 12 | Yes |
| L | Stromal hyperthecosis | Same | Not Visualized | ||||
| 58 | Postmenopausal bleeding | Endometrial cancer | L | Normal ovary | Inclusion cysts ≤2 mm | 11 | No |
| 54 | Postmenopausal bleeding | Endometrial cancer | R | Normal ovarian parenchyma | No follicles or inclusion cysts visible | 11 | No |
| L | Normal ovarian parenchyma | No follicles or inclusion cysts visible | 6 | No | |||
| 55 | Premenopausal menorrhagia | Fibroids and bilateral ovarian cysts | R | Simple corpus luteum cyst | 7-mm follicle, multiple small 2 to 4-mm inclusion glands and cysts including cysts in clusters, dilated lymphatics | 3 | No |
| L | Simple serous cyst | 1- to 2-mm inclusion glands and cysts, dilated lymphatics | 8 | No | |||
| 48 | Irregular bleeding | L ovarian cyst | R | Corpus luteum cyst | Cystic follicles | 5 | No |
| L | Paraovarian serous cystadenoma | Cystic follicles | 2 | No | |||
| 47 | Irregular bleeding | Bilateral adnexal masses | R | Serous cystadenoma | Cystic follicles and inclusion cysts 2–5 mm | 12 | Yes |
| L | Serous cystadenofibroma | Cystic follicles and inclusion cysts 2–5 mm | 6 | No | |||
| 46 | BRCA1 positive, regular cycles | Normal ovaries | R | Normal ovaries | Cystic follicles | 8 | No |
| Normal ovaries | L | Normal ovaries | Cystic follicles | 12 | Yes | ||
| 45 | Irregular Menses | Endometrial hyperplasia | R | Stromal hyperthecosis | Cystic follicles and epithelial inclusion cysts | 10 | No |
| L | Stromal hyperplasia and hyperthecosis | Cystic follicles | 9 | No |
Follicle number on ultrasound indicates all follicles or cysts 2 mm or greater. PCOM is reported on the basis of follicle number ≥12. L, Left; R, right.
Cross-sectional study subjects
U.S. women with PCOS (n = 483) and controls (n = 367) who met the criteria for their respective groups outlined above and as previously described (25) were recruited between 2003 and 2008. Women with PCOS who were over the age of 40 yr had been diagnosed at the Massachusetts General Hospital Reproductive Endocrine Unit between the ages of 23 and 40 yr.
Longitudinal study subjects
Subjects over age 40 yr were recruited (2007–2008) from previous studies (7–15 yr earlier) of women with PCOS (n = 11) without regard to intervening treatment. The total number of eligible PCOS subjects was 97. Of the total, 20 could not be located, 52 did not respond, and eight did not participate. There were no differences at baseline between subjects who did and did not return. Age-matched, regularly cycling control women (n = 15) were a subset of those recruited (2005–2006) and published previously (16). At the initial visit, all women were between the ages of 26 and 42 yr. All subjects had a previous pelvic ultrasound performed by J.M.A. using a Toshiba SAL 727B, 5-MHz convex array transducer. Multiple images were recorded for each ovary.
Study procedures
All cross-sectional and longitudinal subjects with PCOS were studied on d 6 or later in the follicular phase with the exception of one subject who presented in the luteal phase, and her gonadotropins and sex steroids were not included. Control subjects were studied on d 1–8 of the follicular phase. Menopausal subjects were studied at random. Subjects arrived after a 12-h fast and underwent a menstrual history, physical exam (26) and blood sampling, with additional samples at 10 and 20 min to obtain average gonadotropin concentrations.
The second ultrasound for the longitudinal study and all ultrasounds for the cross-sectional study were performed on an ATL HDI 1500, 5-MHz convex array transducer, and multiple images of the ovary were recorded. Ovarian volume was calculated at the time of the ultrasound procedure using length × width × height in centimeters multiplied by 0.5233 (27). Follicles were counted on a fixed image in a plane in which the maximum number of follicles was visualized. PCOM was defined as at least one ovary with 12 or more follicles of 2–10 mm in a single plane or an ovarian volume greater than 10 ml in the absence of a dominant follicle bigger than 10 mm in diameter, a corpus luteum, or a cyst (1,3,4). A consensus was reached on the reading of all ultrasounds by the reviewers (J.M.A., M.K.M., and C.K.W). The maximum ovarian volume and follicle number in both ovaries was used for analysis, excluding the volume of an ovary with a dominant follicle (>10 mm) or a corpus luteum.
Hormonal assays
Gonadotropin, androgen, SHBG, and insulin levels were measured as previously described (28,29). LH and FSH levels are expressed in international units per liter as equivalents of the Second International Reference Preparation 71/223 of human menopausal gonadotropins. The homeostasis model assessment (HOMA) was used to estimate insulin resistance (30).
Data analysis
The cross-sectional data were analyzed using a two-way ANOVA. Linear regression was performed for each variable, and the log ovarian volume in premenopausal and postmenopausal women as a function of age within groups. The most general model was fit and then reduced to eliminate nonsignificant interactions. A significant slope difference demonstrated a group by age interaction.
In addition, a logistic regression model was used to estimate the log odds of PCOS as a function of age, follicle number, log ovarian volume, and testosterone in premenopausal women. The complete first-order model with all interactions was reduced to a simple main-effects model because no interactions were statistically significant. A similar model was used for both pre- and postmenopausal women with menopause status included as a main effect and follicle number set to zero in postmenopausal women. A receiver operator characteristic curve was constructed to evaluate the logistic regression model for predicting PCOS.
The longitudinal data set was analyzed using a random-effects analysis of covariance model. The time of each patient’s baseline visit was set to zero, and the time of the follow-up visit set to years since baseline. The model included a fixed intercept and slope for each group and a random intercept for each patient. The intercept difference was used to estimate a mean baseline difference between age-matched normal and PCOS subjects and the slope difference to estimate an interaction between group and time, that is, a differential aging effect due to PCOS.
All analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC). Data are expressed as mean ± sd unless otherwise indicated. Two sided P values ≤ 0.05 were considered evidence for statistical significance.
Results
Pathology study
The ultrasound-calculated ovarian volume was not different from the pathology volume (2.2 ± 0.9 vs. 2.2 ± 1.1 ml; P = 0.9; n = 6 ovaries). The average coefficient of variation for the two measurements was 10 ± 7% (range 0–17%) when the surgery and ultrasound were performed a maximum of 9 months apart (n = 3).
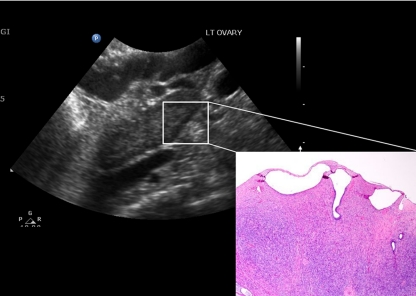
Ovarian morphology was observed in areas free from pathology (Table 1). Perimenopausal subjects (n = 5) had follicle cysts on pathology that were consistent with the ultrasound demonstration of follicles larger than 2 mm. Occasional inclusion cysts were also seen. Postmenopausal women (n = 3) had the ultrasound impression of 11–12 small follicles of 2 mm arranged in a peripheral pattern in the ovary, suggestive of PCOM (Fig. 1), whereas the fourth subject had three to four persistent cysts; one 11 mm and the rest 9 mm or less. However, there were no follicles on pathological examination. Rather, these hypoechoic structures corresponded to serous or inclusion cysts or clusters of inclusion cysts, blood vessels, or dilated lymphatics when examined microscopically in three of four cases and no cystic structures in the fourth (Fig. 1). Therefore, hypoechoic structures on ultrasound were not counted as follicles in the analysis for postmenopausal women.
Figure 1.
Postmenopausal ovary viewed on transvaginal ultrasound and under light microscopy (hematoxylin and eosin stain, ×40 magnification; inset). Ultrasound demonstrates echolucent structures of 2 mm, indicated by the lines. The structures correspond to inclusion cysts on pathological examination (inset).
Anthropomorphic data, hormone levels, and metabolic parameters
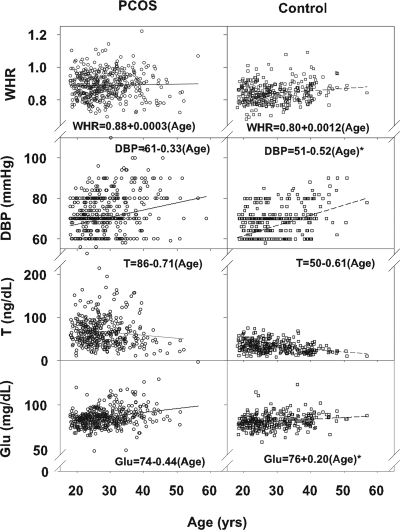
Cross-sectional data were compared between PCOS and control subjects both as continuous variables (Fig. 2) and using an age cutoff of less than 40 or 40 yr or more (Table 2) (25). The body mass index (BMI), systolic blood pressure (SBP), heart rate, and Ferriman-Gallwey score were higher in PCOS than in control subjects (Table 2). In contrast, the waist to hip ratio was higher only in younger PCOS subjects compared with control subjects (Fig. 2). SBP and diastolic blood pressures (DBP) were higher in older PCOS and control subjects compared with younger subjects. In addition, the DBP slope was greater in control subjects than in PCOS subjects (Fig. 2). The Ferriman-Gallwey scores were lower in older compared with younger PCOS subjects but were always higher than the scores in control subjects. The longitudinal data confirmed the higher BMI, SBP, and Ferriman-Gallwey score in PCOS subjects and the decline of the Ferriman-Gallwey score in PCOS subjects over time (Table 3 and supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jem.endojournals.org). Interestingly, SBP increased in control subjects studied longitudinally, whereas PCOS subjects had a higher and stable SBP over time.
Figure 2.
Waist-to-hip ratio (WHR), DBP, testosterone levels (T), and fasting glucose (Glu) in PCOS subjects (first column, open circles) and control subjects (second column, open squares). The linear regression equations are presented, and significant differences in slope are indicated with an asterisk.
Table 2.
Comparison of all parameters in younger (<40 yr) and older (≥40 yr) women with PCOS and control women studied cross-sectionally
| PCOS
|
Control
|
P values | ||||
|---|---|---|---|---|---|---|
| Younger | Older | Younger | Older | PCOS/CTL | Dx/age | |
| Age (yr) | 27.8 ± 5.7 | 46.3 ± 4.5a | 28.1 ± 6.4 | 48.1 ± 6.6a | 1.0 | 0.8 |
| BMI (kg/m2) | 30.6 ± 8.7 | 31.3 ± 8.5 | 24.4 ± 5.1 | 26.7 ± 5.4 | <0.001 | 0.9 |
| Waist to hip ratio | 0.89 ± 0.08 | 0.87 ± 0.09 | 0.84 ± 0.06 | 0.88 ± 0.05a | 0.01 | 0.2 |
| SBP (mm Hg) | 112 ± 16 | 122 ± 16a | 106 ± 14 | 119 ± 8a | 0.02 | 0.1 |
| DBP (mm Hg) | 70 ± 12 | 77 ± 13a | 65 ± 10 | 78 ± 8a | 0.09 | 0.04 |
| Heart rate (beats/min) | 74 ± 10 | 72 ± 11 | 70 ± 8 | 65 ± 9 | <0.001 | 0.7 |
| Ferriman-Gallwey | 14.1 ± 8.6 | 9.9 ± 5.2a | 4.6 ± 2.4 | 5.1 ± 3.2 | <0.001 | 0.02 |
| Testosterone (ng/dl) | 67.7 ± 36.4 | 34.0 ± 18.7a | 32.6 ± 13.4 | 22.7 ± 7.8a | <0.001 | 0.6 |
| Adione (ng/ml) | 3.7 ± 1.3 | 2.4 ± 1.0a | 2.5 ± 1.0 | 1.5 ± 0.4a | <0.001 | 0.1 |
| DHEAS (μg/dl) | 199.8 ± 100.7 | 106.8 ± 65.0a | 177.8 ± 90.0 | 108.9 ± 70.0a | 0.3 | 0.6 |
| SHBG (nmol/liter) | 35.4 ± 19.1 | 48.1 ± 28.9 | 59.1 ± 31.3 | 61.1 ± 32.5 | <0.001 | 0.8 |
| LH (IU/liter) | 27.5 ± 15.8 | 34.5 ± 34.5 | 16.2 ± 18.8 | 20.9 ± 19.7 | <0.001 | 0.1 |
| FSH (IU/liter) | 10.5 ± 2.8 | 38.8 ± 66.8a | 11.1 ± 4.0 | 25.2 ± 30.7a | 0.2 | 0.07 |
| LH/FSH ratio | 2.6 ± 1.5 | 1.6 ± 1.1a | 1.5 ± 1.0 | 1.0 ± 0.5a | <0.001 | 0.5 |
| Cholesterol (mg/dl) | 181.7 ± 35.5 | 198.2 ± 32.6a | 171.2 ± 33.6 | 187.6 ± 24.8a | 0.03 | 0.9 |
| Triglyceride (mg/dl) | 100.8 ± 90.2 | 103.3 ± 66.5 | 69.4 ± 33.9 | 79.4 ± 41.6 | <0.001 | 0.2 |
| HDL (mg/dl) | 52.1 ± 14.9 | 61.1 ± 14.3 | 58.0 ± 14.5 | 60.9 ± 15.0 | 0.05 | 0.6 |
| LDL (mg/dl) | 112.0 ± 31.5 | 114.6 ± 29.7 | 99.9 ± 30.2 | 111.1 ± 25.8 | 0.02 | 0.6 |
| Fasting glucose (mg/dl) | 86.1 ± 16.6 | 94.7 ± 17.3a | 81.7 ± 7.7 | 86.2 ± 7.8a | <0.001 | 0.03 |
| Fasting insulin (μIU/ml) | 11.4 ± 11.1 | 10.5 ± 8.8 | 5.8 ± 7.1 | 6.0 ± 3.2 | <0.001 | 0.9 |
| HOMA | 2.50 ± 2.62 | 2.12 ± 2.01 | 1.22 ± 1.85 | 1.28 ± 0.69 | <0.001 | 0.8 |
| Maximum ovarian volume (ml) | 15.1 ± 6.3 | 8.4 ± 4.7a | 9.0 ± 4.0 | 5.0 ± 3.3a | <0.001 | <0.01 |
| Follicle number | 14.2 ± 3.7 | 8.0 ± 3.7a | 9.6 ± 3.1 | 5.8 ± 3.2a | <0.001 | 0.5 |
Results are expressed as mean ± sd. Data were analyzed using two-way ANOVA. PCOS/CTL indicates P values for differences between PCOS and control subjects, and Dx/age indicates P values for the slope difference to estimate an interaction between diagnosis and age. HDL, High-density lipoprotein; LDL, low-density lipoprotein.
Differences between younger and older women within each group.
Table 3.
Comparison of all parameters in women with PCOS and control women studied longitudinally
| PCOS
|
Control
|
P values
|
||||
|---|---|---|---|---|---|---|
| Younger | Older | Younger | Older | PCOS/CTL | Dx/time | |
| Age (yr) | 36.5 ± 3.3 | 46.3 ± 4.2a | 35.3 ± 5.3 | 46.0 ± 4.5a | 0.5 | 0.1 |
| BMI (kg/m2) | 32.3 ± 10.6 | 32.1 ± 9.3 | 23.7 ± 4.6 | 26.5 ± 5.7 | 0.01 | 0.2 |
| Waist to hip ratio | 0.85 ± 0.08 | 0.87 ± 0.09 | 0.82 ± 0.08 | 0.89 ± 0.05 | 0.7 | 0.7 |
| SBP (mm Hg) | 125 ± 11 | 121 ± 12 | 111 ± 10 | 119 ± 9a | 0.004 | 0.04 |
| DBP (mm Hg) | 77 ± 8 | 79 ± 7 | 73 ± 11 | 77 ± 8 | 0.4 | 0.9 |
| Pulse (beats/min) | 70 ± 12 | 74 ± 12 | 65 ± 8 | 65 ± 8 | 0.4 | 0.6 |
| Ferriman-Gallwey | 12.7 ± 5.9 | 8.0 ± 5.4a | 5.5 ± 2.2 | 5.4 ± 3.3 | <0.001 | 0.02 |
| Testosterone (ng/dl) | 84.0 ± 58.2 | 22.0 ± 18.0 | 31.6 ± 10.3 | 21.3 ± 7.1 | 0.008 | 0.07 |
| Adione (ng/ml) | 2.5 ± 1.2 | 2.2 ± 0.5 | 1.9 ± 0.6 | 1.5 ± 0.4 | 0.1 | 0.3 |
| DHEAS (mcg/dl) | 131.1 ± 50.8 | 84.1 ± 48.9 | 144.8 ± 73.9 | 98.7 ± 53.8 | 0.7 | 0.4 |
| SHBG (nmol/liter) | 43.2 ± 23.7 | 58.3 ± 66.4 | 82.1 ± 36.1 | 55.7 ± 28.8 | 0.02 | 0.8 |
| LH (IU/liter) | 21.4 ± 9.3 | 33.8 ± 28.5 | 13.6 ± 5.5 | 23.7 ± 22.6 | 0.3 | 0.3 |
| FSH (IU/liter) | 8.3 ± 2.2 | 31.5 ± 41.4a | 12.5 ± 2.5 | 27.3 ± 33.5a | 0.5 | 0.1 |
| LH/FSH ratio | 2.7 ± 1.2 | 1.6 ± 1.1 | 1.1 ± 0.5 | 1.0 ± 0.6 | <0.001 | 0.003 |
| Cholesterol (mg/dl) | 199.7 ± 37.5 | 226.5 ± 33.5a | 158.2 ± 26.3 | 190.7 ± 26.7a | 0.07 | 0.8 |
| Triglyceride (mg/dl) | 161.5 ± 93.7 | 173.5 ± 91.0 | 72.8 ± 30.5 | 74.9 ± 27.7 | 0.04 | 0.5 |
| HDL (mg/dl) | 37.6 ± 14.8 | 50.0 ± 12.3 | 50.5 ± 7.8 | 60.3 ± 16.7 | 0.5 | 0.4 |
| LDL (mg/dl) | 128.4 ± 31.7 | 140.0 ± 35.7 | 93.0 ± 26.8 | 115.4 ± 27.7 | 0.1 | 0.7 |
| Fasting glucose (mg/dl) | 92.2 ± 6.4 | 100.0 ± 21.3 | 92.7 ± 4.6 | 86.9 ± 8.3 | 0.8 | 0.1 |
| Fasting insulin (μIU/ml) | 7.2 ± 4.3 | 11.3 ± 4.2 | 4.2 ± 1.4 | 6.0 ± 3.1 | 0.3 | 0.1 |
| HOMA | 1.66 ± 0.99 | 2.50 ± 0.95 | 0.97 ± 0.34 | 1.30 ± 0.69 | 0.3 | 0.1 |
| Maximum ovarian volume (ml) | 15.2 ± 7.4 | 7.1 ± 3.7a | 11.6 ± 4.4 | 5.4 ± 2.2a | 0.07 | 0.2 |
| Follicle number | 12.8 ± 3.2 | 8.1 ± 3.9a | 8.3 ± 1.9 | 6.3 ± 1.8a | 0.001 | 0.007 |
Results are expressed as mean ± sd. Data were analyzed using a random-effects analysis of covariance model. The intercept difference was used to estimate a mean baseline difference between younger PCOS and control subjects (PCOS/CTL P value) and the slope difference to estimate an interaction between group and time (Dx/time P value). HDL, High-density lipoprotein; LDL, low-density lipoprotein.
Differences between older and younger women within the PCOS and control groups.
Testosterone, androstenedione, and LH levels and the LH to FSH ratio were higher and SHBG levels were lower in PCOS compared with control subjects (Table 2), with testosterone higher in PCOS subjects at both younger and older ages compared with controls (Fig. 2). Testosterone, androstenedione, and dehydroepiandrosterone sulfate (DHEAS) were also lower in older compared with younger subjects within the PCOS and control groups. The higher testosterone and LH to FSH ratio at younger and older ages and lower SHBG levels were confirmed in PCOS subjects studied longitudinally (Table 3 and supplemental Table 2). FSH was higher in older compared with younger subjects in the PCOS and control groups and increased similarly in PCOS and control subjects studied longitudinally.
Total cholesterol, triglycerides, low-density lipoprotein, fasting glucose and insulin levels, and HOMA were higher in PCOS subjects compared with control subjects (Table 3). Total cholesterol and fasting glucose were higher in older compared with younger subjects within the PCOS and control groups, and the fasting glucose slope was greater in PCOS compared with control groups (Fig. 2). In subjects studied longitudinally, only triglycerides were higher in PCOS compared with control subjects, whereas total cholesterol increased in both PCOS and control groups studied longitudinally (Table 3 and supplemental Table 2).
Menstrual cycle and ultrasound parameters
In subjects studied longitudinally, four subjects met the criteria for PCOM, four no longer met the criteria, and three could not be assessed at the second visit (1). Four subjects with PCOS had irregular menses, four had regular menses, and three could not be assessed. There was no difference in the number of follicles in PCOS subjects with regular and irregular cycles (8 ± 3 vs. 10 ± 5; P = 0.5).
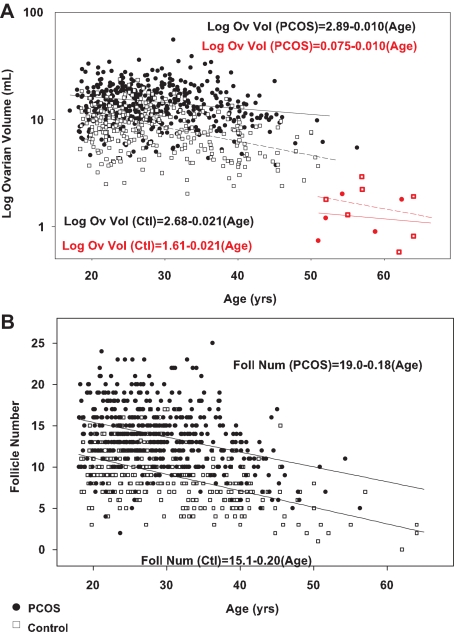
Women with PCOS had a higher ovarian volume and follicle number, and both were lower in older compared with younger subjects within the PCOS and control groups (Table 2 and Fig. 3). Similarly, ovarian volume and follicle number decreased in PCOS and control subjects studied longitudinally, and subjects with PCOS had a greater decrease in follicle number over time than control subjects (Table 3 and supplemental Table 2).
Figure 3.
Log ovarian volume (A) and follicle number (B) as a function of age in PCOS (black circles) and control subjects (open squares) and postmenopausal PCOS (red circles) and control subjects (red squares). Our pathology study demonstrated no follicles in postmenopausal women; therefore, there are no postmenopausal women included in the panel depicting follicle number (B). The slope of the log ovarian volume regression line is significantly different in PCOS and control subjects (P < 0.01).
Using previously defined criteria to predict PCOS in women under 36 yr old, follicle number (1) and ovarian volume (31) demonstrated a sensitivity of 82% for both in the cross-sectional data, similar to previous results. However, the specificity of 66 and 61%, respectively, was lower than in previous data. Using the same criteria in women over 40 yr, the sensitivity (21 and 60%, respectively) was much lower, although the specificity (100 and 96%, respectively) was excellent.
Premenopausal and postmenopausal ovaries were examined separately in cross-sectional data based on an observed data separation and an absence of antral follicles on pathological exam in postmenopausal women. In premenopausal women, log ovarian volume decreased linearly with age in PCOS (log ovarian volume = 2.89 + −0.010 × age) and control subjects (log ovarian volume = 2.68 + −0.021 × age) (Fig. 3A).
In postmenopausal women, ovarian volume was not different in PCOS and control subjects (1.3 ± 0.6 vs. 1.6 ± 0.8 ml; P = 0.4) and falls within the range reported in previous studies (0.9 ± 0.6 to 2.2 ± 0.01 ml) (7,32). Nevertheless, log ovarian volume decreased linearly with age in postmenopausal PCOS (log ovarian volume = 0.75 + −0.010 × age) and control subjects (log ovarian volume = 1.61 + −0.021 × age), with a difference in the slopes (P < 0.01; Fig. 3A). Furthermore, the decrement in the intercept in postmenopausal compared with premenopausal women was greater for women with PCOS than control women (2.14 vs. 1.07; P < 0.0001).
Follicle number decreased linearly with age in women with PCOS (follicle number = 19.0 + −0.18 × age) and control subjects (follicle number = 15.1 + −0.20 × age). The decrease was not different in the two groups (Fig. 3B).
Based on the linear decrease in log ovarian volume, follicle number, and testosterone with age, a cutoff to predict PCOS needs to be age based. PCOS could be predicted by the following equation: log (odds of PCOS) = −10.1302 + 0.0978 × age + 0.2698 × follicle number + 0.6967 × log volume + 0.0632 × testosterone. This combination of age, follicle number, log ovarian volume, and testosterone predicted PCOS with a receiver operator characteristic curve area of 0.90. A log (odds of PCOS) score of 0.51 or higher results in a specificity of 83% and a sensitivity of 83% for predicting PCOS.
Discussion
The pathology, longitudinal, and cross-sectional studies provide a comprehensive look at ovarian aging in well characterized women with PCOS. The data demonstrate that ovarian volume and follicle number decrease longitudinally, extending previous cross-sectional findings (5,6). As a result of the decrease, less than 50% of subjects with PCOS met the criteria for PCOM at follow-up (4). Both the longitudinal and the extensive cross-sectional data demonstrate that the decrement in follicle number with age was parallel but higher in subjects with PCOS compared with regularly cycling controls, whereas the pathology study demonstrates no follicles in postmenopausal ovaries. In contrast, the fall in ovarian volume with age was less pronounced in women with PCOS, although the average ovarian volume was similar in postmenopausal subjects with PCOS and controls, suggesting a greater decrement in ovarian volume in women with PCOS in transition to menopause. Taken together, age-based criteria are necessary to define PCOM.
Incorporating the ovarian and androgen changes with age, the best model to predict PCOS includes ovarian volume, follicle number, testosterone, and age. Although the sensitivity and specificity of the model are not as great as for the parameters previously proposed to predict PCOS, the previous studies apply to reproductive-age women and do not take into account the change in ovarian morphology with age (1,2,4). Indeed, the specificity to predict PCOS was best when incorporating ovarian volume, follicle number, and testosterone in the model. The clinical significance is the possibility that PCOS may be predicted in older reproductive-age women despite uninformative menstrual cycle data, the potential removal of clinically significant hirsutism, or the decrease in androgens with time (33,34).
The change in follicle number with age has previously been modeled only in population studies and control women. Studies using whole ovaries and ovarian biopsies demonstrate a decline in follicle number with age (12), which becomes more rapid after age 40 yr (17). Therefore, when antral follicle count on ultrasound is used as a proxy for total follicle number (10), one expects a similar pattern of follicle decline. However, our ultrasound follicle counts and others demonstrate the best data fit using a linear decline in follicle number (9,35,36,37) rather than quadratic (38), logarithmic (9), or biphasic (10). Our data also demonstrate a linear decline in follicle number with age in women with PCOS, as suggested previously by an inverse correlation between follicle number and age (5,34). Importantly, the follicle number was higher at all ages in PCOS compared with control women, with a parallel decline.
Ovarian volume has been a less consistent correlate of age. The inconsistency does not appear to be related to technique because ovarian volume obtained by ultrasound is accurate when compared with pathology in this study and others (39). The variability likely results from failure to control for follicle development, hormone therapy, and menopausal status. With these limitations in mind, the largest study of ovarian volume assessed ultrasound for ovarian cancer screening (7). The study demonstrated a stable ovarian volume to age 35 yr, the greatest decline between 35 and 55 yr, and a very minor decline after age 55 yr (7). Similar to follicle number, other studies demonstrate a linear (8,40), biphasic (41), or quadratic decline (38). The current study demonstrates a linear decline in ovarian volume in controls and in women with PCOS for the first time, but a higher initial volume, a lesser slope, and a greater decrement in the volume change from premenopause to postmenopause in women with PCOS.
In contrast to the cross-sectional data, we did not demonstrate a difference in the longitudinal decline in ovarian volume between PCOS and control subjects. The longitudinal studies are limited by small numbers and the sequential recruitment of control and PCOS subjects, although the ultrasound machine and technician were the same for both. A correlation between the decrease in follicle number and ovarian volume suggests that the decrease in follicle number may partially explain the decrease in ovarian volume (9,10,11,12). However, the volume does not decrease as markedly as follicle number before age 35 yr (42), and a lesser decline in the ovarian volume despite a similar decline in follicle number in women with PCOS compared with controls suggest that a different ovarian compartment accounts for the difference in slopes. Indeed, the prominent stromal component in the ovaries of women with PCOS (43) may account for the difference.
Our pathology data were limited by the availability of specimens from women with documented PCOS and by the concomitant pathology for which the oophorectomy was performed. Although limited, the pathology was critical to interpret the ultrasound findings. Indeed, the hypoechoic structures on ultrasound in postmenopausal women with PCOS corresponded to inclusion cysts and vascular structures and were therefore not reliable indicators of the presence of follicles. Similarly, previous pathology studies do not demonstrate secondary follicles in postmenopausal ovaries (17). Therefore, we did not analyze follicle number in postmenopausal women. Thus, it is unlikely that PCOM persists into menopause despite previous reports (6).
The longitudinal data confirm and extend previous findings suggesting that menstrual cycles may become regular with age in women with PCOS (6,18). Although previous studies suggest that those women who gain regular menstrual cyclicity have fewer follicles, the current data did not indicate a relationship (5,18). As in previous studies, there was no relationship between weight and cycle regularity (5). Rather, the FSH levels increased similarly in women with PCOS and controls, but the increase may have been sufficient to drive follicle development in PCOS.
Older women with PCOS have a lower Ferriman-Gallwey score, testosterone, androstenedione, and DHEAS compared with younger women, but all values remain higher than in older control women with the exception of DHEAS (33,34,44,45,46). Furthermore, testosterone levels tended to decrease when assessed longitudinally, supporting these cross-sectional studies. Therefore, hyperandrogenemia should be defined on the basis of normative data for both the assay and age of the patient.
As has been suggested previously, the women with PCOS over 35 yr had higher BMI, HOMA, glucose, and triglyceride levels compared with controls (6,45,47,48). Similar to a previous longitudinal study, BMI and triglyceride levels increased in women with PCOS over time (49). SBP and DBP increase over time or with increasing age in controls in contrast to PCOS subjects whose SBP and DBP were higher at all ages examined. Thus, our cross-sectional and longitudinal data provide evidence that women with PCOS have higher levels of metabolic syndrome components across a broad range of age, and our longitudinal data suggest there is no improvement over time; thus, untreated women with PCOS have a longer exposure to these adverse cardiovascular risk factors. More longitudinal data are needed to determine whether the exposure translates into an increased risk of coronary vascular disease.
Log ovarian volume and follicle number decrease linearly with age in women with PCOS and controls necessitating age-based criteria to define PCOM. These parameters, along with testosterone, may distinguish PCOS in women over age 40 yr who have not previously been diagnosed. Although measurement of cardiovascular risk factors in older women assesses risk at one point in time, identifying women with PCOS over 40 yr could alert a physician to a long exposure to adverse cardiovascular risk factors, perhaps prompting more aggressive treatment. Identification may also be useful to phenotype mothers and relatives in genetic studies. Thus, age-based criteria should be incorporated to define PCOM and to diagnose PCOS.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grant U01 HD 4417 and National Center for Research Resources General Clinical Research Centers Program Grant M01-RR-01066.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 21, 2009
Abbreviations: BMI, Body mass index; DBP, diastolic blood pressure; HOMA, homeostasis model assessment; PCOM, polycystic ovarian morphology; PCOS, polycystic ovarian syndrome; SBP, systolic blood pressure.
References
- Jonard S, Robert Y, Cortet-Rudelli C, Pigny P, Decanter C, Dewailly D 2003 Ultrasound examination of polycystic ovaries: is it worth counting the follicles? Hum Reprod 18:598–603 [DOI] [PubMed] [Google Scholar]
- Pache TD, Wladimiroff JW, Hop WC, Fauser BC 1992 How to discriminate between normal and polycystic ovaries: transvaginal US study. Radiology 183:421–423 [DOI] [PubMed] [Google Scholar]
- Adams J, Franks S, Polson DW, Mason HD, Abdulwahid N, Tucker M, Morris DV, Price J, Jacobs HS 1985 Multifollicular ovaries: clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet 2:1375–1379 [DOI] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group 2004 Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19:41–47 [DOI] [PubMed] [Google Scholar]
- Elting MW, Kwee J, Korsen TJ, Rekers-Mombarg LT, Schoemaker J 2003 Aging women with polycystic ovary syndrome who achieve regular menstrual cycles have a smaller follicle cohort than those who continue to have irregular cycles. Fertil Steril 79:1154–1160 [DOI] [PubMed] [Google Scholar]
- Birdsall MA, Farquhar CM 1996 Polycystic ovaries in pre- and post-menopausal women. Clin Endocrinol (Oxf) 44:269–276 [DOI] [PubMed] [Google Scholar]
- Pavlik EJ, DePriest PD, Gallion HH, Ueland FR, Reedy MB, Kryscio RJ, van Nagell Jr JR 2000 Ovarian volume related to age. Gynecol Oncol 77:410–412 [DOI] [PubMed] [Google Scholar]
- Oppermann K, Fuchs SC, Spritzer PM 2003 Ovarian volume in pre- and perimenopausal women: a population-based study. Menopause 10:209–213 [DOI] [PubMed] [Google Scholar]
- Ruess ML, Kline J, Santos R, Levin B, Timor-Tritsch I 1996 Age and the ovarian follicle pool assessed with transvaginal ultrasonography. Am J Obstet Gynecol 174:624–627 [DOI] [PubMed] [Google Scholar]
- Scheffer GJ, Broekmans FJ, Dorland M, Habbema JD, Looman CW, teVelde ER 1999 Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility. Fertil Steril 72:845–851 [DOI] [PubMed] [Google Scholar]
- Erdem A, Erdem M, Biberoglu K, Hayit O, Arslan M, Gursoy R 2002 Age-related changes in ovarian volume, antral follicle counts and basal FSH in women with normal reproductive health. J Reprod Med 47:835–839 [PubMed] [Google Scholar]
- Lass A, Silye R, Abrams DC, Krausz T, Hovatta O, Margara R, Winston RM 1997 Follicular density in ovarian biopsy of infertile women: a novel method to assess ovarian reserve. Hum Reprod 12:1028–1031 [DOI] [PubMed] [Google Scholar]
- Polson DW, Adams J, Wadsworth J, Franks S 1988 Polycystic ovaries: a common finding in normal women. Lancet 1:870–872 [DOI] [PubMed] [Google Scholar]
- Clayton RN, Ogden V, Hodgkinson J, Worswick L, Rodin DA, Dyer S, Meade TW 1992 How common are polycystic ovaries in normal women and what is their significance for the fertility of the population? Clin Endocrinol (Oxf) 37:127–134 [DOI] [PubMed] [Google Scholar]
- Farquhar CM, Birdsall M, Manning P, Mitchell JM, France JT 1994 The prevalence of polycystic ovaries on ultrasound scanning in a population of randomly selected women. Aust NZ J Obstet Gynaecol 34:67–72 [DOI] [PubMed] [Google Scholar]
- Murphy MK, Hall JE, Adams JM, Lee H, Welt CK 2006 Polycystic ovarian morphology in normal women does not predict the development of polycystic ovary syndrome. J Clin Endocrinol Metab 91:3878–3884 [DOI] [PubMed] [Google Scholar]
- Richardson SJ, Senikas V, Nelson JF 1987 Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab 65:1231–1237 [DOI] [PubMed] [Google Scholar]
- Elting MW, Korsen TJ, Rekers-Mombarg LT, Schoemaker J 2000 Women with polycystic ovary syndrome gain regular menstrual cycles when ageing. Hum Reprod 15:24–28 [DOI] [PubMed] [Google Scholar]
- Bean JA, Leeper JD, Wallace RB, Sherman BM, Jagger H 1979 Variations in the reporting of menstrual histories. Am J Epidemiol 109:181–185 [DOI] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Dodson WC, Dunaif A 1999 Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 84:165–169 [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J 1999 Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 22:141–146 [DOI] [PubMed] [Google Scholar]
- Guzick DS 2004 Cardiovascular risk in PCOS. J Clin Endocrinol Metab 89:3694–3695 [DOI] [PubMed] [Google Scholar]
- Zawadzki JK, Dunaif A 1992 Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, eds. Polycystic ovary syndrome. Boston: Blackwell Scientific; 377–384 [Google Scholar]
- Taylor AE, McCourt B, Martin KA, Anderson EJ, Adams JM, Schoenfeld DA, Hall JE 1997 Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab 82:2248–2256 [DOI] [PubMed] [Google Scholar]
- Welt CK, Arason G, Gudmundsson JA, Adams J, Palsdóttir H, Gudlaugsdóttir G, Ingadóttir G, Crowley WF 2006 Defining constant versus variable phenotypic features of women with polycystic ovary syndrome using different ethnic groups and populations. J Clin Endocrinol Metab 91:4361–4368 [DOI] [PubMed] [Google Scholar]
- Ferriman D, Gallwey JD 1961 Clinical assessment of body hair growth in women. J Clin Endocrinol Metab 21:1440–1447 [DOI] [PubMed] [Google Scholar]
- Sample WF, Lippe BM, Gyepes MT 1977 Gray-scale ultrasonography of the normal female pelvis. Radiology 125:477–483 [DOI] [PubMed] [Google Scholar]
- Welt CK, Pagan YL, Smith PC, Rado KB, Hall JE 2003 Control of follicle-stimulating hormone by estradiol and the inhibins: critical role of estradiol at the hypothalamus during the luteal-follicular transition. J Clin Endocrinol Metab 88:1766–1771 [DOI] [PubMed] [Google Scholar]
- Adams JM, Taylor AE, Crowley Jr WF, Hall JE 2004 Polycystic ovarian morphology with regular ovulatory cycles: insights into the pathophysiology of polycystic ovarian syndrome. J Clin Endocrinol Metab 89:4343–4350 [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Jonard S, Robert Y, Dewailly D 2005 Revisiting the ovarian volume as a diagnostic criterion for polycystic ovaries. Hum Reprod 20:2893–2898 [DOI] [PubMed] [Google Scholar]
- Flaws JA, Langenberg P, Babus JK, Hirshfield AN, Sharara FI 2001 Ovarian volume and antral follicle counts as indicators of menopausal status. Menopause 8:175–180 [DOI] [PubMed] [Google Scholar]
- Winters SJ, Talbott E, Guzick DS, Zborowski J, McHugh KP 2000 Serum testosterone levels decrease in middle age in women with the polycystic ovary syndrome. Fertil Steril 73:724–729 [DOI] [PubMed] [Google Scholar]
- Bili H, Laven J, Imani B, Eijkemans MJ, Fauser BC 2001 Age-related differences in features associated with polycystic ovary syndrome in normogonadotrophic oligo-amenorrhoeic infertile women of reproductive years. Eur J Endocrinol 145:749–755 [DOI] [PubMed] [Google Scholar]
- Ng EH, Yeung WS, Fong DY, Ho PC 2003 Effects of age on hormonal and ultrasound markers of ovarian reserve in Chinese women with proven fertility. Hum Reprod 18:2169–2174 [DOI] [PubMed] [Google Scholar]
- Broekmans FJ, Faddy MJ, Scheffer G, te Velde ER 2004 Antral follicle counts are related to age at natural fertility loss and age at menopause. Menopause 11:607–614 [DOI] [PubMed] [Google Scholar]
- van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, Fauser BJ, Themmen AP, te Velde ER 2005 Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril 83:979–987 [DOI] [PubMed] [Google Scholar]
- Tufan E, Elter K, Durmusoglu F 2004 Assessment of reproductive ageing patterns by hormonal and ultrasonographic ovarian reserve tests. Hum Reprod 19:2484–2489 [DOI] [PubMed] [Google Scholar]
- van Santbrink EJ, Hop WC, Fauser BC 1997 Classification of normogonadotropic infertility: polycystic ovaries diagnosed by ultrasound versus endocrine characteristics of polycystic ovary syndrome. Fertil Steril 67:452–458 [DOI] [PubMed] [Google Scholar]
- Giacobbe M, Pinto-Neto AM, Costa-Paiva LH, Martinez EZ 2004 Ovarian volume, age, and menopausal status. Menopause 11:180–185 [DOI] [PubMed] [Google Scholar]
- Bastos CA, Oppermann K, Fuchs SC, Donato GB, Spritzer PM 2006 Determinants of ovarian volume in pre-, menopausal transition, and post-menopausal women: a population-based study. Maturitas 53:405–412 [DOI] [PubMed] [Google Scholar]
- Broekmans FJ, Faddy M, te Velde ER 2005 Ovarian reserve and reproductive age may be determined from measurement of ovarian volume by transvaginal sonography. Hum Reprod 20:1114–1115; author reply 1115–1116 [DOI] [PubMed] [Google Scholar]
- Fulghesu AM, Ciampelli M, Belosi C, Apa R, Pavone V, Lanzone A 2001 A new ultrasound criterion for the diagnosis of polycystic ovary syndrome: the ovarian stroma/total area ratio. Fertil Steril 76:326–331 [DOI] [PubMed] [Google Scholar]
- Dahlgren E, Johansson S, Lindstedt G, Knutsson F, Odén A, Janson PO, Mattson LA, Crona N, Lundberg PA 1992 Women with polycystic ovary syndrome wedge resected in 1956 to 1965: a long-term follow-up focusing on natural history and circulating hormones. Fertil Steril 57:505–513 [DOI] [PubMed] [Google Scholar]
- Loucks TL, Talbott EO, McHugh KP, Keelan M, Berga SL, Guzick DS 2000 Do polycystic-appearing ovaries affect the risk of cardiovascular disease among women with polycystic ovary syndrome? Fertil Steril 74:547–552 [DOI] [PubMed] [Google Scholar]
- Puurunen J, Piltonen T, Jaakkola P, Ruokonen A, Morin-Papunen L, Tapanainen JS 2009 Adrenal androgen production capacity remains high up to menopause in women with polycystic ovary syndrome. J Clin Endocrinol Metab 94:1973–1978 [DOI] [PubMed] [Google Scholar]
- Talbott E, Clerici A, Berga SL, Kuller L, Guzick D, Detre K, Daniels T, Engberg RA 1998 Adverse lipid and coronary heart disease risk profiles in young women with polycystic ovary syndrome: results of a case-control study. J Clin Epidemiol 51:415–422 [DOI] [PubMed] [Google Scholar]
- Wild S, Pierpoint T, Jacobs H, McKeigue P 2000 Long-term consequences of polycystic ovary syndrome: results of a 31 year follow-up study. Hum Fertil 3:101–105 [DOI] [PubMed] [Google Scholar]
- Pasquali R, Gambineri A, Anconetani B, Vicennati V, Colitta D, Caramelli E, Casimirri F, Morselli-Labate AM 1999 The natural history of the metabolic syndrome in young women with the polycystic ovary syndrome and the effect of long-term oestrogen-progestagen treatment. Clin Endocrinol (Oxf) 50:517–527 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.