Abstract
Context: Exceptional longevity is associated with raised serum TSH.
Objective: The aim of this study was to examine whether offspring of people with exceptional longevity have elevated serum TSH and whether specific single nucleotide polymorphisms (SNPs) in the TSH-B gene and TSH receptor (TSHR) gene are associated with this phenotype.
Design/Setting/Patients: We measured serum TSH and free T4 in Ashkenazi Jewish centenarians (n = 232; median age, 97 yr), their offspring (n = 366; median age, 69 yr), and age-matched controls without familial longevity (n = 163; median age, 70 yr). We determined TSH heritability, its distribution, and association with SNPs in the TSH-B and TSHR genes.
Results: Offspring had higher median serum TSH [1.68 mIU/liter (97.5% confidence interval, 0.65 to 4.79 mIU/liter)], compared to controls [1.50 mIU/liter (97.5% confidence interval, 0.63 to 3.93 mIU/liter); P = 0.02], with estimated heritability of 0.33 (P = 0.004). Allele frequency of two SNPs in the promoter/enhancer region of TSHR gene, associated with increased serum TSH, was higher in centenarians and their offspring compared to controls (rs10149689 G allele frequency, 0.57 and 0.53 vs. 0.48; P = 0.001 and P = 0.08; odds ratio, 1.56 and 1.22, respectively; and rs12050077 A allele frequency, 0.57 and 0.53 vs. 0.46; P = 0.0001 and P = 0.01; odds ratio, 1.68 and 1.32, respectively). Linkage disequilibrium between the two SNPs was high (r2 = 0.95), suggesting interaction between them. Furthermore, GA haplotype frequency was significantly higher among centenarians and offspring compared to controls (0.57 and 0.53 vs. 0.46; P = 0.0001 and P = 0.01, respectively).
Conclusions: A heritable phenotype characterized by raised serum TSH is associated with human longevity. Carriers of rs12050077 and rs10149689 SNPs in the TSHR have higher serum TSH, possibly contributing to decreased thyroid function and longevity.
Offspring of centenarians have elevated serum TSH levels and demonstrate two single nucleotide TSH receptor gene polymorphisms that are associated with higher serum TSH.
Several studies in humans suggest decreases in thyroid function with aging, which are considered to be independent of antithyroid antibody levels or age-related comorbidities (1,2,3). We have previously demonstrated that the increased TSH with aging reflects a progressive population shift to higher serum TSH with age that extends even to people who have achieved exceptional longevity (4,5,6). Because increased serum TSH concentrations may reflect decreased thyroid function, we hypothesized that exceptional longevity may be associated with decreased thyroid hormone secretion (6). Furthermore, we have recently reported a significant inverse correlation between serum TSH and free T4 (FT4) in Ashkenazi Jewish centenarians and younger Ashkenazi Jewish controls (6), suggesting a functional feedback in thyroid hormone regulation that results in a relative (subclinical) decrease in thyroid function.
These findings in humans are further supported by animal studies that demonstrate an inverse correlation between extended life span and decreased thyroid function (7,8), suggesting a causal relationship. The precise mechanisms responsible for the effects of hypothyroidism on life span, however, have not been clarified, although multiple actions of decreased thyroid hormone concentrations (including lowering the metabolic rate, lowering core body temperature and oxygen consumption, and reducing reactive oxygen species generation and oxidative damage) may play an important role in longevity (9,10,11,12,13,14).
Hereditary and genetic influences on serum thyroid hormone and TSH concentrations have been reported in twin studies (15,16) and in Mexican-American families (17). In addition, several recent reports show that polymorphisms in several thyroid pathway genes could influence serum thyroid hormone and TSH concentrations (18,19,20).
In this study, we hypothesized that the alterations in thyroid function seen in human longevity may have a genetic basis. Consequently, we examined thyroid function in offspring of individuals with exceptional longevity and searched for genetic markers that may be responsible for the specific pattern of thyroid function tests observed in this population.
This project was part of the Longevity program at Albert Einstein College of Medicine, Bronx, New York.
Subjects and Methods
Population
We studied three distinct populations: 1) 232 Ashkenazi Jewish centenarians (166 women, median age, 97 yr; and 66 men, median age, 97 yr); 2) 366 Ashkenazi Jewish offspring of these centenarians (185 women, median age, 68 yr; and 181 men, median age, 69 yr); and 3) 111 of the offsprings’ Ashkenazi Jewish spouses (control group A; 51 women, median age, 66 yr; and 60 men, median age, 73 yr). An additional control group of 52 Ashkenazi Jews (control group B; 28 women, median age, 70 yr; and 24 men, median age, 75 yr), which was not family-related to the above subjects but was from the same geographic area, was similarly assessed. Two control groups were used to exclude any possibility of environmental factors affecting the thyroid function tests in control group A (subjects lived in the same household as their spouse, the offspring). However, all parameters tested were virtually identical in both control groups (data not shown). Thus, in our report, the controls represent control group A and control group B combined. All participants were recruited randomly.
Stated age was verified by checking birth certificates or U.S. passports. Jewish religion was self-reported (mother and father Jewish), and subjects were considered Ashkenazi Jews if their four grandparents were originally Ashkenazi Jews. Information on medical history, demographic characteristics, and clinical data were obtained uniformly using a structured questionnaire. All subjects underwent a physical examination and were asked to provide a blood sample. Informed written consent was obtained in accordance with the policy of the Committee on Clinical Investigation of the Albert Einstein College of Medicine.
Exclusion criteria included acute or debilitating medical condition, history of thyroid disease, and/or subjects taking thyroid medications. Additionally, 10 participants in the control groups were excluded because their biological parents lived past 85 yr of age.
We chose to study Ashkenazi Jews due to their known genetic homogeneity that is attributed to founder effects. The founders lived in the 16th to 17th centuries in eastern and central Europe. Although this population experienced isolation, inbreeding, and genetic drift, it has undergone rapid growth after immigration to the United States, beginning in the 18th century. In the past, the Ashkenazi population has served as a valuable genetic resource to discover the genetic bases of several Mendelian disorders as well as age-related diseases (21).
Founder populations amplify certain gene variants while maintaining great stretches of uniformity in other DNA sequences. Genes that are very close together exhibit extremely tight linkage (linkage disequilibrium), which is particularly pronounced in isolated populations. Numerous association studies have exploited the properties of linkage disequilibrium, genetically uniform background in the isolated and founder populations, such as the Finnish and Icelandic populations (22,23,24).
TSH and FT4 analyses
Measurements of TSH were performed at the laboratories of Montefiore Medical Center, Bronx, New York, using a solid-phase, two-site chemiluminescent immunometric assay (Immunolite 2000 Third Generation TSH; Siemens Corp., Tarrytown, NY; manufacturer’s reference limits, 0.4–4.0 mIU/liter). FT4 was measured using ELISA (Alpco Diagnostic, Salem, NH; manufacturer’s reference limits, 0.8–1.8 ng/dl). Because this method provides only an estimate of FT4 and not a direct measurement of FT4, all FT4 values represent estimated levels of FT4. Antithyroid antibodies were not determined because of insufficient availability of serum.
Due to logistic and technical restrictions, serum FT4 was analyzed in 174 offspring and 172 controls. However, only 100 offspring and 134 controls had both serum TSH and FT4 determined (inter- and intraassay coefficients of variation were 1.5 and 3% for TSH, and 3 and 6% for FT4).
All blood draws were performed in the morning after an overnight fast.
Analysis of single nucleotide polymorphisms (SNPs)
The TSH gene, also called TSH-B (TSH subunit-β), spans 4.5 kb with three exons and is located on the long arm of chromosome 1. We selected four SNPs (rs10494170, rs12046203, rs12740035, and rs7523360) of TSH-B gene and genotyped them in both Ashkenazi Jewish centenarians and controls. The allelic frequencies of these SNPs between the Ashkenazi Jewish centenarians and controls did not show significant differences. Due to the association between TSH receptor (TSHR) and TSH expression, we further examined SNPs in this gene. SNPs (rs12050077 and rs10149689) were genotyped using the PSQ HS 96A Pyrosequencer, according to the manufacturer’s recommendations (Pyrosequencing, Uppsala, Sweden; www.pyrosequencing.com). Briefly, a PCR product was generated from a primer pair that included one primer covalently coupled to biotin, the biotinylated template was bound to streptavidin-coated Sepharose HP beads, and this mixture was then annealed to a sequencing primer. Stepwise elongation of a sequencing primer strand upon sequential addition of a specified sequence of deoxynucleotide triphosphates and the degradation of nucleotides by apyrase were carried out simultaneously. As the sequencing reaction progressed, the DNA strand was extended, and the sequence was determined from the measured signal output of light upon nucleotide incorporation. The resulting peaks in the pyrogram were analyzed using Pyrosequencing software. Primer sequences are available from the authors upon request. Error rates based on blind replicates were estimated to be 0–5%.
Statistical analysis
All analyses were conducted using Statistical Analysis Software (SAS Institute, Cary NC), version 9.12. Because serum TSH and FT4 were not normally distributed, nonparametric tests including the Kruskal-Wallis one-way ANOVA by ranks and Mann-Whitney rank sum test were used for group comparisons.
χ2 test was performed to compare the difference in TSHR genotype frequencies. Genotype frequencies of all SNPs were found to be in Hardy-Weinberg equilibrium. Because two SNPs (in the TSHR) were involved in an independent new assay, the Bonferroni correction for multiple comparisons was used; thus the threshold for statistical significance was P < 0.025. Given that this is a relatively homogeneous population, tests for population stratification were not performed. The haplotype association test between cases (Ashkenazi Jewish centenarians) and controls was performed using Haploview 4 software (http://www.broad.institut.org/haploview/haploview). Wilcoxon statistics were calculated to test homogeneity between the groups.
Heritability analysis
The phenotypic variance represents a combination of genetic (additive) variance, environmental variance, and the covariance between these two factors. However, we were only interested in the additive effect (narrow sense), which was measured as the covariance (i.e. the slope) of the relationship between the average performance of the offspring over the average performance of their parents multiplied by 2 because there was only one parent involved.
Results
Demographic data
The distribution of our population by age and gender is depicted in Table 1. The median age of offspring (69 yr) was not different from controls (70 yr). However, the median age of female controls, 67 yr [97.5% confidence interval (CI), 52–80], was significantly lower than male controls, 74 yr (97.5% CI, 59–80; P = 0.002).
Table 1.
Age, TSH, and FT4 in female and male controls, offspring, and probands (Ashkenazi Jewish centenarians)
| Controls | Offspring | Probands | |
|---|---|---|---|
| n (females/males) | 163 (79/84) | 366 (185/181) | 232 (166/66) |
| Age (yr) | |||
| All | 70 (53–80) | 69 (59–79) | 97 (95–105) |
| Females | 67 (52–80) | 68 (59–79) | 97 (95–103) |
| Males | 74 (59–80) | 69 (59–79) | 97 (95–103) |
| TSH (mIU/liter) | |||
| All | 1.55 (0.63–3.93) | 1.68 (0.65–4.79)a | 1.97 (0.42–7.15)a |
| Females | 1.60 (0.60–4.7) | 1.72 (0.51–6.3)a | 2.00 (0.53–7.34)a |
| Males | 1.50 (0.55–4.50) | 1.68 (0.65–5.9)a | 1.93 (0.61–6.9)a |
| FT4 (ng/dl) | |||
| All | 1.00 (0.69–1.7) | 1.03 (0.67–2.0) | 1.02 (0.62–2.02) |
| Females | 0.99 (0.74–1.5) | 1.04 (0.66–1.9)a | 1.04 (0.65–2.02)a |
| Males | 1.00 (0.57–1.7) | 1.02 (0.67–2.0) | 0.95 (0.48–2.06)a |
Data are expressed as median (97.5% CI).
P < 0.05 vs. controls.
Serum TSH distribution
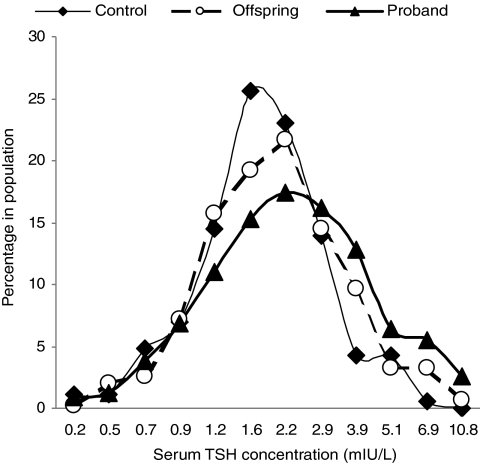
Serum TSH concentrations in the offspring were significantly higher compared with controls [median, 97.5% CI: 1.68 (0.65–4.79) and 1.55 (0.63–3.93) mIU/liter, respectively; P = 0.02; Table 1). Moreover, serum TSH distribution was also shifted to higher TSH concentrations in offspring compared with controls, including the TSH concentrations at peak frequency (Fig. 1), suggesting a population shift phenomenon. TSH distribution in Ashkenazi Jewish centenarians, recently published (4) and shown only for comparative purposes, was further shifted to higher TSH concentrations (Fig. 1) compared with offspring, suggesting a genetic predisposition for higher TSH.
Figure 1.
TSH distribution in thyroid disease-free Ashkenazi Jewish centenarians, their offspring, and controls. The TSH frequency distribution curves were prepared using log-transformed values of TSH concentrations. Bin values were calculated by using the antilog function on the equal interval concentrations. Note: intervals on horizontal axis are not equal; the TSH data point is the upper TSH concentration in bin.
To determine whether medications taken by participants may affect TSH concentration, we applied a linear regression model where serum TSH levels served as a dependent variable, and various medications taken by our study subjects served as independent variables; no significant effect of any medication was detected. Furthermore, stepwise regression of a mixed model, where probability to enter was 0.25 and probability to leave was 0.05, demonstrated that none of the medications had any significant effect on serum TSH. Drug categories and percentage of subjects using the drugs are listed in Table 2, as well as the percentage of subjects taking one or more drugs.
Table 2.
Percentage of subjects taking drugs from various categories and percentage of subjects using medication(s)
| % of total | |
|---|---|
| Drug categories | |
| Blood pressure | 35.43 |
| Heart disease | 21.40 |
| Diabetes mellitus | 5.56 |
| Dyslipidemia | 27.30 |
| Depression | 13.44 |
| Sleep medications | 10.21 |
| Aspirin | 20.83 |
| Anticoagulants | 0.75 |
| No. of drugs taken by same person | |
| 0 | 32.69 |
| 1 | 26.06 |
| 2 | 20.58 |
| 3 | 12.95 |
| 4 | 6.06 |
| 5 | 1.41 |
| 6 | 0.17 |
| 7 | 0.08 |
The differences in age distributions between males vs. females had no effect on the outcome regarding TSH levels, even when plotted logarithmically. In fact, when logistic regression was applied, adjusting for age, the P value was 0.91, indicating total similarity (Fig. 2).
Figure 2.
Logarithmic plot of age distribution in males and females. Dashed line represents females, and continuous line represents males.
Serum FT4 distribution
Serum FT4 concentrations were similar in offspring and controls with a median of 1.03 ng/dl (97.5% CI, 0.67–2.00) in the offspring and 1.00 ng/dl (97.5% CI, 0.69–1.7) in the control group (P = not significant; Table 2).
There was no gender-specific difference between serum FT4 concentrations in males in all groups. Female offspring had higher serum FT4 levels than female controls [1.04 ng/dl (97.5% CI, 0.66–1.9) vs. 0.99 ng/dl (97.5% CI, 0.74–1.5); P = 0.02].
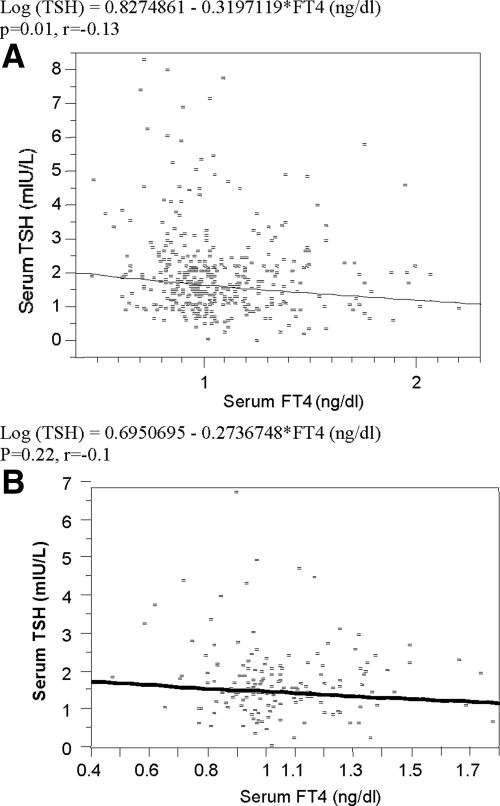
There was a logarithmically inverse correlation between serum TSH concentrations and serum FT4 concentrations in all groups (r = −0.13; P = 0.01; Fig. 3A), but not in the control (r = −0.1; P = 0.22; Fig. 3B).
Figure 3.
The correlation between serum TSH and FT4 concentrations in all groups (A) and in controls (B).
Genetic analyses
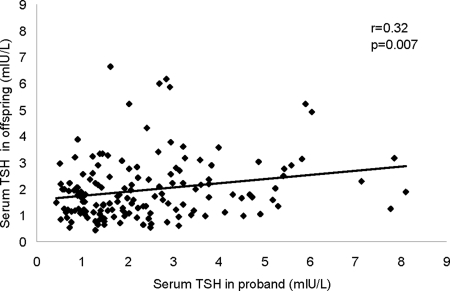
Because both Ashkenazi Jewish centenarians (6) and their offspring had significantly higher serum TSH concentrations compared with controls, we determined whether TSH concentrations in these groups might be inherited. Heritability analysis demonstrated a moderate degree of heritability (h2 =0.32; P = 0.007) for serum TSH in Ashkenazi Jewish centenarians and their offspring (Fig. 4).
Figure 4.
Hereditability analysis of serum TSH in Ashkenazi Jewish centenarians (probands) and their offspring.
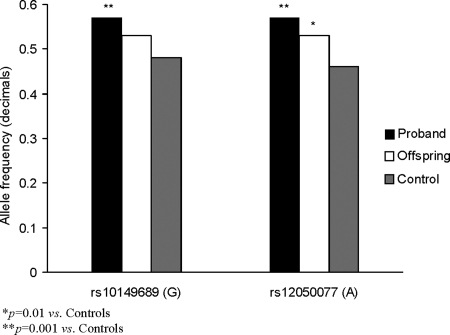
To examine further the role of genetic factors, we performed a case-control genetic analysis of common TSH and TSHR variants (4 and 2, respectively). These SNPs were a part of a larger group of SNPs in another independent dataset comprised of individuals from the same genetic origin. Although the SNPs in the TSH-B in this dataset did not show significant changes between cases and controls, the SNPs in the TSHR did show significant differences. Thus, we chose to examine further those SNPs using a different technology and with an independent dataset. The allelic frequency for two SNPs in the TSHR gene was significantly different comparing Ashkenazi Jewish centenarians and controls [rs10149689 G allele frequency, 0.57 vs. 0.48; P = 0.001; odds ratio (OR), 1.56] and (rs12050077 A allele frequency, 0.57 vs. 0.46; P = 0.0001; OR 1.68). Similarly, allelic frequency also differed between the offspring and controls (rs10149689 G allele frequency, 0.53 vs. 0.48; P = 0.08; OR 1.22; and rs12050077 A allele frequency, 0.53 vs. 0.46; P = 0.01; OR 1.32; Fig. 5). Allelic frequencies for these SNPs were not significantly different between offspring and centenarians.
Figure 5.
GA haplotype prevalence in Ashkenazi Jewish centenarians (probands), their offspring, and controls.
Linkage disequilibrium between the two SNPs was high (r2 = 0.95), suggesting interaction between them. The frequency of GA haplotype was significantly greater among both Ashkenazi Jewish centenarians and their offspring compared with controls (0.57 and 0.53 vs. 0.46; P = 0.0001 and P = 0.01, respectively).
In a dominant model comparing offspring to controls with adjustment for gender, we found that the median TSH in those who had rs10149689 (G allele) and rs12050077 (A allele) was significantly higher than those who had neither of the alleles [median (97.5% CI), 1.42 (0.56 to 3.33) vs. 1.68 (0.63 to 6.06) mIU/liter in controls, P = 0.02; and 1.49 (0.46 to 3.74) vs. 1.70 (0.68 to 5.38) mIU/liter, P = 0.049 in offspring]. Similarly, the median TSH level in the combined groups (control and offspring) associated with GA haplotype was significantly higher compared with the individuals without the GA [median (97.5% CI), 1.78 (0.59 to 7.01) vs.1.54 (0.50 to 5.29) mIU/liter; P = 0.04].
Carriers of the favorable allele in the combined groups (offspring and control) have higher TSH levels [median (97.5% CI), 1.70 (0.59 to 5.34) vs.1.45 (0.50–3.99) mIU/liter; P = 0.005], suggesting that the high prevalence of the TSHR SNPs is associated with higher serum TSH levels. However, when the centenarians’ data were entered into the equation, the differences within the genotype (carriers/noncarriers of the favorable allele) are less significant [median (97.5% CI), 1.89 (0.61–5.07) vs. 1.60 (0.49–4.50) mIU/liter; P = 0.04], suggesting that additional factors are involved. Finally, adjusting for age at recruitment diminished the differences, suggesting that age is a crucial component of elevated TSH.
Regulatory elements prediction
Using enhancer (http://www.cs.helsinki.fi/u/kpalin/EEL) and promoter prediction software (25), we located our SNPs between two enhancer regions and in close proximity to the promoter area. Because this was an association study and the SNPs were located outside the gene, we have predicted that they are in linkage disequilibrium with a regulatory genetic variant that is located either in the enhancer or in the promoter. Thus, these results demonstrate a possible effect on gene expression that leads to an increase TSH levels.
Further studies are needed, however, to clarify this issue.
Discussion
Our data show that offspring of the centenarian group have raised serum TSH concentrations compared with age-matched controls. Furthermore, offspring of the centenarian group who had elevated serum TSH levels also had higher TSH concentrations, suggesting a heritable trait that we documented by heritability analysis. After searching for genetic markers that could be associated with, and possibly responsible for, the raised serum TSH in centenarians and their offspring, we demonstrated for the first time that two SNPs (rs10149689 and rs12050077 of the TSHR), located between enhancer regions and close to the promoter region, were significantly associated with raised TSH concentrations in the Ashkenazi Jewish centenarians and their offspring compared with controls, and showed strong linkage disequilibrium. Our new results raise the possibility that these genetic changes exert an effect on TSHR gene expression that leads to increased TSH and may be, at least in part, transmitted from individuals with exceptional longevity to their offspring.
The inverse correlation between serum TSH and FT4 concentrations in our study groups raises the possibility that mild, subclinical hypothyroidism might contribute to this phenotype. Whether hypothyroidism plays a direct role in human longevity remains to be determined. However, multiple animal studies have shown a cause and effect relationship between thyroid function and life span (7,26). There are several comparative aging studies that involve animals with slow rates of aging. These animals demonstrate only minimal age-related deterioration in physiological capacity and reproduction and are resistant to chronic disease (8). Indeed, extraordinary longevity in animal models may be associated with exceptional antiaging mechanisms, providing unique insights into both the genetics and physiology of longevity. One example of such a mammal is the naked mole-rat (NMR, Rodentia: Bathyergidae; Heterocephalus glaber), which has a life span of more than 28 yr, exceeding any other rodent of its size (8). Other examples of mammal longevity are the Ames and Snell dwarf mice and calorie-restricted (CR) rats (27). Interestingly, all of these animals exhibit at least three common hormonal and metabolic characteristics: reduced thyroid function, (absent TSH and severely reduced thyroid hormone in Ames and Snell dwarf mice, reduced thyroid function in NMR and CR rats), reduced basal metabolic rate, and reduced core body temperature (8,27). A causal relationship between thyroid function and longevity was further demonstrated by experimental induction of hypothyroidism in rats, which resulted in extended life span. Conversely, inducing hyperthyroidism resulted in significantly shorter life span (26,28). Taken together, animal models of longevity demonstrate that hypothyroidism, and the accompanying metabolic consequences of hypothyroidism, play a central physiological role in life extension, suggesting that genetic determinants of decreased thyroid function may modulate life span.
Thyroid hormone has profound impact on metabolic rate and body temperature by various mechanisms, including modulating mitochondrial activity, membrane composition and ion permeability, and substrate cycling (29). The relationship between longevity and hypothyroidism is considered to be due to lower levels of reactive oxygen species production and oxidative stress in response to changes in both obligate and facultative thermogenesis (30).
Our findings are consonant with studies of identical and fraternal twins that show a strong effect of genetic factors on serum free thyroid hormones and TSH. These studies support the idea that the thyroid function set point between free hormones and TSH are genetically determined (16). These findings were further supported in Mexican-American families in the San Antonio Family Heart Study (17), which showed that genes controlled a substantial portion of the variation in thyroid hormones and suggested that identification of such genes could provide important insight into processes that modulate thyroid hormone-related metabolic effects.
A limitation of our study is that, at present, our findings and conclusions are restricted to the Ashkenazi Jewish population and cannot be generalized to other populations. Clearly, genetic studies in non-Ashkenazi populations in North America would be of great interest in this regard. Others have reported on serum TSH in individuals of exceptional longevity, some showing increased concentrations (2,31) and others showing decreased concentrations (32,33). None have reported on genetic changes as in the present work. We suggest that those reported studies be interpreted cautiously because the study populations were small, have different genetic backgrounds from our study groups, and live in areas of variable iodine supply. Another limitation is that, because of lack of availability of serum in some individuals, we could not examine the relationship between FT4 and TSH in the entire control and offspring groups.
In our Ashkenazi populations, the higher serum TSH concentrations in the centenarian group and their offspring suggest that they might have a small degree of hypothyroidism, possibly subclinical hypothyroidism. Because nonthyroidal disease may decrease serum TSH, an alternative, but less likely, possibility is that the centenarians were in better overall health than the control group (34). Subclinical hypothyroidism is defined as raised serum TSH in association with normal FT4, and it occurs in millions of people, particularly the elderly (1). The prevalence of TSH above 4.5 mIU/liter is less than 2% in people without known thyroid disease who are between 20 and 60 yr of age and rises steeply to 15% in individuals older than 80 yr of age (1). Because of population shifts in TSH distribution to higher concentrations with age, the serum TSH of 70% of those elderly individuals with TSH above 4.5 mIU/liter, who are currently considered to have subclinical hypothyroidism, actually falls within their age-specific limits (4). These recent reports suggest that only a minority of elderly individuals with minimally raised serum TSH may have thyroid disease.
Our present findings of a genetic basis for raised TSH in the Ashkenazi population with exceptional longevity, if extended to other populations in future studies, suggest that this change could be protective and, as a corollary to that speculation, that routine levothyroxine treatment might be harmful.
Acknowledgments
The authors thank Ms. Zhao Hu and Mr. Temuri Bodagov for laboratory determinations, and Drs. Govindaraju R. Diddahally, and Swapnil Rajpathak for reviewing and commenting on this article.
Footnotes
This work was supported by Grants AG-027734, AG-18728, RR-12248, DK-20541, and U19 AG023122 from the National Institute on Aging Longevity Consortium and by M01-RR12248 from the National Institutes of Health and the Glenn Foundation.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 16, 2009
For editorial see page 4658
Abbreviations: CI, Confidence interval; FT4, free T4; OR, odds ratio; SNP, single nucleotide polymorphism; TSHR, TSH receptor.
References
- Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE 2002 Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499 [DOI] [PubMed] [Google Scholar]
- Tietz NW, Shuey DF, Wekstein DR 1992 Laboratory values in fit aging individuals—sexagenarians through centenarians. Clin Chem 38:1167–1185 [PubMed] [Google Scholar]
- Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frölich M, Westendorp RG 2004 Thyroid status, disability and cognitive function, and survival in old age. JAMA 292:2591–2599 [DOI] [PubMed] [Google Scholar]
- Surks MI, Hollowell JG 2007 Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 92:4575–4582 [DOI] [PubMed] [Google Scholar]
- Boucai L, Surks MI 2009 Reference limits of serum thyrotropin (TSH) and free thyroxine (free T4) are significantly influenced by race and age in an urban outpatient practice of medicine. Clin Endocrinol (Oxf) 70:788–793 [DOI] [PubMed] [Google Scholar]
- Atzmon G, Barzilai N, Hollowell JG, Surks MI, Gabriely I 2009 Extreme longevity is associated with increased serum thyrotropin. J Clin Endocrinol Metab 94:1251–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffenstein R, Pinto M 2009 Endocrine function in naturally long-living small mammals. Mol Cell Endocrinol 299:101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffenstein R 2005 The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci 60:1369–1377 [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Else PL 2000 Mechanisms underlying the cost of living in animals. Annu Rev Physiol 62:207–235 [DOI] [PubMed] [Google Scholar]
- Silvestri E, Schiavo L, Lombardi A, Goglia F 2005 Thyroid hormones as molecular determinants of thermogenesis. Acta Physiol Scand 184:265–283 [DOI] [PubMed] [Google Scholar]
- Hauck SJ, Hunter WS, Danilovich N, Kopchick JJ, Bartke A 2001 Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Exp Biol Med (Maywood) 226:552–558 [DOI] [PubMed] [Google Scholar]
- Izquierdo JM, Luis AM, Cuezva JM 1990 Postnatal mitochondrial differentiation in rat liver. Regulation by thyroid hormones of the β-subunit of the mitochondrial F1-ATPase complex. J Biol Chem 265:9090–9097 [PubMed] [Google Scholar]
- Wiesner RJ, Kurowski TT, Zak R 1992 Regulation by thyroid hormone of nuclear and mitochondrial genes encoding subunits of cytochrome-c oxidase in rat liver and skeletal muscle. Mol Endocrinol 6:1458–1467 [DOI] [PubMed] [Google Scholar]
- Romanick MA, Rakoczy SG, Brown-Borg HM 2004 Long-lived Ames dwarf mouse exhibits increased antioxidant defense in skeletal muscle. Mech Ageing Dev 125:269–281 [DOI] [PubMed] [Google Scholar]
- Hansen PS, van der Deure WM, Peeters RP, Iachine I, Fenger M, Sørensen TI, Kyvik KO, Visser TJ, Hegedüs L 2007 The impact of a TSH receptor gene polymorphism on thyroid-related phenotypes in a healthy Danish twin population. Clin Endocrinol (Oxf) 66:827–832 [DOI] [PubMed] [Google Scholar]
- Hansen PS, Brix TH, Iachine I, Sørensen TI, Kyvik KO, Hegedüs L 2007 Genetic and environmental interrelations between measurements of thyroid function in a healthy Danish twin population. Am J Physiol Endocrinol Metab 292:E765–E770 [DOI] [PubMed] [Google Scholar]
- Samollow PB, Perez G, Kammerer CM, Finegold D, Zwartjes PW, Havill LM, Comuzzie AG, Mahaney MC, Göring HH, Blangero J, Foley TP, Barmada MM 2004 Genetic and environmental influences on thyroid hormone variation in Mexican Americans. J Clin Endocrinol Metab 89:3276–3284 [DOI] [PubMed] [Google Scholar]
- Sørensen HG, van der Deure WM, Hansen PS, Peeters RP, Breteler MM, Kyvik KO, Sørensen TI, Hegedüs L, Visser TJ 2008 Identification and consequences of polymorphisms in the thyroid hormone receptor α and β genes. Thyroid 18:1087–1094 [DOI] [PubMed] [Google Scholar]
- Peeters RP, van Toor H, Klootwijk W, de Rijke YB, Kuiper GG, Uitterlinden AG, Visser TJ 2003 Polymorphisms in thyroid hormone pathway genes are associated with plasma TSH and iodothyronine levels in healthy subjects. J Clin Endocrinol Metab 88:2880–2888 [DOI] [PubMed] [Google Scholar]
- Peeters RP, van der Deure WM, Visser TJ 2006 Genetic variation in thyroid hormone pathway genes; polymorphisms in the TSH receptor and the iodothyronine deiodinases. Eur J Endocrinol 155:655–662 [DOI] [PubMed] [Google Scholar]
- Bergman A, Atzmon G, Ye K, MacCarthy T, Barzilai N 2007 Buffering mechanisms in aging: a systems approach toward uncovering the genetic component of aging. PLoS Comput Biol 3:e170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnason E, Sigurgislason H, Benedikz E 2000 Genetic homogeneity of Icelanders: fact or fiction? Nat Genet 25:373–374 [DOI] [PubMed] [Google Scholar]
- Bataillon T, Mailund T, Thorlacius S, Steingrimsson E, Rafnar T, Halldorsson MM, Calian V, Schierup MH 2006 The effective size of the Icelandic population and the prospects for LD mapping: inference from unphased microsatellite markers. Eur J Hum Genet 14:1044–1053 [DOI] [PubMed] [Google Scholar]
- Katoh T, Mano S, Ikuta T, Munkhbat B, Tounai K, Ando H, Munkhtuvshin N, Imanishi T, Inoko H, Tamiya G 2002 Genetic isolates in East Asia: a study of linkage disequilibrium in the X chromosome. Am J Hum Genet 71:395–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen S 1999 Promoter 2.0: for the recognition of PolII promoter sequences. Bioinformatics 15:356–361 [DOI] [PubMed] [Google Scholar]
- Ooka H, Shinkai T 1986 Effects of chronic hyperthyroidism on the lifespan of the rat. Mech Ageing Dev 33:275–282 [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A 1996 Dwarf mice and the ageing process. Nature 384:33 [DOI] [PubMed] [Google Scholar]
- Ooka H, Fujita S, Yoshimoto E 1983 Pituitary-thyroid activity and longevity in neonatally thyroxine-treated rats. Mech Ageing Dev 22:113–120 [DOI] [PubMed] [Google Scholar]
- Harper ME, Seifert EL 2008 Thyroid hormone effects on mitochondrial energetics. Thyroid 18:145–156 [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA 2007 Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev 87:1175–1213 [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Nesi B, Pratelli L, Savarino L, Cucinotta D, Cavalli G 2000 Blood micronutrient and thyroid hormone concentrations in the oldest-old. J Clin Endocrinol Metab 85:2260–2265 [DOI] [PubMed] [Google Scholar]
- van den Beld AW, Visser TJ, Feelders RA, Grobbee DE, Lamberts SW 2005 Thyroid hormone concentrations, disease, physical function, and mortality in elderly men. J Clin Endocrinol Metab 90:6403–6409 [DOI] [PubMed] [Google Scholar]
- Magri F, Muzzoni B, Cravello L, Fioravanti M, Busconi L, Camozzi D, Vignati G, Ferrari E 2002 Thyroid function in physiological aging and in centenarians: possible relationships with some nutritional markers. Metabolism 51:105–109 [DOI] [PubMed] [Google Scholar]
- Wartofsky L, Burman KD 1982 Alterations in thyroid function in patients with systemic illness: the “euthyroid sick syndrome.” Endocr Rev 3:164–217 [DOI] [PubMed] [Google Scholar]