Abstract
Context: Obesity is associated with reduced GH.
Objective: The aim of the study was to determine whether reduced GH is associated with increased carotid intima-media thickness (cIMT) in obesity.
Design: A total of 102 normal-weight and obese men and women without known hypopituitarism were studied. Subjects underwent GH stimulation testing with GHRH-arginine. Lipid profile, inflammatory markers, oral glucose tolerance test, abdominal computed tomography, dual-energy x-ray absorptiometry, and cIMT were measured. Relative GH deficiency was defined as peak GH of 4.2 μg/liter or less. Subjects were separated based on BMI and GH testing into three groups: normal weight, obese GH sufficient (GHS), and obese relative GH deficient (GHD). Age, gender, and race were similar between the groups. BMI, percentage body fat, and visceral adiposity did not differ between obese GHS and relative GHD.
Results: Peak GH was associated with cIMT, IGF-I, high-density lipoprotein, low-density lipoprotein, triglycerides, adiponectin, C-reactive protein, and TNF-α (all P < 0.05). Obese GHS subjects had similar cIMT compared to normal-weight subjects (P = not significant), whereas obese GHD subjects had higher cIMT compared to normal-weight subjects (P < 0.05) (normal weight, 0.645 ± 0.023, vs. obese GHS, 0.719 ± 0.021, vs. obese GHD, 0.795 ± 0.063 mm; P = 0.01 by ANOVA). Similar results were seen in sensitivity analyses with less stringent cutoffs (<5, ≤8, <9 μg/liter) to define GHD. In multivariate modeling, peak GH remained significantly associated with cIMT after controlling for age, gender, race, tobacco, blood pressure, cholesterol, and fasting glucose (R2 for model, 0.35; P < 0.0001).
Conclusions: These results suggest that reduced GH secretion is associated with a more abnormal metabolic phenotype in obesity, characterized by increased cIMT, dyslipidemia, insulin resistance, and inflammation.
Reduced growth hormone levels on standard stimulation testing is associated with increased cardiovascular disease risk including increased carotid intima-media thickness, dyslipidemia, insulin resistance, and inflammation in obesity.
Obesity is associated with reduction in both spontaneous (1,2,3) and stimulated GH levels (4,5,6,7,8,9,10). The reduced GH levels associated with obesity can be reversed with weight loss, suggesting a functional or relative deficiency (11). In preliminary studies, we have shown that approximately 25% of obese men fail a standard GHRH-arginine stimulation test with a peak stimulated GH level of 4.2 μg/liter or less (our unpublished observation), whereas 64% of obese men have peak stimulated GH below 9 μg/liter (9).
GH deficiency (GHD) due to pituitary disease is known to be associated with increased cardiovascular disease (CVD) risk (12,13,14,15,16), which can be attenuated with GH replacement (17,18,19,20,21). Furthermore, reduced GH levels are negatively associated with low-density lipoprotein (LDL) cholesterol and triglycerides and positively associated with high-density lipoprotein (HDL) cholesterol in older men and women without structural pituitary disease (22). More recently, Utz et al. (23) demonstrated an association between reduced GH levels and increased carotid intima-media thickness (cIMT) in 45 overweight and obese female subjects. Subjects meeting clinical criteria for GHD (peak stimulated GH <5 μg/liter) had higher cIMT compared with subjects with normal GH stimulation. However, subjects classified as having relative GHD were older, with larger body mass index (BMI) and trunk fat than GH-sufficient (GHS) subjects in the study of Utz et al. (23). Therefore, it remains unknown whether relative GHD is associated independently with increased CVD risk in obese subjects, comparing obese GHS and GHD subjects similar in age, BMI, and central adiposity. In addition, the study of Utz et al. (23) was limited to women and similar studies have not been done in men.
To determine whether reduced peak stimulated GH levels contribute to increased CVD risk independent of age or BMI in both men and women, we investigated peak stimulated GH levels and CVD risk markers including cIMT in 102 normal-weight and obese men and women. Relative GHD was defined using a strict cutoff criteria of 4.2 μg/liter or less on standard GHRH-arginine stimulation testing. This cutoff value was previously validated as having the most discriminative power to diagnose GHD due to hypothalamic-pituitary disease in obese men and women in a large sample of hypopituitary and normal subjects (24). We hypothesized that reduced GH secretion would be associated with unfavorable cardiac risk markers including increased cIMT in obese subjects with relative GHD.
Subjects and Methods
Study subjects
A total of 102 normal-weight (BMI <25 kg/m2; n = 33) and obese (BMI ≥30 kg/m2; n = 69) men and women from the Boston community were recruited between November 2007 and March 2009. Data from a subset (n = 22) were previously published in a study evaluating the association of central adiposity to GH secretion in normal-weight and obese men (10). Normal-weight and obese male and female subjects between the ages of 18 and 55 who were otherwise healthy and without known pituitary dysfunction including dysfunction of the adrenal, GH, thyroid, or gonadal axes were selected. Subjects receiving GH, anabolic steroids, glucocorticoids, testosterone, hormone replacement, hormonal contraception, or any medication known to affect GH were excluded. Subjects with known diabetes mellitus, hemoglobin level less than 11 g/dl, creatinine above 1.5 mg/dl, aspartate aminotransferase more than 2.5-fold above the upper limit of normal, and chronic illness such as HIV were also excluded. Subjects with a history and physical exam suggestive of pituitary dysfunction were also excluded. Written informed consent was obtained from each subject before testing, in accordance with the Committee on the Use of Humans as Experimental Subjects of the Massachusetts Institute of Technology and the Subcommittee on Human Studies at the Massachusetts General Hospital.
Biochemical assessment
GH stimulation testing was performed using standard GHRH-arginine stimulation as previously reported (10). Briefly, after an overnight fast, sermorelin acetate (GHRH 1-29) (Geref; Serono Laboratories, Inc., Norwell, MA) was administered iv at a dose of 1 μg/kg. Subsequently, arginine hydrochloride (30 g/300 ml) was administered at a dose of 0.5 g/kg (maximum, 30 g) via iv pump at 600 ml/h over 30 min. GH levels were assessed at 0, 30, 45, 60, 90, and 120 min after sermorelin administration. Serum GH was measured using the Beckman Access Ultrasensitive human GH assay, a paramagnetic particle, chemiluminescent immunoassay (Beckman Coulter, Chaska, MN). The analytical sensitivity of the assay is 0.002 μg/liter. The intraassay variation ranges from 1.90–2.78%, and the interassay variation ranges from 1.77–2.65%. Subjects also underwent oral glucose tolerance tests (OGTTs) using standard 75-g oral glucose challenge and measurement of fasting cholesterol profile on a separate visit. Glucose and lipid levels were determined using standard methodology in the Massachusetts Institute of Technology clinical laboratory. Insulin was measured by a paramagnetic-particle chemiluminescence immunoassay using the Beckman Access Immunoassay System (Beckman Coulter). The analytical sensitivity of the assay is 0.03 IU/ml, and the precision is 3–5.6%. IGF-I was measured using an ELISA (Immuno Diagnostic Systems, Inc., Fountain Hills, AZ) with a detection limit of 3.1 μg/liter, an intraassay variability of 5.6%, and interassay variability of 5.5%. Adiponectin, C-reactive protein (CRP), and TNF-α were also measured using commercially available ELISA kits (R&D Systems, Inc., Minneapolis, MN; Diagnostic Systems Laboratories, Inc., Webster, TX; and Invitrogen Corporation, Carlsbad, CA, respectively).
Anthropometric assessment
Height and body weight were obtained after an overnight fast. Total body fat percentage was determined by dual x-ray absorptiometry testing using a Hologic-4500 densitometer (Hologic, Inc., Waltham, MA). In addition, 1-cm cross-sectional abdominal computed tomography (CT) scans were performed at the level of L4 to assess the distribution of abdominal sc adipose tissue (SAT) and abdominal visceral adipose tissue (VAT) as previously described (25).
cIMT
Measurement of cIMT was performed as previously described (26). Briefly, ultrasound images were obtained using a high resolution 5–12 MHz linear array transducer (HDI 5000 SONOS CT; ATL Ultrasound, Bothell, WA). Digital images were captured using a high-quality video frame capture card. Edge detection and mean cIMT calculation were accomplished with an in-house computer program. The published reproducibility of the technique is excellent with a sd of 0.007 mm (27). The average cIMT over the length of the measured segments on the left carotid artery is reported.
Statistical analysis
Continuous variables were tested for normality of distribution with the use of Wilk-Shapiro test and examination of the histogram distribution. Variables that were normally distributed were compared using the Student’s t test, and variables that were not normally distributed were compared using the nonparametric Wilcoxon rank sum test. Nominal variables were compared using the χ2 test. The effect of GH status on cIMT was determined using ANOVA followed by Tukey-Kramer post hoc test and Student’s t test for significant ANOVA. Univariate regression analysis was performed comparing peak stimulated GH levels with measures of CVD risk factors as well as overall and regional adiposity using the Pearson correlation coefficient. Multivariate regression analysis with standard least squares modeling was also performed, including peak stimulated GH, age, gender, race, tobacco use, blood pressure, cholesterol, fasting glucose, and other metabolic parameters related to peak GH on univariate regression as covariates and cIMT as the dependent variable. Models including VAT were constructed to assess whether effects were independent of increased VAT. Gender was controlled for in all analyses including ANOVA and multivariate modeling. Statistical analysis was performed using JMP Statistical Database Software (SAS Institute, Inc., Cary, NC). Statistical significance was determined as P < 0.05.
Results
Clinical characteristics and GH parameters of study subjects
The subjects ranged in age from 18 to 55 yr. Thirty-three normal-weight men and women [age, 44.0 yr, interquartile range (IQR), 28.5–49.0 yr; BMI, 22.5 ± 0.3 kg/m2] and 69 obese men and women [age, 43.0 (IQR, 36.5–47.5) yr; BMI, 38.1 ± 0.8 kg/m2] were studied. Obese subjects were stratified into GHS (n = 55; mean peak GH, 10.3 ± 1.0 μg/liter) or relative GHD (n = 14; mean peak GH, 2.8 ± 0.3 μg/liter) based on peak stimulated GH of 4.2 μg/liter or less. All normal-weight subjects had peak stimulated GH above 4.2 μg/liter (mean peak GH, 44.7 ± 4.9 μg/liter; range, 6.1–96.7 μg/liter). There were no significant differences in age, gender, and race among the three groups (normal weight, obese GHS, and obese GHD). The age distributions broken down by decade were not significantly different in the normal-weight, obese GHS, and obese GHD groups (P = 0.16). Among the whole group, 14.7% were 18–30 yr of age, 21.6% were 31–40 yr of age, 46.1% were 41–50 yr of age, and 17.7% were 51–55 yr of age. In addition, the BMI, percentage body fat, SAT, and VAT were not different between obese GHS and obese GHD groups (Table 1). Twenty-two additional overweight subjects (BMI, 25–30 kg/m2) were recruited and characterized in terms of GH response to GHRH-arginine (see Additional analyses among overweight subjects).
Table 1.
Baseline characteristics of study subjects stratified by BMI and GH status (n = 102)
| Normal weight | Obese GHS | Obese GHD | P value | P value adjusted for gender | |
|---|---|---|---|---|---|
| n | 33 | 55 | 14 | ||
| Age (yr) | 44.0 (28.5–49.0) | 43.0 (36.0–48.0) | 45.0 (40.0–47.75) | 0.68 | |
| Gender, no. of males (%) | 21 (64) | 28 (51) | 11 (78) | 0.12 | |
| Race, n (%) | 0.32 | 0.16 | |||
| Caucasian | 21 (64) | 26 (47) | 8 (57) | ||
| Not Caucasian | 12 (36) | 29 (53) | 6 (43) | ||
| Tobacco (pack years) | 5.3 ± 1.8 | 8.6 ± 1.9 | 2.7 ± 1.5 | 0.21 | 0.10 |
| Blood pressure (mm Hg) | |||||
| Systolic | 118 ± 2 | 119 ± 2 | 126 ± 4 | 0.20 | 0.008 |
| Diastolic | 74 ± 1 | 75 ± 1 | 78 ± 3 | 0.06 | 0.0005 |
| Body composition | |||||
| Height (cm) | 173.2 ± 1.6 | 169.7 ± 1.1 | 174.5 ± 3.0 | 0.02 | <0.0001 |
| BMI (kg/m2) | 22.5 ± 0.3 | 37.2 ± 0.8a | 40.7 ± 2.3b | <0.0001 | <0.0001 |
| SAT by abdominal CT (cm2) | 129 ± 11 | 464 ± 20a | 527 ± 44b | <0.0001 | <0.0001 |
| VAT by abdominal CT (cm2) | 51 ± 7 | 177 ± 11a | 216 ± 16b | <0.0001 | <0.0001 |
| % Total fat by DEXA | 19.1 ± 1.3 | 35.5 ± 1.2a | 34.1 ± 2.0b | <0.0001 | <0.0001 |
| Metabolic parameters | |||||
| Total cholesterol (mg/dl) | 174 ± 6 | 183 ± 4 | 181 ± 10 | 0.67 | 0.42 |
| HDL cholesterol (mg/dl) | 58 ± 3 | 45 ± 2a | 44 ± 2b | <0.0001 | 0.0001 |
| LDL cholesterol (mg/dl) | 101 ± 5 | 115 ± 4 | 117 ± 8 | 0.12 | 0.06 |
| Triglycerides (mg/dl) | 75 ± 7 | 126 ± 11a | 134 ± 22b | 0.01 | 0.03 |
| Fasting glucose (mg/dl) | 86 ± 2 | 95 ± 3 | 94 ± 2 | 0.10 | 0.05 |
| 2-h glucose on OGTT (mg/dl) | 98 ± 7 | 128 ± 7a | 122 ± 8 | 0.03 | 0.07 |
| HbA1c (%) | 5.5 ± 0.04 | 5.8 ± 0.07a | 5.7 ± 0.09 | 0.006 | 0.009 |
| Fasting insulin (μIU/ml) | 3.1 ± 0.4 | 8.9 ± 1.0a | 8.9 ± 1.5b | <0.0001 | <0.0001 |
| Basal GH (μg/liter) | 0.8 ± 0.2 | 0.1 ± 0.03a | 0.1 ± 0.06b | 0.0005 | 0.0007 |
| Peak GH on stimulation test (μg/liter) | 44.7 ± 4.9 | 10.3 ± 1.0a | 2.8 ± 0.3b,c | <0.0001 | <0.0001 |
| IGF-I (μg/liter)d | 93 ± 8 | 77 ± 5 | 58 ± 7b | 0.02 | 0.003 |
Relative GHD is defined as peak stimulated GH of 4.2 μg/liter or less on standard GHRH-arginine stimulation test. Results are presented as mean ± sem except for age, which is presented as median (IQR). Statistical significance was determined by ANOVA, followed by Tukey-Kramer and Student’s t test for significant ANOVA. P value for ANOVA and ANOVA adjusted for gender are reported. Age is reported as median (IQR) with analysis provided by nonparametric Wilcoxon rank sum test. For determination of significance for race, a χ2 test was performed for Caucasian or not. DEXA, dual x-ray absorptiometry.
P < 0.05 for obese GHS vs. normal weight.
P < 0.05 for obese GHD vs. normal weight.
P < 0.05 for obese GHD vs. obese GHS.
Corresponding IGF-I sd scores for the three groups are: normal weight, −0.6 ± 0.2; obese GHS, −0.9 ± 0.1; and obese GHD, −1.3 ± 0.2.
Effects of gender on GH stimulation
Normal-weight women had higher peak stimulated GH compared with normal-weight men (normal-weight women, 58.1 ± 10.0 vs. normal-weight men, 37.0 ± 4.6 μg/liter; P = 0.04). When stratified by GH status, obese GHS women had higher peak stimulated GH compared with obese GHS men (obese GHS women, 12.7 ± 1.8 vs. obese GHS men, 8.0 ± 0.8 μg/liter; P = 0.02), whereas obese GHD women had similar peak stimulated GH levels compared with obese GHD men (obese GHD women, 2.4 ± 0.6 vs. obese GHD men, 2.9 ± 0.3 μg/liter; P = 0.44).
GH and cIMT
Peak stimulated GH to GHRH-arginine stimulation testing was negatively associated with cIMT by univariate analysis (r = −0.35; P = 0.0003; Fig. 1) among all subjects (including normal-weight, obese GHS, and obese GHD subjects; n = 102), and this remained significant, controlling for gender (P = 0.0008). A significant trend to increase was observed among the three groups (normal-weight, obese GHS, and obese GHD) by overall ANOVA (P = 0.01) and by ANOVA adjusted for gender (P = 0.004). Between group analysis demonstrated that obese GHS subjects had similar cIMT compared with normal-weight controls, whereas obese GHD subjects had higher cIMT compared with normal-weight controls [normal weight, 0.645 ± 0.023, vs. obese GHS, 0.719 ± 0.021, vs. obese GHD, 0.795 ± 0.063 mm; P = not significant (NS) between normal-weight and obese GHS; P < 0.05 between normal-weight and obese GHD] (Fig. 1). cIMT was higher in men compared with women (men, 0.735 ± 0.022, vs. women, 0.663 ± 0.024 mm; P = 0.03).
Figure 1.
Peak GH and cIMT. A, Peak stimulated GH is negatively associated with cIMT on univariate analysis. Men are depicted with open diamonds, whereas women are depicted with closed squares. B, Relative GHD was determined by peak stimulated GH of 4.2 μg/liter or less on standard GHRH-arginine stimulation test. cIMT is significantly different across groups by overall ANOVA (P = 0.01). Between-group comparisons with Tukey-Kramer post hoc tests show that cIMT is similar between normal-weight and obese GHS subjects (P = NS) but significantly different between normal-weight and obese subjects with relative GHD (P < 0.05).
Relationship of peak stimulated GH to measures of CVD risk and anthropometrics
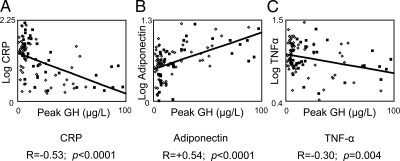
Univariate analysis was performed among all subjects (including normal-weight, obese GHS, and obese GHD subjects; n = 102). Peak stimulated GH was negatively associated with traditional CVD risk factors such as systolic and diastolic blood pressure, LDL cholesterol, and triglycerides, and positively associated with HDL cholesterol (Table 2). Peak stimulated GH was also negatively associated with fasting glucose and insulin, 2-h glucose on OGTT, and glycosylated hemoglobin (HbA1c) (Table 2). Peak stimulated GH was negatively associated with CRP and TNF-α and positively associated with adiponectin (Fig. 2 and Table 2). All of these associations remained significant controlling for gender (Table 2).
Table 2.
Univariate analysis of peak stimulated GH to various parameters among all subjects (normal weight, obese GHS, and obese GHD; n = 102)
| Parameter | r | P | P value adjusted for gender |
|---|---|---|---|
| Age | −0.37 | 0.0001 | 0.0003 |
| Systolic BP | −0.30 | 0.002 | 0.006 |
| Diastolic BP | −0.27 | 0.007 | 0.02 |
| Body composition | |||
| BMI | −0.62 | <0.0001 | <0.0001 |
| SAT | −0.59 | <0.0001 | <0.0001 |
| VAT | −0.65 | <0.0001 | <0.0001 |
| % Total body fat | −0.44 | <0.0001 | <0.0001 |
| Metabolic parameters | |||
| Total cholesterol | −0.17 | 0.09 | 0.12 |
| HDL cholesterol | +0.42 | <0.0001 | <0.0001 |
| LDL cholesterol | −0.26 | 0.009 | 0.02 |
| Triglycerides | −0.36 | 0.0002 | 0.0002 |
| Fasting glucose | −0.27 | 0.005 | 0.009 |
| 2-h glucose on OGTT | −0.31 | 0.002 | 0.0007 |
| HbA1c | −0.25 | 0.01 | 0.008 |
| Fasting insulin | −0.39 | 0.0001 | 0.0004 |
| IGF-I | +0.44 | <0.0001 | <0.0001 |
| Adiponectin | +0.56 | <0.0001 | <0.0001 |
| CRP | −0.35 | 0.0005 | 0.0002 |
| TNF-α | −0.26 | 0.01 | 0.02 |
BP, Blood pressure.
Figure 2.
The association of peak stimulated GH on standard GHRH-arginine stimulation test and inflammatory markers by univariate analysis. Men are depicted with open diamonds, whereas women are depicted with closed squares. A, Peak stimulated GH is negatively associated with serum CRP. B, Peak stimulated GH is positively associated with serum adiponectin. C, Peak stimulated GH is negatively associated with serum TNF-α.
Multivariate regression analysis of the relationship between peak stimulated GH and cIMT
Multivariate regression analysis was performed using standard least squares modeling among all subjects (including normal-weight, obese GHS, and obese GHD subjects; n = 102). Peak stimulated GH remained significantly associated with cIMT after controlling for traditional CVD risk factors including age, gender, race, tobacco use, systolic blood pressure, total cholesterol, and fasting glucose (β = −0.001; P = 0.046; R2 for overall model, 0.35; P < 0.0001 for overall model) (Table 3). The significant relationship between peak stimulated GH and cIMT also remained strong when controlling for VAT along with BMI and metabolic variables of HDL cholesterol, LDL cholesterol, triglycerides, fasting glucose, fasting insulin, and CRP (β = −0.002; P = 0.05; R2 for overall model, 0.21; P = 0.02 for overall model).
Table 3.
Multivariate regression analysis of peak stimulated GH and traditional CVD risk factors to cIMT among all subjects (normal weight, obese GHS and obese GHD; n = 102)
| Parameters | Estimate | se | P |
|---|---|---|---|
| Peak stimulated GH | −0.001 | 0.0007 | 0.046 |
| Age | 0.006 | 0.002 | 0.001 |
| Gender (male or not) | 0.02 | 0.02 | 0.18 |
| Race (Caucasian or not) | −0.05 | 0.01 | 0.002 |
| Tobacco | 0.002 | 0.001 | 0.15 |
| Systolic blood pressure | 0.0002 | 0.001 | 0.84 |
| Total cholesterol | 0.0002 | 0.0004 | 0.67 |
| Fasting glucose | 0.0006 | 0.0008 | 0.46 |
R2 for overall model is 0.35 (P < 0.0001).
Sensitivity analyses for the relationship between GHD and cIMT using less stringent cutoff values to define relative GHD
The relationship between GHD and cIMT was analyzed using different cutoff points with similar results. Regardless of the cutoff point used, including peak stimulated GH of 4.2 or less, less than 5, 8 or less , and less than 9 μg/liter, cIMT was higher in obese GHD subjects compared with normal-weight subjects, whereas cIMT of obese GHS subjects was not significantly different from normal-weight subjects. These results remained significant after controlling for gender (Table 4).
Table 4.
cIMT stratified by BMI and GH status using different GH cutoff values
| Different cutoffs | Normal weight | Obese GHS | Obese GHD | P | P value adjusted for gender |
|---|---|---|---|---|---|
| Peak GH ≤4.2 μg/liter | 0.645 ± 0.023 (n = 33) | 0.719 ± 0.021 (n = 55) | 0.795 ± 0.063 (n = 14)a | 0.01 | 0.004 |
| Peak GH <5 μg/liter | 0.645 ± 0.023 (n = 33) | 0.721 ± 0.024 (n = 46) | 0.761 ± 0.042 (n = 23)a | 0.03 | 0.006 |
| Peak GH ≤8 μg/liter | 0.645 ± 0.023 (n = 33) | 0.663 ± 0.025 (n = 22) | 0.767 ± 0.028 (n = 47)a,b | 0.002 | 0.001 |
| Peak GH <9 μg/liter | 0.645 ± 0.023 (n = 33) | 0.652 ± 0.026 (n = 20) | 0.768 ± 0.027 (n = 49)a,b | 0.001 | 0.0006 |
Statistical analysis performed by ANOVA and by ANOVA adjusted for gender followed by Tukey-Kramer for significant ANOVA. There were no differences between obese GHS vs. normal weight among any of the cutoffs examined.
P < 0.05 for obese GHD vs. normal weight.
P < 0.05 for obese GHD vs. obese GHS.
Additional analyses among overweight subjects
An additional 22 (BMI, 25–30 kg/m2) subjects [men, n = 16 (73%); age, 45.5 (IQR, 40.0–52.3) yr; BMI, 26.6 ± 0.3 kg/m2] were recruited and characterized in terms of peak GH response to GHRH-arginine testing. All 22 subjects had peak stimulated GH above 4.2 μg/liter (range, 5.6–46.3 μg/liter).
Discussion
This study demonstrates a significant association between reduced peak stimulated GH and cIMT, as well as other cardiometabolic risk factors including a number of inflammatory indices, in otherwise healthy obese men and women without known hypothalamic/pituitary disease. In addition, we show that a subset of obese subjects with relative GHD defined based on a strict cutoff criteria for GH on GHRH-arginine testing demonstrated increased cardiovascular risk.
Carmichael et al. (22) previously demonstrated a negative association between GH and LDL cholesterol and triglycerides and a positive association with HDL cholesterol in older men and women between the ages of 50 and 90 yr. In our study, we extend these results to younger men and women. In addition, we also demonstrate association of peak stimulated GH with other CVD risk factors of blood pressure, fasting glucose and insulin, 2-h glucose on OGTT, HbA1c, as well as inflammatory markers that have not previously been explored. Furthermore, the negative association demonstrated between peak stimulated GH and cIMT in 45 overweight and obese women by Utz et al. (23) is now confirmed in our larger study that includes both men and women. In our study, age, gender, and race; and within the obese groups (obese GHS vs. obese GHD), BMI, percentage body fat, SAT, and VAT did not differ, making it unlikely that this result is secondary to confounders such as age, gender, race, BMI, and body fat distribution, which may independently affect CVD risk. Moreover, this finding was confirmed on multivariate regression analysis controlling for CVD risk factors of age, gender, race, tobacco use, blood pressure, total cholesterol, and fasting glucose and in a separate model by controlling for BMI, VAT, and other metabolic variables including the inflammatory marker CRP. To our knowledge, this is the first time that the relationship between peak GH and cIMT has been shown to remain significant in modeling including traditional risk factors known to contribute highly to cIMT.
We chose to define relative GHD of obesity using a peak stimulated GH of 4.2 μg/liter or less for this study. In a study comparing six different GH stimulation tests, Biller et al. (28) demonstrated that a peak stimulated GH of 4.1 μg/liter or less with the GHRH-arginine stimulation test conferred the best discriminatory power to diagnose true GHD of pituitary disease in her study population. In a study of more than 600 hypopituitary and normal subjects, Corneli et al. (24) demonstrated that a peak stimulated GH cutoff of 4.2 μg/liter or less on standard GHRH-arginine stimulation test provided the best discrimination between subjects with true GHD and those subjects without GHD among obese subjects. Given the close agreement between these studies, we used a cutoff of peak stimulated GH of 4.2 μg/liter or less to define GHD in our study. Furthermore, in our study, the relationship between peak stimulated GH and cIMT demonstrated a significant negative linear association on univariate analysis, suggesting a continuum. Therefore, it is not surprising that this relationship between relative GHD and increased cIMT was significant regardless of the different GH cutoff points used in the analysis (peak stimulated GH ≤4.2, <5, ≤8, or <9 μg/liter). The exact cutoff point appropriate for the diagnosis of relative GHD is yet to be determined and will require further study with a larger population; however, our results suggest that reduced peak stimulated GH is associated with increased cIMT, regardless of the cutoff point selected.
We also demonstrate a novel association between peak stimulated GH and the inflammatory markers adiponectin and TNF-α. Utz et al. (23) had previously shown a strong negative association between peak stimulated GH and CRP in her study of overweight and obese women, which we have also confirmed in our study of both men and women. In combination, these three makers suggest a profile consistent with increased chronic inflammation. Because both adiponectin (26) and CRP (29,30,31) have been associated with increased cIMT, these inflammatory markers may mediate some of the effects of reduced peak stimulated GH on cardiometabolic risk markers including cIMT in obesity. Our data suggest that reduced peak stimulated GH is associated with a more severe metabolic phenotype including dyslipidemia, insulin resistance, and increased inflammatory markers among obese patients.
We have previously shown a significant association between peak stimulated GH and VAT (10). Because VAT is positively associated with cIMT (32), the relationship between peak stimulated GH and cIMT identified in this study may be mediated, in part, by VAT. However, because our two obese groups (GHS and relative GHD) had a similar degree of adiposity and VAT, our data suggest that a VAT-independent effect of GH on cIMT may exist. Furthermore, controlling for VAT in our multivariate model still resulted in a strong association between peak stimulated GH and cIMT, suggesting that this association is robust and, at least in part, independent of VAT.
Our study included both men and women, and peak stimulated GH response to GHRH-arginine was higher among normal-weight women than men and obese GHS women than men, but similar between obese GHD women compared with men. Importantly, the proportions of men and women were not significantly different between the groups, and our results relating peak GH to cardiovascular parameters remained highly significant controlling for gender.
The primary goal of the study was to evaluate the effects of relative GHD, as defined by peak stimulated GH of 4.2 μg/liter or less, on cardiovascular, metabolic, and inflammatory risk markers among obese patients. Therefore, the primary comparison of CVD risk markers in the study was purposely limited to a comparison between obese GHS, obese GHD, and a normal-weight control group. We investigated peak GH response to GHRH-arginine in an additional group of overweight patients (BMI, 25–30 kg/m2) and found that all of these subjects demonstrated a normal response to GHRH-arginine, suggesting that relative GHD occurs in a subset of obese patients but not among overweight patients per se.
A limitation of our study is its observational design. Although strong associations have been identified, causality cannot be determined from an observational study. However, data from studies in which GH is administered to obese patients with improvements in VAT and lipids (33,34,35) and from studies in hypopituitary patients in which GH administration improves cardiovascular risk parameters (17,18,20,21) support the data from this study that relative GHD may be playing an independent role to increase CVD risk markers in obesity.
In summary, our study demonstrates a strong association between reduced GH secretion and increased cIMT in obese men and women. In addition, our study also highlights the more severe metabolic phenotype associated with reduced GH secretion in obesity, including unfavorable lipid profile, insulin resistance, and increased inflammation. It remains unknown whether the reduced GH levels associated with obesity confer higher CVD event rates. Nonetheless, our findings raise the intriguing question of whether a pathophysiological entity of relative GHD exists in a large subpopulation of obesity and whether treatment with physiological GH, GH secretagogues, or GHRH in this subpopulation of obese patients may be useful to reduce CVD risk. Indeed, several studies have demonstrated a benefit to treatment of obesity with recombinant GH in adults (33,34,35), and more studies will be necessary in the future to determine whether such strategies will be useful. Our results also suggest that therapies to target traditional risk factors in this group, including lipid-lowering and insulin-sensitizing agents, may be useful. Additional studies are needed to determine the optimal treatment strategies to reduce CVD risk and whether such strategies actually reduce CVD event rates in this population.
Footnotes
These studies conducted at the Clinical Research Center of the Massachusetts Institute of Technology and the Massachusetts General Hospital were funded by a grant to the Harvard Catalyst (UL1 RR025758) from the National Center for Research Resources, National Institutes of Health (NIH). This work was also supported in part by the NIH Grants 1R01 HL085268-01A1 (to S.K.G.) and K24 DK064545-06 (to S.K.G.).
Clinical Trials Registration no.: NCT00562796.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 16, 2009
Abbreviations: BMI, Body mass index; cIMT, carotid intima-media thickness; CRP, C-reactive protein; CT, computed tomography; CVD, cardiovascular disease; GHD, GH deficiency or GH-deficient; GHS, GH-sufficient; HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NS, not significant; OGTT, oral glucose tolerance test; SAT, sc adipose tissue; VAT, visceral adipose tissue.
References
- Iranmanesh A, Lizarralde G, Veldhuis JD 1991 Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab 73:1081–1088 [DOI] [PubMed] [Google Scholar]
- Riedel M, Hoeft B, Blum WF, von zur Mühlen A, Brabant G 1995 Pulsatile growth hormone secretion in normal-weight and obese men: differential metabolic regulation during energy restriction. Metabolism 44:605–610 [DOI] [PubMed] [Google Scholar]
- Van Dam EW, Roelfsema F, Helmerhorst FH, Frölich M, Meinders AE, Veldhuis JD, Pijl H 2002 Low amplitude and disorderly spontaneous growth hormone release in obese women with or without polycystic ovary syndrome. J Clin Endocrinol Metab 87:4225–4230 [DOI] [PubMed] [Google Scholar]
- Vizner B, Reiner Z, Sekso M 1983 Effect of l-dopa on growth hormone, glucose, insulin, and cortisol response in obese subjects. Exp Clin Endocrinol 81:41–48 [DOI] [PubMed] [Google Scholar]
- Williams T, Berelowitz M, Joffe SN, Thorner MO, Rivier J, Vale W, Frohman LA 1984 Impaired growth hormone responses to growth hormone-releasing factor in obesity. A pituitary defect reversed with weight reduction. N Engl J Med 311:1403–1407 [DOI] [PubMed] [Google Scholar]
- Kopelman PG, Noonan K, Goulton R, Forrest AJ 1985 Impaired growth hormone response to growth hormone releasing factor and insulin-hypoglycaemia in obesity. Clin Endocrinol (Oxf) 23:87–94 [DOI] [PubMed] [Google Scholar]
- Ghigo E, Procopio M, Boffano GM, Arvat E, Valente F, Maccario M, Mazza E, Camanni F 1992 Arginine potentiates but does not restore the blunted growth hormone response to growth hormone-releasing hormone in obesity. Metabolism 41:560–563 [DOI] [PubMed] [Google Scholar]
- Cordido F, Alvarez-Castro P, Isidro ML, Casanueva FF, Dieguez C 2003 Comparison between insulin tolerance test, growth hormone (GH)-releasing hormone (GHRH), GHRH plus acipimox and GHRH plus GH-releasing peptide-6 for the diagnosis of adult GH deficiency in normal subjects, obese and hypopituitary patients. Eur J Endocrinol 149:117–122 [DOI] [PubMed] [Google Scholar]
- Bonert VS, Elashoff JD, Barnett P, Melmed S 2004 Body mass index determines evoked growth hormone (GH) responsiveness in normal healthy male subjects: diagnostic caveat for adult GH deficiency. J Clin Endocrinol Metab 89:3397–3401 [DOI] [PubMed] [Google Scholar]
- Makimura H, Stanley T, Mun D, You SM, Grinspoon S 2008 The effects of central adiposity on growth hormone (GH) response to GH-releasing hormone-arginine stimulation testing in men. J Clin Endocrinol Metab 93:4254–4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen MH, Hvidberg A, Juul A, Main KM, Gotfredsen A, Skakkebaek NE, Hilsted J, Skakkebae NE 1995 Massive weight loss restores 24-hour growth hormone release profiles and serum insulin-like growth factor-I levels in obese subjects. J Clin Endocrinol Metab 80:1407–1415 [DOI] [PubMed] [Google Scholar]
- Evans LM, Davies JS, Goodfellow J, Rees JA, Scanlon MF 1999 Endothelial dysfunction in hypopituitary adults with growth hormone deficiency. Clin Endocrinol (Oxf) 50:457–964 [DOI] [PubMed] [Google Scholar]
- Johansson JO, Landin K, Tengborn L, Rosén T, Bengtsson BA 1994 High fibrinogen and plasminogen activator inhibitor activity in growth hormone-deficient adults. Arterioscler Thromb 14:434–437 [DOI] [PubMed] [Google Scholar]
- Leonsson M, Hulthe J, Oscarsson J, Johannsson G, Wendelhag I, Wikstrand J, Bengtsson BA 2002 Intima-media thickness in cardiovascularly asymptomatic hypopituitary adults with growth hormone deficiency: relation to body mass index, gender, and other cardiovascular risk factors. Clin Endocrinol (Oxf) 57:751–759 [DOI] [PubMed] [Google Scholar]
- Markussis V, Beshyah SA, Fisher C, Sharp P, Nicolaides AN, Johnston DG 1992 Detection of premature atherosclerosis by high-resolution ultrasonography in symptom-free hypopituitary adults. Lancet 340:1188–1192 [DOI] [PubMed] [Google Scholar]
- Sesmilo G, Miller KK, Hayden D, Klibanski A 2001 Inflammatory cardiovascular risk markers in women with hypopituitarism. J Clin Endocrinol Metab 86:5774–5781 [DOI] [PubMed] [Google Scholar]
- Maison P, Griffin S, Nicoue-Beglah M, Haddad N, Balkau B, Chanson P 2004 Impact of growth hormone (GH) treatment on cardiovascular risk factors in GH-deficient adults: a metaanalysis of blinded, randomized, placebo-controlled trials. J Clin Endocrinol Metab 89:2192–2199 [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Verhovec R, Zizek B, Prezelj J, Poredos P, Clayton RN 1999 Growth hormone (GH) treatment reverses early atherosclerotic changes in GH-deficient adults. J Clin Endocrinol Metab 84:453–457 [DOI] [PubMed] [Google Scholar]
- Johannsson G, Bengtsson BA, Andersson B, Isgaard J, Caidahl K 1996 Long-term cardiovascular effects of growth hormone treatment in GH-deficient adults. Preliminary data in a small group of patients. Clin Endocrinol (Oxf) 45:305–314 [DOI] [PubMed] [Google Scholar]
- Johansson JO, Landin K, Johannsson G, Tengborn L, Bengtsson BA 1996 Long-term treatment with growth hormone decreases plasminogen activator inhibitor-1 and tissue plasminogen activator in growth hormone-deficient adults. Thromb Haemost 76:422–428 [PubMed] [Google Scholar]
- Sesmilo G, Biller BM, Llevadot J, Hayden D, Hanson G, Rifai N, Klibanski A 2000 Effects of growth hormone administration on inflammatory and other cardiovascular risk markers in men with growth hormone deficiency. A randomized, controlled clinical trial. Ann Intern Med 133:111–122 [DOI] [PubMed] [Google Scholar]
- Carmichael JD, Danoff A, Milani D, Roubenoff R, Lesser ML, Livote E, Reitz RE, Ferris S, Kleinberg DL 2006 GH peak response to GHRH-arginine: relationship to insulin resistance and other cardiovascular risk factors in a population of adults aged 50–90. Clin Endocrinol (Oxf) 65:169–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz AL, Yamamoto A, Hemphill L, Miller KK 2008 Growth hormone deficiency by growth hormone releasing hormone-arginine testing criteria predicts increased cardiovascular risk markers in normal young overweight and obese women. J Clin Endocrinol Metab 93:2507–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corneli G, Di Somma C, Baldelli R, Rovere S, Gasco V, Croce CG, Grottoli S, Maccario M, Colao A, Lombardi G, Ghigo E, Camanni F, Aimaretti G 2005 The cut-off limits of the GH response to GH-releasing hormone-arginine test related to body mass index. Eur J Endocrinol 153:257–264 [DOI] [PubMed] [Google Scholar]
- Rietschel P, Hadigan C, Corcoran C, Stanley T, Neubauer G, Gertner J, Grinspoon S 2001 Assessment of growth hormone dynamics in human immunodeficiency virus-related lipodystrophy. J Clin Endocrinol Metab 86:504–510 [DOI] [PubMed] [Google Scholar]
- Lo J, Dolan SE, Kanter JR, Hemphill LC, Connelly JM, Lees RS, Grinspoon SK 2006 Effects of obesity, body composition, and adiponectin on carotid intima-media thickness in healthy women. J Clin Endocrinol Metab 91:1677–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R, Kaufhold J, Hemphill LC, Lees RS, Karl WC 2000 Anisotropic edge-preserving smoothing in carotid B-mod ultrasound for improved segmentation and intima-media thickness (IMT) measurement. Comput Cardiol 27:37–40 [Google Scholar]
- Biller BM, Samuels MH, Zagar A, Cook DM, Arafah BM, Bonert V, Stavrou S, Kleinberg DL, Chipman JJ, Hartman ML 2002 Sensitivity and specificity of six tests for the diagnosis of adult GH deficiency. J Clin Endocrinol Metab 87:2067–2079 [DOI] [PubMed] [Google Scholar]
- Sitzer M, Markus HS, Mendall MA, Liehr R, Knorr U, Steinmetz H 2002 C-reactive protein and carotid intimal medial thickness in a community population. J Cardiovasc Risk 9:97–103 [DOI] [PubMed] [Google Scholar]
- Talbott EO, Zborowski JV, Boudreaux MY, McHugh-Pemu KP, Sutton-Tyrrell K, Guzick DS 2004 The relationship between C-reactive protein and carotid intima-media wall thickness in middle-aged women with polycystic ovary syndrome. J Clin Endocrinol Metab 89:6061–6067 [DOI] [PubMed] [Google Scholar]
- Corrado E, Rizzo M, Muratori I, Coppola G, Novo S 2006 Association of elevated fibrinogen and C-reactive protein levels with carotid lesions in patients with newly diagnosed hypertension or type II diabetes. Arch Med Res 37:1004–1009 [DOI] [PubMed] [Google Scholar]
- Lear SA, Humphries KH, Kohli S, Frohlich JJ, Birmingham CL, Mancini GB 2007 Visceral adipose tissue, a potential risk factor for carotid atherosclerosis: results of the Multicultural Community Health Assessment Trial (M-CHAT). Stroke 38:2422–2429 [DOI] [PubMed] [Google Scholar]
- Mekala KC, Tritos NA 2009 Effects of recombinant human growth hormone therapy in obesity in adults: a meta analysis. J Clin Endocrinol Metab 94:130–137 [DOI] [PubMed] [Google Scholar]
- Johannsson G, Mårin P, Lönn L, Ottosson M, Stenlöf K, Björntorp P, Sjöström L, Bengtsson BA 1997 Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab 82:727–734 [DOI] [PubMed] [Google Scholar]
- Franco C, Brandberg J, Lönn L, Andersson B, Bengtsson BA, Johannsson G 2005 Growth hormone treatment reduces abdominal visceral fat in postmenopausal women with abdominal obesity: a 12-month placebo-controlled trial. J Clin Endocrinol Metab 90:1466–1474 [DOI] [PubMed] [Google Scholar]