Abstract
The cases of four newborn infants with congenital rickets are reported. All infants were native Canadian: three were Cree and one was Inuit. One had a narrow chest and pulmonary hypoplasia, two had clinical and radiological signs of rickets with craniotabes, thickened wrists, and prominent costochondral junctions, and one had perinatal asphyxia and hydrops. All had hypocalcemia, hypophosphatemia and secondary hyperparathyroidism. Serum 25-hydroxyvitamin D levels were low in three of the infants. The four mothers had evidence of vitamin D deficiency. All infants recovered following treatment with 5000 IU oral vitamin D daily.
Keywords: Congenital rickets, Hyperparathyroidism, Hypocalcemia, 25-hydroxyvitamin D
Abstract
On rend compte du cas de quatre nouveau-nés atteints de rachitisme congénital. Tous étaient originaires du Canada. Trois étaient Cris et un, Inuit. L’un avait le thorax étroit et une hypoplasie pulmonaire, deux présentaient des signes cliniques et radiologiques de rachitisme accompagné de craniotabès, de poignets épaissis et d’un chapelet costal, et le dernier souffrait d’asphyxie périnatale et d’anasarque. Tous étaient atteints d’hypocalcémie, d’hypophosphatémie et d’hyperparathyroïdie secondaire. Le taux de 25-hydroxyvitamine D sérique était faible chez trois des nouveau-nés. Les quatre mères manifestaient des signes de carence en vitamine D. Tous les enfants se sont rétablis après un traitement quotidien de 5 000 UI de vitamine D par voie orale.
Rickets in infants and children caused by a diet inadequate in vitamin D was common in the industrialized world until about the middle of the last century. Improved public health measures and the fortification of liquid cows’ milk and infant formulas with vitamin D resulted in the virtual elimination of the disease in these countries. A few cases of rickets in children and osteomalacia in adults have been reported among immigrant populations in Europe (1), and in Canada rickets continues to be seen with distressing frequency among the aboriginal population living in communities in Manitoba, northwestern Ontario and Nunavut (2,3). Identified risk factors include residence in northern latitudes with decreased sunlight exposure and, therefore, inadequate dermal synthesis of vitamin D; little milk consumption after weaning; insufficient intake of both vitamin D and calcium; and generally low socioeconomic status. Herein we report our experience in the past three years with four infants who presented at birth with rickets caused by maternal vitamin D deficiency (VDD).
CASE PRESENTATIONS
The clinical and radiological findings of the four patients, who were all seen by one or more of the authors, are summarized in Table 1. All infants were aboriginal Canadians; three were Cree and one was Inuit. Patient 1 had a number of unusual features and is presented in more detail below. Patients 2 and 3 had signs of clinical rickets at birth and patient 4 had perinatal asphyxia and hydrops. The latter three infants also had low birthweights and experienced intrauterine growth retardation. Their mothers had little or no prenatal care, did not consume milk, and had not taken vitamin or calcium supplements during their pregnancies. None were known to be drug abusers. All four infants had low levels of serum calcium and phosphorus when they were first assessed (Table 2), three had elevated alkaline phosphatase activity and all had elevated levels of parathyroid hormone (PTH). All but patient 2 had low serum 25-hydroxyvitamin D levels. Patients 1, 2 and 3 had radiological evidence of rickets. Patient 4 had no radiological long bone changes, but had abnormally thin ribs. The mother of patient 3 was not evaluated until eight months postpartum, and she had a low level of serum 25-hydroxyvitamin D. The other three mothers were evaluated postpartum; two had abnormally low levels of serum 25-hydroxyvitamin D and two mothers had hypocalcemia. All patients were treated with 5000 IU/day vitamin D orally and all recovered clinically and radiologically.
TABLE 1.
Summary of clinical and radiological findings in four infants with congenital rickets
| Patient | Sex | Race | Gestation | Birthweight (g) | Clinical presentation | Initial x-ray |
|---|---|---|---|---|---|---|
| 1 | M | Cree | 34 weeks | 2490 | Respiratory distress | Osteopenia Cupped radial metaphyses Narrow chest Subperiosteal resorption Hypoplastic lungs |
| 2 | M | Cree | 25–28 weeks | 866 | Very low birthweight | Osteopenia |
| Abnormally wide cranial sutures | Rickets in wrist, knee and skull | |||||
| 3 | F | Inuit | 36–38 weeks | 1796 | Clinical rickets | Rib fractures Demineralized skeleton Wide splayed metaphyses |
| 4 | F | Cree | 29 weeks | 900 | Perinatal asphyxia | Thin ribs |
| Hydrops |
TABLE 2.
Summary of biochemical findings in four infants with congenital rickets and their mothers
| Patient | Ca mmol/L | Infant (postnatal age in days) | 25-OHD nmol/L | Ca mmol/L | P mmol/L | Mother ALP units/L | PTH ng/L | 25-OHD nmol/L | ||
|---|---|---|---|---|---|---|---|---|---|---|
| P mmol/L | ALP units/L | PTH ng/L | ||||||||
| 1 | 1.93 (1) | 1.37 (1) | 221 (1) | 1321 (1) | – | 1.90 | 1.03 | 139 | 72 | 37 |
| 2.20 (6) | 1.26 (6) | 270 (6) | 861 (6) | 27 (6) | ||||||
| 2.02 (13) | 1.59 (13) | 435 (13) | 606 (13) | |||||||
| 2.37 (66) | 1.36 (66) | 213 (66) | 28 (66) | |||||||
| 2 | 1.63 (1) | 1.22 (1) | 233 (5) | 572 (5) | 50 (3) | 2.16 | 1.22 | – | 28 | 59 |
| 2.08 (2) | 0.46 (3) | 406 (75) | 375 (14) | |||||||
| 2.16 (3) | 0.34(8) | 336 (89) | 371 (28) | |||||||
| 2.32 (8) | ||||||||||
| 3 | 1.88 (3) | 1.34 (3) | 410 (3) | 175 (3) | <15 (3) | 2.40 | 1.16 | – | 24 | 27 |
| 520 (23) | ||||||||||
| 4 | 1.48 (1) | 0.90 (1) | 111 (1) | 401 (1) | <15 (1) | 1.96 | 0.95 | – | 34 | 17 |
| 2.18 (2) | 351 (60) | |||||||||
| Reference | ||||||||||
| Range | 2.10 – 2.60 | 1.62 – 2.52 | 117 – 352 | 0 – 65 | 35 – 200 | 2.10 – 2.60 | 0.81 – 1.45 | 30 – 120 | 0 – 65 | 35 – 200 |
ALP Serum alkaline phosphatase; Ca Serum calcium; 25-OHD Serum 25-hydroxyvitamin D; P Serum phosphorous; PTH Parathyroid hormone
Patient 1 case presentation
The mother of this baby drank no milk and took no vitamin supplements during her pregnancy. She had a seizure disorder that was treated with carbamazepine. At 32 weeks’ gestation a fetal assessment examination showed that the fetus had short bowed legs, a small chest and polyhydramnios. At birth the baby required intubation and ventilation. The chest was narrow and bell-shaped. Cranial sutures were widely open but otherwise there were no clinical signs of rickets.
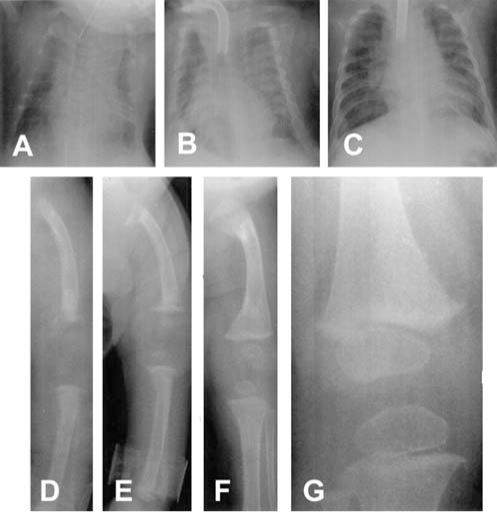
Radiographs at birth showed a narrow chest, splayed ribs and pulmonary hypoplasia. There was generalized osteoporosis, cupping and fraying of the radial metaphyses, and subperiosteal bone resorption in the femora, tibiae and humeri, suggestive of hyperparathyroidism. The baby had hypocalcemia, hypophosphatemia and a low level of serum 25-hydroxyvitamin D. Serum PTH was greatly elevated (Table 2). The mother also had hypocalcemia, low serum 25-hydroxyvitamin D and mildly elevated serum PTH. The patient was treated with calcium and 5000 IU/day vitamin D. Between one and four months of age, radiographic examinations of the skeleton showed significant improvement. The subperiosteal resorption had resolved, and the distal radial cupping had greatly diminished, but there was evidence of healing buckle fractures in the proximal and distal femora bilaterally. The abnormal biochemical findings had resolved by one month of age and the serum PTH was normal at six weeks. The baby required ventilatory support from birth until 15 months of age, essentially because of a highly compliant chest wall and tracheomalacia. A tracheostomy was performed at two months of age. The infant was discharged home at 18 months of age on maintenance doses of vitamin D, at which time he was growing normally and bone x-rays were normal. A composite of serial radiographs is shown in Figure 1. Clinical follow-up at one and two years showed normal growth and no clinical, biochemical and radiological signs of rickets.
Figure 1).
Chest (A, B, C) and lower limb (D, E, F, G) radiographs of patient 1. Marked osteoporosis, a very narrow chest and thin ribs, fraying of the ends of the long bones and subperiosteal bone resorption were evident at birth (A,D). Following treatment with 5000 IU vitamin D daily, radiographs at one month of age (E) showed fractures at the ends of the long bones with resolution of the subperiosteal bone resorption. Improved mineralization and healing of rickets were evident by four months of age (B,F). The bones were considered normal at 15 months (C) and three years of age (G)
DISCUSSION
The four babies were treated with 5000 IU/day vitamin D and they all recovered rapidly from rickets, thus excluding vitamin D-dependent rickets (4,5). None had intestinal malabsorption, or hepatic or renal disease that accounted for the rickets. In patient 1, a diagnosis of primary hyperparathyroidism was initially entertained because of the greatly elevated serum PTH level and widespread subperiosteal bone resorption. However, the baby had hypocalcemia, rather than hypercalcemia, and had a low level of serum 25-hydroxyvitamin D, as did the mother, who had many risk factors for VDD, including treatment with an anticonvulsant drug that increases the urinary excretion of calcitroic acid, which leads to VDD. Moreover, the baby rapidly improved clinically, radiologically and biochemically with 5000 IU/day of vitamin D, thus making it clear that the hyperparathyroidism was secondary to VDD.
There are few other published cases of congenital rickets caused by maternal VDD (6–11). Serum PTH levels were rarely documented. In addition, Gradus et al (12) reported a rather similar infant to our patient 1, but that infant’s mother had maternal hypoparathyroidism.
The only source of vitamin D available to the fetus is that which is derived from the mother, and the vitamin freely crosses the placenta, particularly during the second half of pregnancy. Infants of mothers with VDD have reduced concentrations of 25-hydroxyvitamin D in their cord blood samples (13). Although we have seen many young infants with VDD rickets over the past three decades, characteristically presenting with hypocalcemic seizures (2,3), we have not previously seen babies who have evidence of the disease at birth. Some of our earlier patients who presented at a month or two of age might indeed have had congenital rickets, although we did not see them at birth. Lebrun et al (14) reported in a 1987 study that 76% of mothers in two northern Manitoba communities with a high incidence of rickets had serum 25-hydroxyvitamin D levels below the normal range. A more recent cross-sectional survey (15) in these same communities documented VDD in 85% of 115 pregnant women.
VDD rickets is an anomaly in today’s world. It is a completely preventable disease that can cause severe and life-threatening illness in young infants and should no longer be seen in industrialized countries. The Indian and Inuit Health Committee of the Canadian Paediatric Society (16) recommended that all infants, and pregnant and lactating mothers should receive 400 IU/day vitamin D. This is especially important for those people who live in northern communities where it is recommended that breastfed or bottlefed infants and children up to two years of age should receive 800 IU/day during the winter months. Compliance with these recommendations is very poor in our experience. Despite the efforts of physicians and other health care professionals, public health authorities and local governments, rickets is seen as often today in many aboriginal communities in Manitoba, northwestern Ontario and Nunavut as it was 30 years ago. Our recent experience with the four infants reported in the present article seems to suggest an escalation of the problem. When compliance with a daily vitamin D supplement for pregnant women and young infants is poor, we again recommend that consideration be given to the administration of an annual or semiannual large prophylactic dose of oral vitamin D to pregnant women and young infants (3). A dose of 100,000 IU produces a rise of 25-hydroxyvitamin D, which is sustained for approximately six months. This measure has been found to be an effective, efficient, inexpensive and safe means of prophylaxis against VDD, both in some of our northern communities (B Martin, personal communication) as well as in other high-risk populations (17).
REFERENCES
- 1.Mughal MZ, Salama H, Greenaway T, Laing I, Mawer EB. Florid rickets associated with prolonged breast feeding without vitamin D supplementation. BMJ. 1999;318:39–40. doi: 10.1136/bmj.318.7175.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haworth JC, Dilling LA. Vitamin-D-deficient rickets in Manitoba, 1972–84. CMAJ. 1986;134:237–41. [PMC free article] [PubMed] [Google Scholar]
- 3.Haworth JC, Dilling LA, deGroot W, Greenberg CR, Longstaffe SEA, Moffatt MEK. Vitamin D deficiency in Manitoba and northwest Ontario. Can J Pediatr. 1995;2:331–5. [Google Scholar]
- 4.Fraser D, Kooh SW, Kind HP, Holick MF, Tanaka Y, DeLuca HF. Pathogenesis of hereditary vitamin-D-dependent rickets. An inborn error of vitamin D metabolism involving defective conversion of 25-hydroxyvitamin D to 1a, 25-dihydroxyvitamin D. N Engl J Med. 1973;289:817–22. doi: 10.1056/NEJM197310182891601. [DOI] [PubMed] [Google Scholar]
- 5.Brooks MH, Bell NH, Love L, et al. Vitamin-D-dependent rickets type II. Resistance of target organs to 1,25-dihydroxyvitamin D. N Engl J Med. 1978;298:996–9. doi: 10.1056/NEJM197805042981804. [DOI] [PubMed] [Google Scholar]
- 6.Moncrieff M, Fadahunsi TO. Congenital rickets due to maternal vitamin D deficiency. Arch Dis Child. 1974;49:810–1. doi: 10.1136/adc.49.10.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blond MH, Gold F, Pierre F, et al. La rachitisme carential du foetus. A propos d’un cas. J Gynecol Obstet Biol Reprod. 1997;26:834–6. [PubMed] [Google Scholar]
- 8.Kirk J. Congenital rickets – a case report. Austr Paediatr J. 1982;18:291–3. [PubMed] [Google Scholar]
- 9.Sann L, David L, Thomas A, Frederich A, Chapuy MC, Francois R. Congenital hyperparathyroidism and vitamin D deficiency secondary to maternal hypoparathyroidism. Acta Paediatr Scand. 1976;65:381–5. doi: 10.1111/j.1651-2227.1976.tb04901.x. [DOI] [PubMed] [Google Scholar]
- 10.Ford JA, Davidson DC, McIntosh WB, Fyfe WM, Dunnigan MG. Neonatal rickets in Asian immigrant population. BMJ. 1973;3:211–2. doi: 10.1136/bmj.3.5873.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park W, Paust H, Kaufmann HJ, Offermann G. Osteomalacia of the mother – rickets of the newborn. Eur J Pediatr. 1987;146:292–3. doi: 10.1007/BF00716477. [DOI] [PubMed] [Google Scholar]
- 12.Gradus D, LeRoith D, Karplus M, Zmora E, Grief M, Bar-Ziv J. Congenital hyperparathyroidism and rickets: Secondary to maternal hypocalcemia. Isr J Med Sci. 1981;17:705–8. [PubMed] [Google Scholar]
- 13.Brooke OG, Brown IR, Cleeve HJ, Sood A. Observations on the vitamin D state of pregnant Asian women in London. Br J Obstet Gynecol. 1981;88:18–26. doi: 10.1111/j.1471-0528.1981.tb00931.x. [DOI] [PubMed] [Google Scholar]
- 14.Lebrun JB, Moffatt MEK, Mundy RJT, et al. Vitamin D deficiency in a Manitoba community. Can J Public Health. 1993;84:394–6. [PubMed] [Google Scholar]
- 15.Smith PJ.Vitamin D deficiency in three northern Manitoba communities. PhD Thesis. Winnipeg: University of Manitoba, 1999
- 16.Indian and Inuit Health Committee, Canadian Paediatric Society Vitamin D supplementation for northern native communities. CMAJ. 1988;138:229–30. [PMC free article] [PubMed] [Google Scholar]
- 17.Stephens WP, Klimiuk PS, Berry JL, Mawer EB. Annual high-dose vitamin D prophylaxis in Asian immigrants. Lancet. 1981;ii:1199–201. doi: 10.1016/s0140-6736(81)91439-2. [DOI] [PubMed] [Google Scholar]