Incidence of type 2 diabetes has now reached epidemic proportions, afflicting 8% of adults in the United States. More worrisome are predictions based upon current trends indicating incidence of 30%: one of every three children born in the year 2000 to develop diabetes in their lifetime (1). Type 2 diabetes is characterized by chronic hyperglycemia resultant from the combined dysfunction of insulin secretion from pancreatic β-cells in the face of peripheral insulin resistance. Thus, type 2 diabetic patients require multifactorial treatment strategies to regain and maintain euglycemia, which can strain both patient compliance and patient’s pocketbooks. The answer to this dilemma is to devise a magic pill that could simultaneously coordinate increases in insulin secretion with peripheral glucose clearance. Moreover, because obesity trends pair with those of type 2 diabetes, wouldn’t it be nice if such a magic pill could induce weight loss, too?
Glucagon-Like Peptide-1 (GLP-1) Is the Answer
Within the last decade, such a magic pill substance was discovered: the incretin hormone GLP-1. A seminal study from 2002 determined that continuous infusion of GLP-1 decreased fasting hyperglycemia, improved insulin sensitivity and β-cell function, and reduced body weight (2). These observations correlated beautifully with data demonstrating a paucity of GLP-1 release from the L cells of type 2 diabetic patients (3,4,5,6). Importantly, unlike insulin injection or oral sulfonylurea therapies, GLP-1 injection enhances insulin secretion but without risk of hypoglycemic episodes. Because GLP-1 specifically amplifies insulin release triggered by nutrient stimulation (7), as opposed to triggering secretion itself absent of nutrient stimulation, GLP-1 does not inappropriately elevate insulin secretion under fasting conditions. Rather, GLP-1 action works in sync with the regulated insulin secretion machinery, increasing the quantity of insulin released only when needed. To exert effects upon weight loss, GLP-1 action is coupled through decreases in gastric emptying, intestinal motility, and food intake. Despite its wonder-drug profile, development of GLP-1 therapy has met with challenges due to its inordinately short half-life (2 min) resulting from degradation upon secretion from the L cell of the intestine by the serine protease dipeptidyl peptidase-IV, and a propensity for patient intolerance due to nausea. Interestingly, efforts to increase endogenous GLP-1 levels by elongating half-life via inhibition of dipeptidyl peptidase-IV have not yielded the desired weight loss effect that is seen with GLP-1 administration (8,9), and as such, agonists to boost GLP-1 release are being sought (10,11). Given the hyperinsulinemia associated with prediabetes and insulin resistance, the current study by Lim et al. (12) raises the question as to whether hyperinsulinemia might be a direct cause of impaired GLP-1 release in type 2 diabetic patients.
The GLP-1 Release Pathway
Under normal conditions, the L cell of the intestine senses nutrients such as glucose and fats through microvilli on the apical surface and responds by triggering the fusion of GLP-1-containing secretory granules with the basolateral plasma membrane to release the processed bioactive GLP-1 peptides. Glucose-stimulated GLP-1 release is biphasic, initially rising within 10–15 min of food intake, peaking by 40 min (13). In a previous study, Lim and colleagues (14) discovered that, surprisingly, acute insulin stimulation could also trigger GLP-1 secretion from the L cell, concurrent with the activation of MAPK kinase (MEK)-1/2 and on to ERK1. Lacking, however, was a pathway describing how the insulin signal led to mobilization of the GLP-1 secretory granules within L cells. Based upon analogies drawn between islet β-cell and intestinal L cell release patterns, Lim et al. (12) sought to determine whether filamentous actin (F-actin) remodeling was involved in insulin-stimulated GLP-1 release from L cells.
F-Actin Remodeling in the L Cell
F-actin remodeling to promote vesicle/granule exocytosis events is a conserved mechanism where the ratio of polymerized F-actin to depolymerized globular actin (G-actin) transiently changes to effect mobilization or repositioning of secretory granules with respect to surface membranes. F-actin remodeling is considered a requisite event in processes prominent in the regulation of euglycemia, including glucose-stimulated insulin release (islet β-cells) (15,16) and insulin-stimulated glucose uptake/GLUT4 vesicle translocation (adipose and skeletal muscle tissues) (17,18). Common to F-actin remodeling in these processes is the use of small Rho family GTPases, in particular Cdc42, and its downstream effector protein p21-activated kinase (Pak1). Dissimilar is the impact of F-actin depolymerization upon these processes. For example, conversion of polymerized F-actin to monomeric G-actin using agents such as latrunculin or cytochalasin will potentiate insulin release from β-cells but inhibit glucose uptake into adipocytes. Lim et al. (12) show that L cells respond to latrunculin akin to that of the islet β-cells by potentiating GLP-1 release. Furthermore, a recent study suggests that the mechanisms of glucose-stimulated GLP-1 release and glucose-stimulated insulin release share a biphasic pattern of release (19) wherein intracellularly localized granules require mobilization to the cell surface to support the second phase of release. Considerable support exists for the concept that F-actin remodeling may function as a permissive barrier, corralling granules to simultaneously mobilize and position granules to support release, but only in response to appropriate stimuli (20). In another capacity, F-actin remodeling has also been suggested to facilitate the distal docking/fusion steps of exocytosis in 3T3-L1 adipocytes and β-cells, because their t-SNARE (target membrane soluble N-ethylmaleimide-sensitive factor attachment protein receptor) isofor syntaxin 4 can interact with F-actin directly, or indirectly via actin-binding proteins (21,22,23).
Actin remodeling events in β-cells involve upstream signaling via Rho family GTPases Cdc42 and Rac1. In this issue, Lim et al. (12) elegantly demonstrate a novel pathway within intestinal L cells, whereby acute insulin stimulation triggers Cdc42 activation and Pak1 activation (Fig. 1), although Rac1 appears to be dispensable in this process. Signaling through this Cdc42-Pak1 axis was a requirement for actin remodeling in L cells and the remodeling essential for insulin-stimulated GLP-1 release. Interestingly, the Cdc42-Pak1 axis was also required for activation of MEK1/2 and ERK1/2, placing Cdc42 as a critical proximal regulator of insulin action in L cells. The cell-type specificity for Cdc42 activation by insulin is notable, given that not all cells that express insulin receptors exhibit insulin-induced Cdc42 activation (Wang, Z., and D. C. Thurmond, unpublished data), suggesting involvement of signaling intermediates that are cell-type specific upstream of Cdc42 in the L cell pathway. Actin remodeling has been captured by microscopic methods in other cell types, although capture of net changes by biochemical methods has been more challenging (24). Remarkably, significant changes in F-actin to G-actin ratios were detect able in this L cell system, and in a time-dependent and dynamic manner. From a cell biology perspective, the L cell may present novel opportunities to study actin remodeling in a quantifiable manner. It will be important in future studies to determine whether insulin-stimulated GLP-1 release is also biphasic.
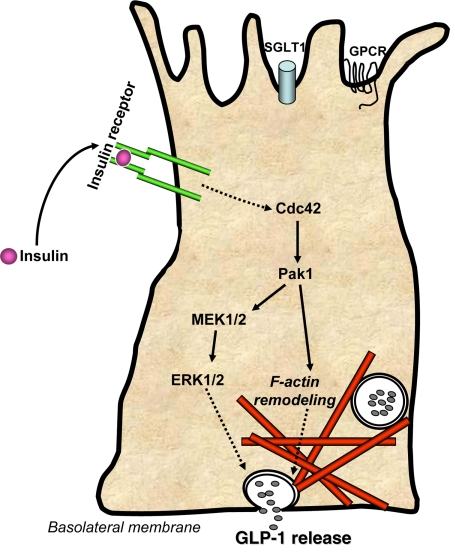
Figure 1.
Schematic model of insulin-induced GLP-1 release from intestinal L cells. Insulin binds to insulin receptors present on L cells to trigger the activation of Cdc42 within 10–15 min. Cdc42 expression is required for subsequent activation of Pak1 and MEK1/2 signaling cascades. Pak1 activation proceeds to induce F-actin remodeling, whereas MEK1/2 signals downstream to ERk1/2, with both pathways resulting in release of GLP-1. Insulin signaling may occur at alternate surfaces based upon reports of insulin receptor localization (25,26). Nutrient sensing through sodium-dependent glucose transporter-1 (SGLT1) and G protein-coupled receptors (GPCR) occurs as nutrients pass cell in the intestinal luminal surface (top).
Perspectives for the Future
How will characterization of this new pathway help treat type 2 diabetes? Clearly, this marks the first stage of pathway characterization for the role of insulin upon the L cells. Surprisingly, insulin stimulation under these acute experimental conditions actually increased GLP-1 release, consistent with the concept that the chronic hyperinsulinemia characteristic of early diabetes and insulin resistance may exert damaging effects upon L cell function via a feed-forward cycle. Mechanistically, one could speculate that under the hyperglycemic conditions of prediabetes, insulin release from β-cells is amplified, and then insulin triggers GLP-1 release from L cells. In turn, GLP-1 travels to β-cells to potentiate more insulin release, and the cycle repeats until euglycemia is restored or until both cell types exhaust into dysfunction and type 2 diabetes ensues. Given these scenarios, would uncoupling this new element of insulin action in L cells be a good or bad target for therapeutic intervention to combat type 2 diabetes?
Footnotes
Work in the Thurmond laboratory has been supported by research grants from the National Institutes of Health (DK076614 and DK067912) and a Career Development Award from the American Diabetes Association (1-03-CD-10).
Disclosure Summary: The author has nothing to disclose.
For article see page 5249
Abbreviations: F-actin, Filamentous actin; G-actin, globular actin; GLP-1, glucagon-like peptide-1; MEK, MAPK kinase; Pak1, p21-activated kinase.
References
- American Diabetes Association 2009 Increase diabetes spending now to save in the long run. Professional Section Quarterly: Summer. Alexandria, VA: American Diabetes Association; 1 [Google Scholar]
- Zander M, Madsbad S, Madsen JL, Holst JJ 2002 Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and β-cell function in type 2 diabetes: a parallel-group study. Lancet 359:824–830 [DOI] [PubMed] [Google Scholar]
- Jones IR, Owens DR, Moody AJ, Luzio SD, Morris T, Hayes TM 1987 The effects of glucose-dependent insulinotropic polypeptide infused at physiological concentrations in normal subjects and type 2 (non-insulin-dependent) diabetic patients on glucose tolerance and B-cell secretion. Diabetologia 30:707–712 [DOI] [PubMed] [Google Scholar]
- Mannucci E, Ognibene A, Cremasco F, Bardini G, Mencucci A, Pierazzuoli E, Ciani S, Fanelli A, Messeri G, Rotella CM 2000 Glucagon-like peptide (GLP)-1 and leptin concentrations in obese patients with type 2 diabetes mellitus. Diabet Med 17:713–719 [DOI] [PubMed] [Google Scholar]
- Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ 2001 Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 86:3717–3723 [DOI] [PubMed] [Google Scholar]
- Vilsbøll T, Knop FK, Krarup T, Johansen A, Madsbad S, Larsen S, Hansen T, Pedersen O, Holst JJ 2003 The pathophysiology of diabetes involves a defective amplification of the late-phase insulin response to glucose by glucose-dependent insulinotropic polypeptide-regardless of etiology and phenotype. J Clin Endocrinol Metab 88:4897–4903 [DOI] [PubMed] [Google Scholar]
- Tolhurst G, Reimann F, Gribble FM 2009 Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol 587:27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrén B, Gomis R, Standl E, Mills D, Schweizer A 2004 Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care 27:2874–2880 [DOI] [PubMed] [Google Scholar]
- Ahrén B, Simonsson E, Larsson H, Landin-Olsson M, Torgeirsson H, Jansson PA, Sandqvist M, Båvenholm P, Efendic S, Eriksson JW, Dickinson S, Holmes D 2002 Inhibition of dipeptidyl peptidase IV improves metabolic control over a 4-week study period in type 2 diabetes. Diabetes Care 25:869–875 [DOI] [PubMed] [Google Scholar]
- Lovshin JA, Drucker DJ 2009 Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 5:262–269 [DOI] [PubMed] [Google Scholar]
- Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K 2009 TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10:167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim GE, Xu M, Sun J, Jin T, Brubaker PL 2009 The Rho guanosine 5′-triphosphatase, cell division cycle 42, is required for insulin-induced actin remodeling and glucagon-like peptide-1 secretion in the intestinal endocrine L cell. Endocrinology 150:5249–5261 [DOI] [PubMed] [Google Scholar]
- Holst JJ 2007 The physiology of glucagon-like peptide 1. Physiol Rev 87:1409–1439 [DOI] [PubMed] [Google Scholar]
- Lim GE, Huang GJ, Flora N, LeRoith D, Rhodes CJ, Brubaker PL 2009 Insulin regulates glucagon-like peptide-1 secretion from the enteroendocrine L cell. Endocrinology 150:580–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson CS, Håkansson J, Salehi A, Bengtsson M, Galvanovskis J, Partridge C, SörhedeWinzell M, Xian X, Eliasson L, Lundquist I, Semb H, Rorsman P 2009 Impaired insulin exocytosis in neural cell adhesion molecule−/− mice due to defective reorganization of the submembrane F-actin network. Endocrinology 150:3067–3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmond DC, Gonelle-Gispert C, Furukawa M, Halban PA, Pessin JE 2003 Glucose-stimulated insulin secretion is coupled to the interaction of actin with the t-SNARE (target membrane soluble N-ethylmaleimide-sensitive factor attachment protein receptor protein) complex. Mol Endocrinol 17:732–742 [DOI] [PubMed] [Google Scholar]
- Kanzaki M, Pessin JE 2001 Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling. J Biol Chem 276:42436–42444 [DOI] [PubMed] [Google Scholar]
- Khayat ZA, Tong P, Yaworsky K, Bloch RJ, Klip A 2000 Insulin-induced actin filament remodeling colocalizes actin with phosphatidylinositol 3-kinase and GLUT4 in L6 myotubes. J Cell Sci 113:279–290 [DOI] [PubMed] [Google Scholar]
- Ohara-Imaizumi M, Aoyagi K, Akimoto Y, Nakamichi Y, Nishiwaki C, Kawakami H, Nagamatsu S 18 September 2009 Imaging exocytosis of single glucagon-like peptide-1 containing granules in a murine enteroendocrine cell line with total internal reflection fluorescent microscopy. Biochem Biophys Res Commun 10.1016/j.bbrc.2009.09.043 [DOI] [PubMed] [Google Scholar]
- Wang Z, Thurmond DC 2009 Mechanisms of biphasic insulin-granule exocytosis: roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci 122:893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell JL, Luo W, Oh E, Wang Z, Thurmond DC 2008 Filamentous actin regulates insulin exocytosis through direct interaction with syntaxin 4. J Biol Chem 283:10716–10726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Jedrychowski MP, Gygi SP, Pilch PF 2006 Role of insulin-dependent cortical fodrin/spectrin remodeling in glucose transporter 4 translocation in rat adipocytes. Mol Biol Cell 17:4249–4256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JA, Burchfield JG, Blair DH, Mele K, Ng Y, Vallotton P, James DE, Hughes WE 2009 Identification of a distal GLUT4 trafficking event controlled by actin polymerization. Mol Biol Cell 20:3918–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins AK, Thurmond DC 2003 Glucose regulates the cortical actin network through modulation of Cdc42 cycling to stimulate insulin secretion. Am J Physiol Cell Physiol 285:C698–C710 [DOI] [PubMed] [Google Scholar]
- Ménard D, Corriveau L, Beaulieu JF 1999 Insulin modulates cellular proliferation in developing human jejunum and colon. Biol Neonate 75:143–151 [DOI] [PubMed] [Google Scholar]
- Buts JP, De Keyser N, Marandi S, Maernoudt AS, Sokal EM, Rahier J, Hermans D 1997 Expression of insulin receptors and of 60-kDa receptor substrate in rat mature and immature enterocytes. Am J Physiol 273:G217–G226 [DOI] [PubMed] [Google Scholar]