Abstract
Resveratrol is a natural polyphenolic compound that activates nicotinamide adenosine dinucleotide-dependent deacetylase SIRT1. Resveratrol has recently been shown to exert potent antidiabetic actions when orally delivered to animal models of type 2 diabetes. However, the tissue(s) mediating these beneficial effects is unknown. Because SIRT1 is expressed in central nervous system (CNS) neurons known to control glucose and insulin homeostasis, we hypothesized that resveratrol antidiabetic effects are mediated by the brain. Here, we report that long-term intracerebroventricular infusion of resveratrol normalizes hyperglycemia and greatly improves hyperinsulinemia in diet-induced obese and diabetic mice. It is noteworthy that these effects are independent of changes in body weight, food intake, and circulating leptin levels. In addition, CNS resveratrol delivery improves hypothalamic nuclear factor-κB inflammatory signaling by reducing acetylated-RelA/p65 and total RelA/p65 protein contents, and inhibitor of nuclear factor-κB α and IκB kinase β mRNA levels. Furthermore, this treatment leads to reduced hepatic phosphoenolpyruvate carboxykinase 1 mRNA and protein levels and ameliorates pyruvate-induced hyperglycemia in this mouse model of type 2 diabetes. Collectively, our results unveiled a previously unrecognized key role for the CNS in mediating the antidiabetic actions of resveratrol.
Oral delivery of the natural pholyphenolic compound resveratrol exerts anti-diabetic actions; these results show that these effects are in part mediated by the brain.
According to the World Health Organization, the incidence of type 2 diabetes mellitus (T2DM) is rapidly rising, with more than 150 million people already affected. Because of its serious associated complications (e.g. heart disease, retinopathy, neuropathy, nephropathy) T2DM is a huge threat to human health. The primary defects underlying T2DM are still poorly understood. However, the distinct and refractory responses to currently available antidiabetic drugs and the discovery of polymorphisms associated with T2DM in several genes suggest that this condition is heterogeneous with respect to primary dysfunctions (1). Despite these issues, the concomitant surge of T2DM and adoption of high-calorie (HC) feeding habits in industrialized countries leaves little doubt that the latter is an important factor in the pathogenesis of diabetes (2). This contention is supported by the fact that long-term HC intake induces T2DM in experimental mammalian organisms (3).
Resveratrol (a natural polyphenolic molecule found in grapes) has recently been shown to exert potent antidiabetic actions when orally delivered in rodent models of T2DM (4,5). Resveratrol activates nicotinamide adenine dinucleotide-dependent deacetylase SIRT1, a nuclear protein thought to mediate some of the beneficial metabolic effects of calorie-restriction (6,7). Although resveratrol also activates other metabolically important enzymes [e.g. AMP-activated protein kinase (AMPK)] (5,8), its antidiabetic actions are thought to be mediated by SIRT1. Indeed, when more potent and selective SIRT1 activators were orally delivered to diabetic rodent models, similar metabolic improvements were seen (9). The molecular basis of SIRT1 activators actions have been proposed to involve enhanced fuel oxidation in liver, skeletal muscle, and adipose tissue (9,10). However, the critical sites mediating these effects have not been established.
Specialized neurons within the central nervous system (CNS) sense physiological changes in circulating hormonal and nutrients levels to maintain metabolic homeostasis (11). An example of these CNS-peripheral feedback loop systems includes crosstalk between pancreatic β-cells, hypothalamic neurons, and liver. Indeed, proper insulin-mediated suppression of hepatic glucose production requires functional hypothalamic insulin receptors (12,13). Furthermore, glucose and leptin sensing in hypothalamic neurons have been shown to be required for normal glucose homeostasis (14,15,16,17). Because HC diet impairs these central sensing mechanisms, pharmacological interventions aimed at reversing these CNS defects have been proposed as reasonable approaches in the fight against T2DM (18).
Because of the established importance of the CNS in coordinating systemic glucose and insulin homeostasis (12,19,20,21,22,23,24,25,26) and the fact that SIRT1 is expressed in hypothalamic neurons known to govern peripheral glucose metabolism (e.g. melanocortin neurons) (27), we hypothesized that resveratrol antidiabetic effects could be mediated by the brain. To test this hypothesis, we assessed the metabolic consequences of central delivery of resveratrol in diet-induced obese and diabetic mice.
Materials and Methods
Mice and stereotaxic surgery
C57BL/6 male mice were housed with food and water available ad libitum in light- and temperature-controlled environments. Care of mice was within the Institutional Animal Care and Use Committee guidelines, and all the procedures were approved by the University of Texas Southwestern Medical Center Institutional Animal Care and Use Committee. Three groups of mice were generated. One group (chow-fed) was fed ad libitum on a regular chow diet until mice were killed. This group did not undergo any surgical procedure. The second group (HC-saline) was fed ad libitum on a regular chow diet up to 8 wk of age and then was switched and maintained on a high-calorie diet (HC; catalog no. D12331 (58% kcal from fat); Research Diets, New Brunswick, NJ). After 14 wk on this diet, mice underwent the following surgical procedure. A cannula was positioned stereotaxically into the cerebral lateral ventricles (−0.34 mm from bregma; ±1 mm lateral; −2.3 mm from skull) and a small osmotic minipump (model 1004; Alzet, Cupertino, CA) implanted sc was attached via a catheter to the cannula for intracerebroventricular infusion. The catheter was 25% longer than the distance between the site of placement of the minipump and the cannula, to allow free movement of the neck. In these mice, the minipump was filled with a sterile saline solution. After surgery, mice were kept on the same HC diet and were killed 5 wk later. The third group (HC-resveratrol) was treated like the second group, but the minipump implanted in these mice was filled with resveratrol (R5010; Sigma, St. Louis, MO) dissolved in sterile saline (final resveratrol concentration, 0.03 μg/μl). Because a volume of 0.11 μl/h was infused intracerebroventricularly, HC-resveratrol mice received a dose of approximately 79.2 ng resveratrol a day. This daily dose of resveratrol is approximately 8.5 million or approximately 152 million times lower, respectively, compared with the dose orally delivered in two previous studies (4,5). Tail vein blood was collected from fed or overnight-fasted mice. Blood was assayed for glucose level using OneTouch FastTake glucometer (LifeScan Inc., Milpitas, CA), and serum was collected after blood centrifugation and assayed for leptin and insulin levels using commercially available kits (Crystal Chem. Inc., Downers Grove, IL).
Assessment of mRNA and protein content
Five weeks after surgery, HC-saline and HC-resveratrol mice and age-matched, chow-fed controls were killed 4 h before the onset of the dark cycle and tissues quickly removed. Brains were processed as follows. After hypothalamus was collected, the remaining brain tissue was divided in two parts by a coronal cut at the border between the midbrain and hypothalamus. The rostral and caudal parts are referred to as forebrain (without hypothalamus) and hindbrain, respectively. These procedures were performed under a stereomicroscope. Tissues were quickly frozen in liquid nitrogen and subsequently stored at −80 C. RNAs were extracted using TRIzol reagent (Invitrogen, Carlsbad, CA). cDNA was generated by SuperScript II (Invitrogen) and used with SYBR Green PCR master mix (Applied Biosystems, Foster City, CA) for quantitative real-time PCR analysis. Inhibitor of nuclear factor-κBα (Iκ-Bα), phosphoenolpyruvate carboxykinase 1 (Pepck), glucose-6-phosphatase (G6Pase), pro-opiomelanocortin (Pomc), agouti-related protein (Agrp), neuropeptide Y (Npy), and IκB kinase complex (IKKβ) mRNA contents were normalized either to β-actin or to 36B4 mRNA contents. Sequences of deoxyoligonucleotide primers were as follows: Iκ-Bα (5′-cggaggacggagactcgtt and 5′-ccatggtcagcggcttct), Pepck (5′-cgcaagctgaagaaatatgacaa and 5′-tcgatcctggccacatctc), G6Pase (5′-cctaagctacaccatcagccg and 5′-ctccacagaaaggaccaggtcag), Pomc (5′-gaggccactgaacatctttgtc and 5′-gcagaggcaaacaagattgg), Agrp (5′-cggccacgaacctctgtag and 5′-ctcatcccctgcctttgc), Npy (5′-ctactccgctctgcgacact and 5′-agtgtctcagggctggatctc), IKKβ (Applied Biosystems; assay on demand Mm00833995_m1), β-actin (5′-catcgtgggccgctcta and 5′-cacccacataggagtccttctg), and 36B4 (5′-cactggtctaggacccgagaag and 5′-ggtgcctctgaagattttcg). All assays were performed at least three times using an Applied Biosystems Prism 7900HT sequence detection system. Proteins were extracted by homogenizing samples in lysis buffer [20 mm Tris, 5 mm EDTA, 1% (vol/vol) Nonidet P-40, and protease inhibitors (P2714-1BTL; Sigma)], then resolved by SDS-PAGE and finally transferred to a nitrocellulose membrane by electroblotting. Acetylated p53 was detected using rabbit polyclonal antisera against the lysine 379 of mouse p53 (Cell Signaling Technology, Danvers, MA). The following antibodies were used for Western blotting: rabbit polyclonal antisera against the acetyl-lysine 379 of mouse p53 (2070; Cell Signaling Technology), acetylated and total RelA/p65 protein contents were determined by using rabbit polyclonal antisera against the lysine 310 RelA/p65 (3045; Cell Signaling Technology), rabbit monoclonal antisera against RelA/p65 (C22B4; Cell Signaling Technology), rabbit polyclonal antisera against PEPCK (Cayman Chemical Co., Ann Arbor, MI). β-Actin, used as loading control, was detected with rabbit polyclonal antisera (Abcam, Cambridge, MA). Protein quantification analysis was done as described previously (27).
Assessment of triglycerides and glycogen content
Triglycerides and glycogen from liver biopsies obtained from mice on day of killing were assayed using L-Type TG H kit (Wako Chemicals USA Inc., Richmond, VA) and Glycogen assay kit (Biovision Research Products, Mountain View, CA) accordingly to the manufacturer’s direction as described previously (28).
Pyruvate tolerance test
This test was conducted 5 wk after the surgery in mice that were fasted for 5 h. Mice received an ip injection of pyruvate as described previously (29).
Statistical methods
Data sets were analyzed for statistical significance using Prism software (GraphPad Software, San Diego, CA) for a two-tailed unpaired Student’s t test or one-way ANOVA (Tukey’s post test) as indicated in each figure legend.
Results
CNS resveratrol delivery activates brain SIRT1
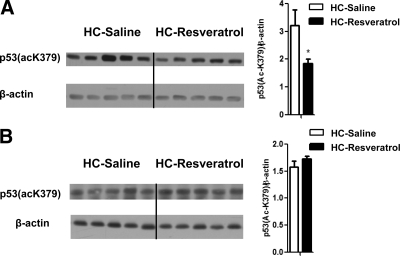
Resveratrol is known to enhance SIRT1 enzymatic activity in the liver and other peripheral tissues, an effect suggested to underlie its positive metabolic actions (4,10). Thus, we tested whether our CNS resveratrol delivery activated SIRT1 only in the brain. The acetylation status of SIRT1 target p53 (30,31,32) has been widely used as a measure for SIRT1 activity in vivo (9,27,33,34,35). Therefore, we assessed the levels of acetylated lysine 379 in p53 [p53(acK379)] in brain and liver of HC-resveratrol and HC-saline mice. Using forebrain protein lysate samples and Western blot assays, we found that the amount of p53(acK379) was lower in HC-resveratrol compared with HC-saline mice, suggesting that central resveratrol delivery enhanced brain SIRT1 enzymatic activity (Fig. 1A). Notably, no differences in the levels of hepatic p53(acK379) were observed between these two groups (Fig. 1B), suggesting that CNS resveratrol administration was restricted to the brain.
Figure 1.
CNS resveratrol administration activates SIRT1 only in the brain. Acetylated lysine (K) 379 in p53 and β-actin (used as loading control) protein levels were assessed in the forebrain (without the hypothalamus) (A) and liver (B) of HC-saline and HC-resveratrol mice by Western blot analysis. Error bars, sem. Statistical analyses were done using two-tailed unpaired Student’s t test. *, P < 0.05.
CNS resveratrol delivery does not affect body weight and food intake
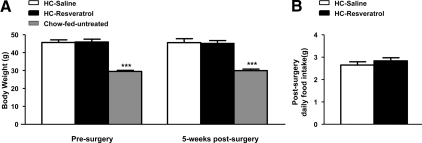
Because oral administration of resveratrol has been suggested to protect against diet-induced obesity (5), we assessed whether CNS resveratrol delivery improved body weight in diet-induced obese mice. Before surgery, HC-saline and HC-resveratrol groups had a similar degree of obesity (Fig. 2A). After 5 wk of treatment, CNS resveratrol delivery did not ameliorate this diet-induced obesity; both groups remained equally overweight compared with chow-fed mice (Fig. 2A). Leptinemia was not different between HC-saline and HC-resveratrol mice (serum leptin levels in fed mice after 5 wk of treatment, mean ± sem: HC-saline, 30.96 ± 3.46 ng/ml; HC-resveratrol, 26.54 ± 4.42 ng/ml; n = 7), suggesting that body adiposity was unchanged by CNS resveratrol treatment. Because transgenic-mediated SIRT1 overexpression concomitantly reduces food intake and energy expenditure with no effects on body weight (36), we measured food intake over the postsurgery period but found no differences between HC-saline and HC-resveratrol groups (Fig. 2B). Body weight and food intake were not changed by CNS resveratrol administration in a second cohort (supplemental Fig. 1, A and B, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). Collectively, our results suggest that CNS resveratrol administration does not affect body weight and food intake in obese male mice.
Figure 2.
CNS resveratrol administration does not improve energy balance. A, Body weight before and 5 wk after surgery in HC-saline and HC-resveratrol C57BL/6 male mice. B, Daily food intake in the postsurgery period in HC-saline and HC-resveratrol mice. The chow-fed group consists of age-matched, C57BL/6 male mice fed ad libitum on a regular chow diet that did not undergo any surgical procedure. Error bars, sem. Statistical analyses were done using two-tailed unpaired Student’s t test or one-way ANOVA (Tukey’s post test) when two or three groups were compared, respectively (n = 7 for each group). ***, P < 0.001 vs. HC-saline mice.
CNS resveratrol delivery normalizes diet-induced hyperglycemia
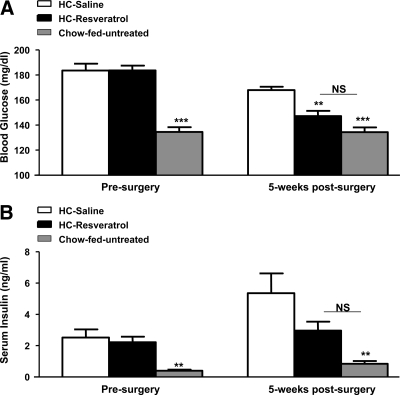
Because oral administration of resveratrol or transgenic-mediated SIRT1 overexpression ameliorates diet-induced diabetes (4,5,36), we assessed whether CNS resveratrol delivery improves glucose homeostasis in diet-induced diabetic mice. Before surgery, HC-saline and HC-resveratrol groups were similarly hyperglycemic (Fig. 3A). It is noteworthy that CNS resveratrol administration significantly reduced blood glucose levels. Indeed, HC-resveratrol mice displayed normal glycemia, whereas HC-saline controls were still hyperglycemic after the 5-wk treatment period (Fig. 3A). Insulin homeostasis was also significantly ameliorated. Before surgery, HC-saline and HC-resveratrol groups were similarly hyperinsulinemic (Fig. 3B). Remarkably, HC-resveratrol mice had insulinemia almost within normal values, whereas HC-saline control mice were still hyperinsulinemic after treatment (Fig. 3B). It is noteworthy that insulinemia continued to rise over time in the HC-saline group (∼110% increase; P = 0.06 after surgery vs. presurgery), an effect that was attenuated by CNS resveratrol treatment (∼30% increase; P = 0.3 after surgery vs. presurgery). The reduced insulinemia in face of normal glycemia suggests enhanced insulin sensitivity in HC-resveratrol mice. Noteworthy, glycemia was normalized and insulinemia improved by CNS resveratrol administration in a second cohort also (supplemental Fig. 2, A and B). Collectively, our data demonstrate that CNS resveratrol administration greatly ameliorates parameters of glucose/insulin homeostasis in diet-induced obese and diabetic mice.
Figure 3.
CNS resveratrol administration improves glucose homeostasis. Blood glucose (A) and serum insulin levels (B) before and 5 wk after surgery in fed HC-saline and HC-resveratrol C57BL/6 male mice. The chow-fed group consists of age-matched, C57BL/6 male mice fed ad libitum on a regular chow diet that did not undergo any surgical procedure. Error bars, sem. Statistical analyses were done using one-way ANOVA (Tukey’s post test). (n = 7 for each group.) **, P < 0.01; ***, P < 0.001 vs. HC-saline mice. NS, Not statistically different.
CNS resveratrol delivery dampens diet-induced nuclear factor-κB (NF-κB) signaling in brain
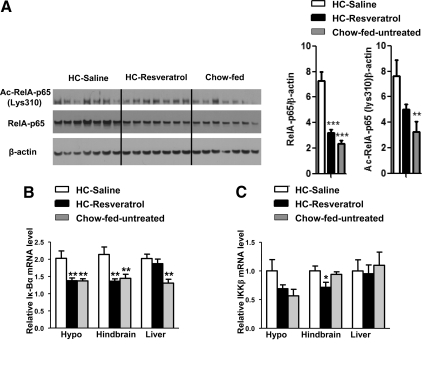
To identify possible mechanisms through which CNS resveratrol infusion improves glucose homeostasis, we assessed mRNA content of neuropeptides known to regulate glucose balance, including Pomc, AgRP, and Npy. Hypothalamic mRNA levels of Pomc, AgRP, and Npy were indistinguishable between HC-resveratrol and HC-saline groups (supplemental Fig. 3). We also assessed markers of inflammation because it has been reported that HC diet triggers hypothalamic expression of NF-κB targets (e.g. Iκ-Bα and IKKβ) (37). Because SIRT1 deacetylates RelA/p65 and hence inhibits NF-κB (38), we directly assessed this pathway. The amounts of acetylated and total RelA/p65 in hypothalamus of HC-saline mice were elevated compared with chow-fed control mice (Fig. 4A). It is noteworthy that both these parameters were reduced to normal levels by CNS resveratrol administration (Fig. 4A). Moreover, Iκ-Bα mRNA level was increased in the hypothalamus of HC-saline mice (Fig. 4B), as expected (37). Remarkably, CNS resveratrol infusion normalized hypothalamic Iκ-Bα mRNA level (Fig. 4B). Resveratrol action was restricted to the CNS because Iκ-Bα mRNA level was also normalized in hindbrain but remained elevated in the liver of HC-resveratrol mice (Fig. 4B). To further assess this pathway, we measured IKKβ mRNA contents. As reported previously (37), the level of IKKβ mRNA tended to be higher in the hypothalamus but (for reasons yet to be established) not in hindbrain and liver of HC-saline mice compared with chow-fed control mice (Fig. 4C). Consistent with our aforementioned results suggesting a brain-restricted antiinflammatory action, CNS resveratrol delivery tended to reduce IKKβ mRNA level in hypothalamus and diminished it in hindbrain but not in liver of HC-fed mice (Fig. 4C). Collectively, our data indicate that CNS resveratrol administration exerts anti-inflammatory effects in the brain of diet-induced obese mice.
Figure 4.
CNS resveratrol administration improves brain NF-kB signaling. A, RelA/p65, Ac-RelA/p65(K310) and β-actin (used as loading control) protein levels (determined by Western blot analysis) were assessed in hypothalamus of HC-saline, HC-resveratrol, and chow-fed, age-matched, C57BL/6 male mice (n = 7 for each group). Iκ-Bα (B) and IKKβ (C) mRNA level in hypothalamus, hindbrain, and liver (determined by quantitative real-time PCR). Error bars, sem. Statistical analyses were done using one-way ANOVA (Tukey’s post test). *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. HC-saline mice.
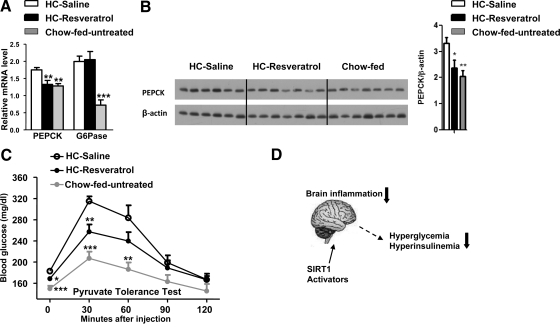
CNS resveratrol delivery improves hepatic PEPCK expression and pyruvate-induced hyperglycemia
To identify tissues underlying the antidiabetic actions of central resveratrol perfusion, we focused on the liver mainly because: 1) inappropriately elevated hepatic glucose output contributes to hyperglycemia in T2DM (39), 2) insulin effects on hepatic glucose production are partially mediated by the CNS (12,20), and 3) enhanced NF-κB signaling (that is rescued by CNS resveratrol delivery) hampers brain insulin sensitivity in HC-fed mice (37). Thus, we first explored hepatic gluconeogenic transcriptional program in HC-resveratrol and control mice. The first and last catalytic reactions in the gluconeogenic pathway are mediated by PEPCK and G6Pase, respectively. As expected, Pepck and G6Pase mRNA levels were elevated in livers of HC-saline mice compared with chow-fed controls (Fig. 5A). It is noteworthy that CNS resveratrol administration reduced hepatic Pepck (but not G6Pase) mRNA to normal levels (Fig. 5A). To independently assess for Pepck expression, we also performed Western blot analyses. In agreement with the mRNA data, liver PEPCK protein level was increased in HC-saline but normalized in HC-resveratrol mice (Fig. 5B). To test whether these changes may have resulted in improved hepatic gluconeogenesis, we performed a pyruvate tolerance test. The pyruvate-induced increase in blood glucose levels in HC-resveratrol mice was between the normal (chow-fed untreated) and the pathological diabetic (HC-saline) states (Fig. 5C). Combined with the fact that liver glycogen contents were not different between HC-saline and HC-resveratrol mice (from fed mice after 5 wk of treatment, mean ± sem: HC-saline, 25.33 ± 2.50 mg glycogen/g liver; HC-resveratrol, 27.26 ± 2.66 mg glycogen/g liver; n = 7), these data would indicate that hepatic gluconeogenesis may have been improved by CNS resveratrol treatment. Increased lipid contents negatively affect insulin sensitivity (28,40,41). Thus, we explored whether improved glucose balance may have been due to reduced lipid accumulation in the liver. However, no differences in triglyceride content and mRNA level of several lipogenic genes in liver of HC-saline and HC-resveratrol mice were observed (data not shown). Collectively, our data suggest that the deleterious effects of HC diet on hepatic PEPCK expression (and perhaps gluconeogenesis) are ameliorated by CNS resveratrol administration.
Figure 5.
CNS resveratrol administration improves hepatic PEPCK expression and Pyruvate-induced hyperglycemia. Pepck and G6Pase mRNA level (determined by quantitative real-time PCR) (A) and PEPCK and β-actin (used as loading control) protein levels (determined by Western blot analysis) (B) were assessed in liver of HC-saline, HC-resveratrol, and chow-fed, age-matched, C57BL/6 male mice (n = 6–7 for each group). C, Blood glucose levels over time after a pyruvate tolerance test in a different cohort 5 wk after treatment (n = 7 for each group). Error bars, sem. Statistical analyses were done using one-way ANOVA (Tukey’s post test). *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. HC-saline mice. D, A proposed model of action through which brain-specific SIRT1 activators may suppress diet-induced brain inflammation leading to improved hyperglycemia and hyperinsulinemia.
Discussion
The high incidence of T2DM combined with the refractory responses to available medications urge for developing better antidiabetic therapies. Recent studies have shown that pharmacological stimulation of the nicotinamide adenine dinucleotide-dependent protein deacetylase SIRT1 by resveratrol or other activators improves many of the hallmark dysfunctions in T2DM (e.g. hyperglycemia, hyperinsulinemia, glucose intolerance, enhanced hepatic glucose production) (4,5,9). It is noteworthy that very similar metabolic improvements were seen when SIRT1 was mildly overexpressed by transgenesis in mouse models of T2DM (36). These findings are intriguing but somewhat counterintuitive given the known metabolic actions of SIRT1 in peripheral tissues. For example, after fasting, SIRT1 is induced in liver to trigger glucose production (42). Conversely, oligonucleotide-mediated suppression of SIRT1 expression in liver decreases hepatic glucose production (43). Moreover, SIRT1 antagonizes insulin actions on adipose tissue by stimulating free fatty acid secretion from adipocytes (44). Thus, direct action of resveratrol in these tissues seems unlikely to explain its antidiabetic effects. In contrast, SIRT1 activation enhances insulin sensitivity in skeletal muscle (45). Accordingly, diet-induced diabetic mice that were orally treated with resveratrol display enhanced muscle oxidative capacity, an effect that may account, at least in part, for the antidiabetic actions of resveratrol (5). However, it is not clear whether resveratrol-mediated myofiber remodeling overrides the expected glucose-rising effects of resveratrol action on the liver. Our results suggest a more complex mechanism pinpointing the brain as a key site for mediating resveratrol effects on glucose homeostasis. We speculate that resveratrol affects neuronal circuitries known to govern whole-body glucose metabolism [for example, hypothalamic neurons (12,19,20,23,25,26)] and that this central actions restrain hyperglycemia and hyperinsulinemia in diet-induced diabetic mice. Our data would also suggest that the liver may be involved, but future additional studies (e.g. hyperinsulinemic/euglycemic clamps analyses) are required to determine the tissues underlying the metabolic improvements elicited by CNS resveratrol perfusion.
Many brain sites are known to control glucose homeostasis, including neurons within the hypothalamus and the caudal brain stem (12,19,20,21,22,23,24,25,26,46). In our experiment, resveratrol was delivered to virtually the whole brain, thus rendering difficult to determine the anatomical regions required for mediating its effects. Some clues to address this point may come from the fact that genetically engineered mice lacking NF-κB signaling selectively in mediobasal hypothalamus are protected against the diet- induced glucose unbalance (37). In addition, mediobasal hypothalamic neurons mediate insulin effects on hepatic gluconeogenesis (12,13). Moreover, resveratrol does not penetrate the blood-brain barrier very efficiently (47), suggesting that if the brain is involved in mediating the glycemia-lowering effects of orally delivered resveratrol (as our data suggest), then circumventricular brain sites (as, for example, in some mediobasal hypothalamic areas) may be involved. Combined with the fact that CNS resveratrol delivery dampens diet-induced brain inflammation (Fig. 4) and improves hepatic PEPCK expression (Fig. 5, A and B), it is tempting to speculate that some mediobasal hypothalamic neurons may be, at least in part, the anatomical sites for mediating resveratrol effects on glucose homeostasis. Experiments in which resveratrol is delivered only to mediobasal hypothalamic nuclei of diabetic animal models are therefore warranted to address this issue.
In addition to SIRT1, resveratrol stimulates AMPK in peripheral tissues and CNS neurons (5,8). AMPK is a metabolic-sensor protein activated in conditions of reduced energy availability. In skeletal muscle, AMPK activation enhances oxidative metabolism, an effect through which the AMPK-activator metformin is thought to lower blood glucose levels (48,49). However, in the brain, activation of AMPK seems to exert opposite effects on body energy metabolism. Indeed, it has been reported that increased hypothalamic AMPK activity antagonizes the anorectic effects of leptin hence leading to hyperphagia and obesity (50). The fact that we did not observe any changes in food intake or body weight in mice that received CNS resveratrol delivery (Fig. 2 and supplemental Fig. 1) suggests that AMPK pathway was not activated. This is corroborated by the fact that phosphorylation statuses of AMPK and its target acetyl-coenzyme A carboxylase in brain of HC-resveratrol and HC-saline mice were not different (data not shown). However, because of the difficulties inherent in accessing AMPK activity in vivo, especially in a heterogeneous tissue such as the brain, we cannot rule out the possibility that CNS resveratrol delivery led to small changes in this pathway. It is also noteworthy that reduced hypothalamic NF-κB signaling is expected to enhance leptin sensitivity in diet-induced obese mice (37), an effect that should lead to changes in food intake and body weight. However, despite improved NF-κB signaling, HC-resveratrol mice did not display improved obesity (Fig. 2 and supplemental Fig. 1). This conundrum may be explained by the fact that our CNS delivery lasted only 5 wk, a period perhaps not sufficient to unmask effects on body weight. Alternatively, it may be possible that CNS resveratrol delivery activated AMPK and that this effect antagonized the expected improved leptin sensitivity via suppression of the NF-κB signaling. Our results do not rule out either possibility. In addition, other molecular targets may be involved in mediating central resveratrol effects. Future work in animals lacking SIRT1 in a neuron-specific manner is clearly needed to determine whether SIRT1 is required and what neuronal groups mediate central resveratrol effects on glucose/insulin balance.
In conclusion, we identified the brain as a key site for mediating resveratrol antidiabetic actions. We found that CNS resveratrol delivery improves diet-induced brain inflammation, a mechanism that we speculate contributes to the improved hyperglycemia and hyperinsulinemia. As proposed in Fig. 5D, we suggest that the development of brain-specific SIRT1 activators may exert similar effects and therefore represent a new strategy in the fight against T2DM.
Supplementary Material
Acknowledgments
We thank Wenhao Li for technical assistance, Dr. Joyce Repa for quantitative real-time PCR primers, and The University of Texas Southwestern (UTSW) Medical Center Mouse Metabolic Phenotyping Core.
Footnotes
This work was supported by American Heart Association Post-Doctoral Fellowship (to G.R.) and Scientist Development Grant (to R.C.), the National Institutes of Health Grants DK080836 (to R.C.) and DK53301, DK081185 (to J.K.E.), the American Diabetes Association Grant 1-07-RA-41 (to L.G. and J.K.E.), and the Smith Family Foundation Pinnacle Program Project Award (to J.K.E.). The University of Texas Southwestern (UTSW) Medical Center Mouse Metabolic Phenotyping Core is supported by National Institutes of Health Grant DK081182.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 9, 2009
Abbreviations: Agrp, Agouti-related peptide; AMPK, AMP-activated protein kinase; CNS, central nervous system; G6Pase, glucose-6-phosphatase; HC, high calorie; Iκ-Bα, inhibitor of nuclear factor-κB α; IKKβ, IκB kinase complex; NF-κB, nuclear factor-κB; Npy, neuropeptide Y; Pepck, phosphoenolpyruvate carboxykinase 1; Pomc, pro-opiomelanocortin; T2DM, type 2 diabetes mellitus.
References
- O'Rahilly S, Barroso I, Wareham NJ 2005 Genetic factors in type 2 diabetes: the end of the beginning? Science 307:370–373 [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M 2001 Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344:1343–1350 [DOI] [PubMed] [Google Scholar]
- Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN 1988 Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37:1163–1167 [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA 2006 Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J 2006 Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127:1109–1122 [DOI] [PubMed] [Google Scholar]
- Michan S, Sinclair D 2007 Sirtuins in mammals: insights into their biological function. Biochem J 404:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA 2003 Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425:191–196 [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J 2007 Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA 104:7217–7222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH 2007 Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450:712–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J 2008 Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 8:347–358 [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW 2006 Central nervous system control of food intake and body weight. Nature 443:289–295 [DOI] [PubMed] [Google Scholar]
- Obici S, Zhang BB, Karkanias G, Rossetti L 2002 Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8:1376–1382 [DOI] [PubMed] [Google Scholar]
- Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Brüning JC 2007 Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 5:438–449 [DOI] [PubMed] [Google Scholar]
- Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC Jr, Lowell BB, Elmquist JK 2005 The hypothalamic arcuate nucleus: a key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metab 1:63–72 [DOI] [PubMed] [Google Scholar]
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB 2007 Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449:228–232 [DOI] [PubMed] [Google Scholar]
- Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW 2005 Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab 2:411–420 [DOI] [PubMed] [Google Scholar]
- Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M, Ashcroft F, Minokoshi Y, Roeper J, Seino S 2001 ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci 4:507–512 [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Marcus JN 2003 Rethinking the central causes of diabetes. Nat Med 9:645–647 [DOI] [PubMed] [Google Scholar]
- Pocai A, Obici S, Schwartz GJ, Rossetti L 2005 A brain-liver circuit regulates glucose homeostasis. Cell Metab 1:53–61 [DOI] [PubMed] [Google Scholar]
- Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L 2005 Hypothalamic K(ATP) channels control hepatic glucose production. Nature 434:1026–1031 [DOI] [PubMed] [Google Scholar]
- Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L 2001 Central melanocortin receptors regulate insulin action. J Clin Invest 108:1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L 2002 Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51:271–275 [DOI] [PubMed] [Google Scholar]
- Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L 2002 Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci 5:566–572 [DOI] [PubMed] [Google Scholar]
- Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L 2005 Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes 54:3182–3189 [DOI] [PubMed] [Google Scholar]
- Pocai A, Lam TK, Obici S, Gutierrez-Juarez R, Muse ED, Arduini A, Rossetti L 2006 Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J Clin Invest 116:1081–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TK, Gutierrez-Juarez R, Pocai A, Rossetti L 2005 Regulation of blood glucose by hypothalamic pyruvate metabolism. Science 309:943–947 [DOI] [PubMed] [Google Scholar]
- Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, Anderson J, Elmquist JK, Coppari R 2008 Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci 28:9989–9996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna CR, Huntgeburth M, Coppari R, Choi CS, Lin J, Krauss S, Barbatelli G, Tzameli I, Kim YB, Cinti S, Shulman GI, Spiegelman BM, Lowell BB 2006 Hypomorphic mutation of PGC-1β causes mitochondrial dysfunction and liver insulin resistance. Cell Metab 4:453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH 2008 Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA 105:9793–9798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W 2001 Negative control of p53 by Sir2α promotes cell survival under stress. Cell 107:137–148 [DOI] [PubMed] [Google Scholar]
- Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA 2001 hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107:149–159 [DOI] [PubMed] [Google Scholar]
- Langley E, Pearson M, Faretta M, Bauer UM, Frye RA, Minucci S, Pelicci PG, Kouzarides T 2002 Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J 21:2383–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Chen J, Lou Z 2008 DBC1 is a negative regulator of SIRT1. Nature 451:583–586 [DOI] [PubMed] [Google Scholar]
- Kim EJ, Kho JH, Kang MR, Um SJ 2007 Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell 28:277–290 [DOI] [PubMed] [Google Scholar]
- Zhao W, Kruse JP, Tang Y, Jung SY, Qin J, Gu W 2008 Negative regulation of the deacetylase SIRT1 by DBC1. Nature 451:587–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks AS, Kon N, Knight C, Matsumoto M, Gutiérrez-Juárez R, Rossetti L, Gu W, Accili D 2008 SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab 8:333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D 2008 Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell 135:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW 2004 Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23:2369–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden M, Bernroider E 2003 Hepatic glucose metabolism in humans–its role in health and disease. Best Pract Res Clin Endocrinol Metab 17:365–383 [DOI] [PubMed] [Google Scholar]
- Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI 2003 Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300:1140–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI 2004 Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P 2005 Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434:113–118 [DOI] [PubMed] [Google Scholar]
- Erion DM, Yonemitsu S, Nie Y, Nagai Y, Gillum MP, Hsiao JJ, Iwasaki T, Stark R, Weismann D, Yu XX, Murray SF, Bhanot S, Monia BP, Horvath TL, Gao Q, Samuel VT, Shulman GI 2009 SirT1 knockdown in liver decreases basal hepatic glucose production and increases hepatic insulin responsiveness in diabetic rats. Proc Natl Acad Sci USA 106:11288–11293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L 2004 Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature 429:771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q 2007 SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab 6:307–319 [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT, Li AJ 2006 Hindbrain catecholamine neurons control multiple glucoregulatory responses. Physiol Behav 89:490–500 [DOI] [PubMed] [Google Scholar]
- Abd El-Mohsen M, Bayele H, Kuhnle G, Gibson G, Debnam E, Kaila Srai S, Rice-Evans C, Spencer JP 2006 Distribution of [3H]trans-resveratrol in rat tissues following oral administration. Br J Nutr 96:62–70 [DOI] [PubMed] [Google Scholar]
- Chen ZP, Stephens TJ, Murthy S, Canny BJ, Hargreaves M, Witters LA, Kemp BE, McConell GK 2003 Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes 52:2205–2212 [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC 2005 The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310:1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferré P, Birnbaum MJ, Stuck BJ, Kahn BB 2004 AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428:569–574 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.