Abstract
Estradiol (E2) rapidly and strongly induces vascular endothelial growth factor (VEGF) transcription in uterine endometrial epithelial cells in vivo. We have shown that this is mediated by both the estrogen receptor-α and hypoxia-inducible factor (HIF)-1α. By contrast, E2 induces little or no VEGF expression in cultured breast or endometrial cancer cells, which lack HIF-1α due to the abnormally high concentration of oxygen (∼20%) to which they are exposed. To test the hypothesis that restoring HIF-1α in cultured cells would restore the ability of E2 to induce VEGF expression, we treated human endometrial cancer cells (ECC-1) with cobalt chloride (CoCl2;100 μm), which prevents oxygen-induced HIF-1α degradation. HIF-1α was absent in untreated ECC-1 cells but detectable by 4 h after treatment with CoCl2 alone, as was a significant increase in VEGF mRNA. E2 plus CoCl2 induced detectable HIF-1α expression at 2 h and an even higher level than that induced by CoCl2 alone at 4 h; this HIF-1α was localized in the nuclei. This was accompanied by increasing VEGF expression, with the increase at 4 h severalfold higher than that induced by CoCl2 alone and was concurrent with recruitment of both HIF-1α and estrogen receptor-α to the VEGF promoter. These results confirm that HIF-1α plays an essential role in E2-induced expression of VEGF. Through the induction of increased microvascular permeability and the consequent exudation of plasma growth factors, VEGF in turn may play an essential role in cancer cell proliferation in vivo.
Prevention of oxygen-induced degradation of the transcription factor HIF-1α in cultured endometrial cancer cells by treatment with CoCl2 restores strong 17β-estradiol induction of VEGF gene expression, as occurs in normal endometrial epithelial cells in vivo.
Vascular endothelial growth factor (VEGF) is a potent inducer of increased microvascular permeability and angiogenesis (1,2), processes that are essential for the normal development of the uterine endometrium during the reproductive cycle (3). The ovarian steroid hormone 17β-estradiol (E2), the primary stimulus of cyclic endometrial growth, rapidly induces VEGF expression in the uterus in vivo (4,5,6,7). This induction of VEGF is not blocked by protein synthesis inhibitors (4,5). Thus, E2-induced VEGF expression is a primary/immediate early gene response, indicating that VEGF plays an essential role in E2’s subsequent effects on the uterus. We have recently proposed (8) that the rapid induction of VEGF expression, which occurs in the luminal epithelial cells, is a key event in E2-induced growth of the endometrium as well as of other E2 target tissues. In this new model, rather than VEGF being only a secondary mediator of E2-induced growth through its induction of angiogenesis, it instead acts as an immediate mediator of E2-induced epithelial cell proliferation, an estromedin, through its rapid effects on stromal microvascular permeability, which bathes epithelial cells in plasma growth factors and other proteins essential for proliferation. We have also shown that E2 induction of VEGF expression in the uterus requires the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, which leads to the simultaneous recruitment of both hypoxia-inducible factor 1 (HIF-1) to the hypoxia response element (HRE) and estrogen receptor (ER)-α to proximal Sp1 binding sites on the VEGF gene promoter (6,7). When that pathway is blocked, HIF-1 is not recruited and VEGF expression does not occur (7). This represents the first demonstration of a specific role for HIF-1, which is increasingly recognized as playing broad and critical roles in normal development, postnatal physiology, and cancer and many other diseases (9,10,11), in a biological action of E2. Given that E2 and its receptor, the PI3K/Akt pathway, HIF-1, and VEGF have all individually been linked to cancer, the demonstration that they are in fact directly linked to each other has important implications for our understanding of how E2 promotes cancer.
Recognition of the centrality of VEGF in E2’s effects, both physiological and pathological, has been hampered, however, by the fact that cultured, ERα-positive cancer cells, the most commonly used model for the study of E2 action, show only weak or no VEGF expression in response to E2 in most studies (12,13,14,15,16,17,18,19,20). This clearly contrasts with the rapid, robust induction of VEGF by E2 in the uterus and with its strong induction by hypoxia in cultured cells. E2 also strongly induces VEGF in rat mammary tumors in vivo (21). The failure of most studies to detect significant induction of VEGF expression by E2 in cultured breast or endometrial cancer cells is matched by several recent expression profiling studies, which also reported either weak or no VEGF induction by E2 (22,23,24,25,26).
The absence of a VEGF response to E2 in vitro does not fit with the robust E2 induction of VEGF expression in vivo, the strong induction of VEGF by hypoxia in vitro, or what one would predict for E2-responsive cancer cells, given the well-established role of VEGF in tumor growth. This led us to examine the nature of this difference between normal epithelial cells and cancer cells. Based on our findings in the normal uterus, we hypothesized that the reason for the failure of E2 to strongly induce VEGF expression in cultured cells was the absence of the labile α-subunit of HIF-1. Culturing cells under standard conditions (i.e. in 95% air-5% CO2) exposes them to 20% oxygen, a supraphysiological concentration (normal tissue levels are on the order of 3–5%) that reduces HIF-1α to undetectable levels (6,27). It seems to be widely overlooked that HIF-1α is in fact normally present at low levels in cells in vivo (28,29), including the uterus (6). Furthermore, an elevated level of HIF-1α is characteristic of breast and endometrial tumor cells in vivo (30,31). It is highly likely therefore that the absence of HIF-1 in cultured cells seriously compromises gene expression in response to E2 and other hormones. This may in part explain the minimal overlap, just 11%, recently observed between genes induced by E2 in human breast tumor xenografts in vivo and the identical cells in culture (22). We undertook studies therefore to determine whether restoring HIF-1α would restore strong E2-induced VEGF expression in cultured cancer cells. Because our previous studies of E2-induced VEGF expression were carried out using the rodent uterus and isolated endometrial luminal epithelial cells (6,7,8), we used ECC-1 cells, a human endometrial carcinoma cell line, for these studies.
Materials and Methods
Cells and treatments
ECC-1 human endometrial cancer cells were generously provided by Dr. George Olt (Pennsylvania State College of Medicine, Milton S. Hershey Medical Center, Hershey, PA). Cells were plated in 10-cm dishes and grown in DMEM/F-12 medium (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (SH30088.03; Hyclone Laboratories, Logan, UT) and penicillin-streptomycin (100 U per 100 μg/ml; Invitrogen, Carlsbad, CA) in an atmosphere of 95% air-5% CO2, resulting in an oxygen concentration of approximately 20%. When the cells reached approximately 95% confluence, the growth medium was replaced with phenol-red-free DMEM/F-12 medium (Mediatech) with 10% charcoal-stripped fetal bovine serum (SH30068.03; Hyclone Laboratories) and penicillin-streptomycin for 24 h before treatment. MCF-7 and ZR-75 cells were obtained from Dr. Angela Brodie (Department of Pharmacology and Experimental Therapeutics, University of Maryland School of Medicine, Baltimore, MD) and American Type Culture Collection (Manassas, VA), respectively, and cultured as described previously (6).
Cobalt chloride crystals (Sigma, St. Louis, MO) were dissolved in molecular grade water and sterile filtered. Cells were left untreated (0 h) or treated with 1, 10, or 100 μm CoCl2 or vehicle for 2–72 h. Cells were also treated with 10 or 100 nm 17β-estradiol (Sigma) or vehicle (1% EtOH in phenol red-free medium, as above) for 2 or 4 h.
Western blot analysis
Cells were rinsed twice in cold PBS and collected in radioimmunoprecipitation buffer (8) by scraping the plates with a plastic scraper. The lysate was transferred to a 1.5-ml microcentrifuge tube, rotated on an orbital mixer at 4 C for 15 min, and centrifuged at 16,000 × g at 4 C for 15 min. The supernatant was collected and stored at −80 C. Protein concentration was determined using the BCA protein assay kit (Pierce, Rockford, IL). Equal amounts of protein (10–40 μg) were loaded and transferred to membranes for each experiment. Western blot analysis was done as described previously (8) with the following primary antibodies: mouse monoclonal antibody to HIF-1α (1:250, no. 610958; BD Biosciences, Palo Alto, CA); mouse monoclonal antibody to HIF-1β (1:1000, no. 611079; BD Biosciences); or rabbit polyclonal antibody to Akt (1:1000, no. 9272; Cell Signaling, Danvers, MA) and the appropriate secondary antibodies, described previously (8).
Nuclear and cytoplasmic extracts
Cells were rinsed and gently scraped from the plates in cold PBS containing protease and phosphatase inhibitors, as above, and were then centrifuged at 200 × g for 5 min. Nuclear and cytoplasmic extracts were obtained using the Nuclear Complex Co-IP kit (Active Motif, Carlsbad, CA) per the manufacturer’s directions; all of the reagents listed below were supplied in the kit. To isolate the nuclei, the cell pellet was resuspended in 1× hypotonic buffer and incubated on ice for 15 min. Detergent was then added and the samples gently pipetted up and down five times. The nuclei were pelleted by centrifugation for 30 sec at 16,000 × g at 4 C. The supernatant (cytoplasmic fraction) was collected and aliquots stored at −80 C. The nuclear pellet was resuspended in complete digestion buffer. Enzymatic shearing cocktail was then added and incubated at 4 C for 90 min. EDTA was added to stop the reaction and the digest was centrifuged for 10 min at 16,000 × g at 4 C. This nuclear fraction supernatant was aliquoted and stored at −80 C. All fractions were processed for Western blots, as above, and probed for HIF-1α, HIF-1β, and Akt (as above) or with rabbit polyclonal antibodies to either ERα (1:300, no. sc-542; Santa Cruz Biotechnology, Santa Cruz, CA) or phospho-ERαSer167 (1:250, no. sc-101676; Santa Cruz Biotechnology) with appropriate secondary antibodies (8).
RNA extraction and reverse transcription
Cells were rinsed with cold PBS and lysed with cold RLT buffer (QIAGEN, Valencia, CA) plus 1% β-mercaptoethanol (Sigma). The plates were then scraped with a plastic scraper and the lysate transferred to QiaShredder tubes (QIAGEN) for homogenization. The homogenates were purified, concentration was determined, and samples were diluted and reverse transcribed, as described previously (6).
PCR
The levels of mRNAs were measured by real-time RT-PCR using a DNA Opticon system (MJ Research, Boston, MA), as described previously (6,7). The following primers were used: human HIF-1α +1555 to +1705, forward, 5′-TTCACCTGAGCCTAATAGTCC-3′ and reverse 5′-CAAGTCTAAATCTGTGTCCTG-3′ (GenBank accession no. U22431.1); human VEGF +25 to +358, forward, 5′-CTGCTGTCTTGGGTGCATTGG-3′ and reverse, 5′-GTTTGATCCGCATAATCTGCAT-3′ (GenBank accession no. NM003376); human progesterone receptor (PR) +2342 to +2596, forward, 5′-AGCCCACAATACAGCTTCGAG-3′ and reverse, 5′-TTTCGACCTCCAAGGACCAT-3′ (GenBank accession no. X51730); human pS2/trefoil factor 1 (TFF1) +371 to +507, forward, 5′-ACTTCTGCAGGGATCTGCC-3′ and reverse, 5′-CAATCTGTGTTGTGAGCCGA-3′ (GenBank accession no. X05322); human adrenomedullin +250 to +660, forward, 5′-AAGAAGTGGAATAAGTGGGCT-3′ and reverse, 5′-TGGCTTAGAAGACACCAGAGT-3′ (GenBank accession no. NM001124.1); and 18S rRNA, described previously (6,7,8).
Chromatin immunoprecipitation (ChIP)
Cells were grown on 15-cm plates to 95% confluence and treated with E2 or vehicle for 4 h in the absence or presence of CoCl2, as above. Cells were washed in PBS and fixed in 1% formaldehyde for 10 min at 37 C. Fixed cells were quickly rinsed with ice-cold PBS and collected by scraping in 1 ml of PBS plus protease inhibitors (Roche, Mannheim, Germany). Cells were pelleted by centrifuging for 5 min at 16,000 × g at 4 C, resuspended in lysis buffer (6), incubated on ice for 10 min, and sonicated as described previously (6,7).
Immunoprecipitation for ERα was carried out using Dynabeads protein G magnetic beads (Invitrogen). The beads were washed three times using PBS + BSA (5 mg/ml) per the manufacturer’s instructions. For each sample, 1 μg of mouse monoclonal human ERα antibody (Ab-10; NeoMarkers/Lab Vision, Fremont, CA) in 5 μl of buffer was added to 50 μl of the bead suspension and incubated overnight at 4 C for antibody binding. The chromatin was diluted 1:10 in dilution buffer (6) and incubated overnight with the antibody-magnetic bead complexes. The bead-antibody-chromatin complexes were washed six times in radioimmunoprecipitation buffer [50 mm HEPES (pH 7.6), 1 mm EDTA, 0.7% Na deoxycholate, 1% Nonidet P-40, 0.5 m LiCl, protease inhibitor tablets (Roche)] and twice in Tris/EDTA buffer (pH 7.6), eluted for 30 min at room temperature, and reverse cross-linked overnight at 65 C. Finally, the samples were purified using the QIAquick PCR purification kit (QIAGEN). Immunoprecipitation for HIF-1α was carried out as described previously (6,7) with agarose protein G beads (16-266; Upstate, Billerica, MA). For each sample, 5 μg of rabbit polyclonal human HIF-1α antibody (sc-10790 X; Santa Cruz) were used.
The yield of target region DNA in each sample after ChIP was analyzed by both conventional and real-time PCR, as described previously (6,7). The following primers were used for PCR analysis: human VEGF promoter containing Sp1 sites: −222 to +139, forward, 5′-TCAGGCTGTGAACCTTGG-3′ and reverse, 5′-TATCAAATTCCAGCACCGAG-3′; human VEGF promoter containing HRE, −1216 to −883, forward, 5′-TTGGGCTGATAGAAGCCTTG-3′ and reverse, 5′-TGGCACCAAGTTTGTGGAGC-3′.
Statistical analysis
Statistical analyses were done using factorial ANOVA and appropriate post hoc tests (StatView, version 4.5; Abacus Concepts, Berkeley, CA).
Results
E2 does not stimulate VEGF expression in ECC-1 cells cultured in 95% air (20% oxygen)
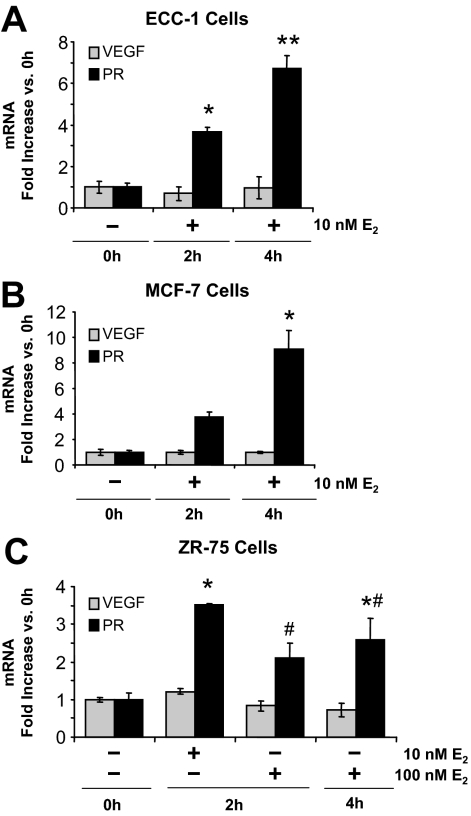
As shown in Fig. 1A, E2 has no effect on VEGF expression in ECC-1 cancer cells cultured under standard conditions (95% air), either at 2 or 4 h. We likewise observed no response from either MCF-7 or ZR-75 human breast cancer cells (Fig. 1, B and C). All of these cells, however, showed the expected induction of the PR gene by E2. This result contrasts with the rapid, strong induction of VEGF expression by E2 in the normal uterus (4,6) and rat mammary tumors in vivo (21).
Figure 1.
VEGF and PR mRNA expression in cancer cell lines grown in 95% air-5% CO2. ECC-1, MCF-7, and ZR-75 cells were treated with vehicle (0 h) or 10 or 100 nm E2 for 2 or 4 h, as indicated. VEGF and PR mRNA expression were measured by real-time RT-PCR, as described in Materials and Methods. A, ECC-1 cells (mean ± sem, n = 6 replicate cultures/group; *, P < 0.01, **, P < 0.0001 vs. all other groups). B, MCF-7 cells (mean ± sem, n = 5 replicate cultures/group; *, P < 0.001 vs. all other groups). C, ZR-75 cells (mean ± sem, n = 5 replicate cultures/group; *, P < 0.05, #, P < 0.05 vs. all other groups).
CoCl2 restores HIF-1α in ECC-1 cells
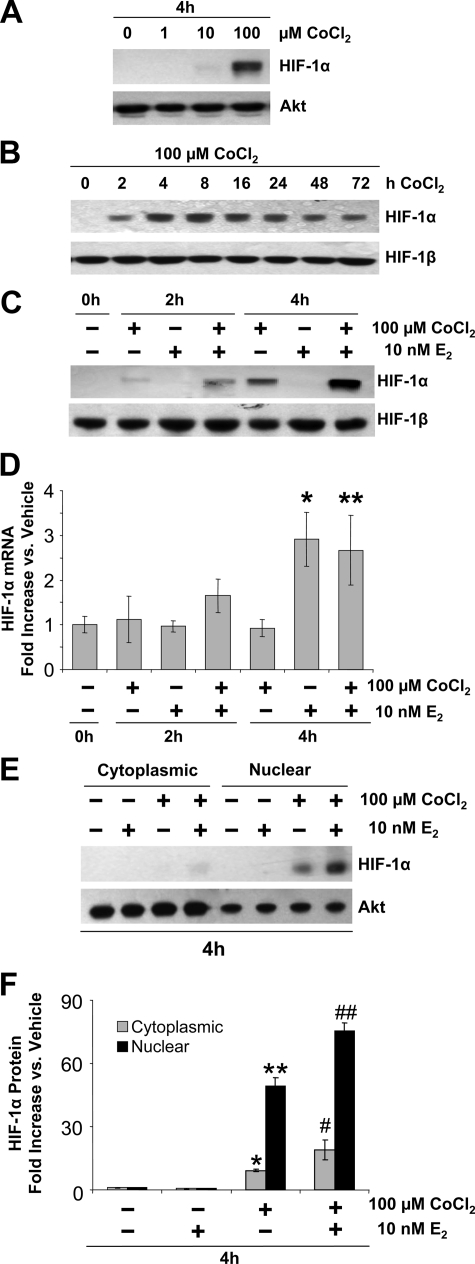
We hypothesized that the failure of E2 to induce VEGF expression in ECC-1 and other human cancer cell lines is due to an absence of HIF-1α when cells are cultured in 95% air (20% oxygen). As shown in Fig. 2, A–C, ECC-1 cells cultured in 95% air have no detectable HIF-1α. We then determined whether the addition of CoCl2, which inhibits HIF-1α degradation (32), would restore HIF-1α to the cells. As shown in Fig. 2, A and B, 1 μm CoCl2 had no effect, but detectable HIF-1α was induced by 10 μm CoCl2, and 100 μm CoCl2 strongly induced HIF-1α by 4–8 h; HIF-1α levels remained relatively steady through 48 h and had declined slightly by 72 h. This result indicates that even though HIF-1α protein is undetectable in ECC-1 cells in 95% air, the HIF-1α gene is constitutively expressed, as it is in normal cells in vivo. When the excessive rate of degradation induced by 20% oxygen is reduced by CoCl2, therefore, HIF-1α protein rapidly accumulates. In all subsequent experiments, 100 μm CoCl2 was used.
Figure 2.
Effect of CoCl2 and E2 on HIF-1α levels in ECC-1 cells. A, Dose response to CoCl2 on HIF-1α protein. Cells were treated with 0 (vehicle), 1, 10, or 100 μm CoCl2 for 4 h, and HIF-1α and total Akt protein levels were examined by Western blot analysis (30 μg protein/lane). B, Effect of CoCl2 over time on HIF-1α protein. Cells were treated with 100 μm CoCl2 for 0–72 h, and HIF-1α and HIF-1β protein levels were examined by Western blot analysis (30 μg of protein/lane). C, Effect of E2 plus CoCl2. Cells were treated with vehicle, 10 nm E2, 100 μm CoCl2, or the combination of the two for 2 or 4 h, and HIF-1α and HIF-1β protein levels were examined by Western blot analysis (40 μg protein/lane). D, Cells were treated as described in C and HIF-1α and 18S mRNA levels measured by RT-PCR. Real-time RT-PCR results (mean ± sem, n = 6 replicate cultures/group; *, P < 0.001 vs. vehicle, 2 h CoCl2, 2 h E2, 2 h CoCl2 + E2 and 4 h CoCl2; **, P < 0.01 vs. vehicle, 2 h CoCl2, 2 h E2, and 4 h CoCl2). E and F, Cytoplasmic and nuclear localization of HIF-1α protein induced by CoCl2, E2, or CoCl2 plus E2 (concentrations as in C) for 4 h. HIF-1α and Akt protein levels were examined in nuclear and cytoplasmic fractions by Western blot analysis. E, Representative gels (10 μg protein/lane). F, Quantification of HIF-1α protein by densitometry after normalization to Akt (fold increase compared with vehicle; means ± sem, n = 5 replicate cultures/group; *, P < 0.01, **, P < 0.0001, #, P < 0.05, ##, P < 0.0001 vs. all other groups).
Inhibition of HIF-1α degradation by CoCl2 reveals E2 stimulation of HIF-1α expression
As seen in Fig. 2C, when ECC-1 cells were treated with both 100 μm CoCl2 and 10 nm E2, the level of HIF-1α protein increased above that induced by CoCl2 alone. This result is consistent with previously reported results in the uterus (6), in which HIF-1α was already present before E2 treatment but had increased 2.5-fold by 2 h after E2 injection. Thus, the ability of E2 to stimulate HIF-1α expression is unmasked when the abnormally high rate of HIF-1α degradation caused by 20% oxygen is reduced by CoCl2.
In the uterus, the E2 induction of HIF-1α protein is matched by an increase in HIF-1α mRNA, indicating that E2 induces either increased transcription of the HIF-1α gene or an increase in HIF-1α mRNA stability. As shown in Fig. 2D, E2 also increased the level of HIF-1α mRNA in ECC-1 cells. CoCl2 by contrast had no effect on the level of HIF-1α mRNA, either alone or in the presence of E2, consistent with the fact that it acts by inhibiting HIF-1α protein degradation.
The HIF-1α induced by CoCl2 and E2 is localized in the nucleus
To determine the subcellular location of HIF-1α protein in ECC-1 cells after CoCl2 and E2 treatment, nuclear and cytoplasmic protein extracts were prepared. As shown in Fig. 2, E and F, nearly all of the HIF-1α induced in response to either CoCl2 alone or CoCl2 plus E2 was localized within the nucleus.
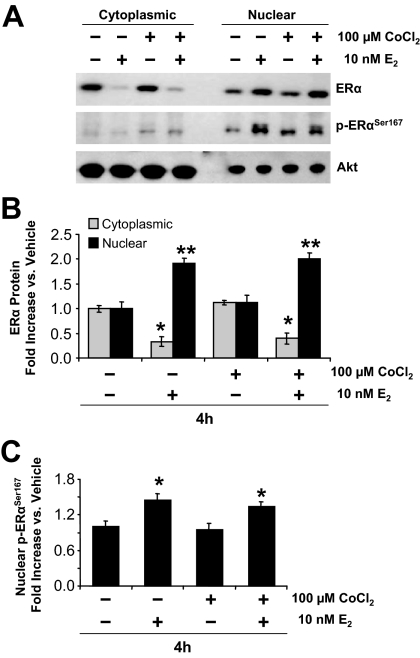
In contrast to HIF-1α, ERα was initially localized in both the cytoplasmic and nuclear compartments and E2 induced a major shift from cytoplasm to nucleus (Fig. 3, A and B). E2 also induced a significant increase in the level of ERα phosphorylated on serine167, an event associated with increased ERα binding to DNA and transcriptional activity (Fig. 3C).
Figure 3.
Effect of CoCl2 and E2 on ERα expression, subcellular localization, and phosphorylation in ECC-1 cells. A, Cytoplasmic and nuclear localization of total ERα, p (phospho)-ERαSer167, and Akt, determined by Western blot analysis (10 μg protein/lane). B, Quantification of total ERα by densitometry after normalization to Akt (mean ± sem, n = 5 replicate cultures/group; *, P < 0.0001, **, P < 0.001 vs. all other groups). C, Quantification of p-ERα Ser167 by densitometry after normalization to Akt (mean ± se, n = 5 replicate cultures/group; *, P < 0.01 vs. all other groups).
The presence of HIF-1α unmasks E2-induced VEGF expression
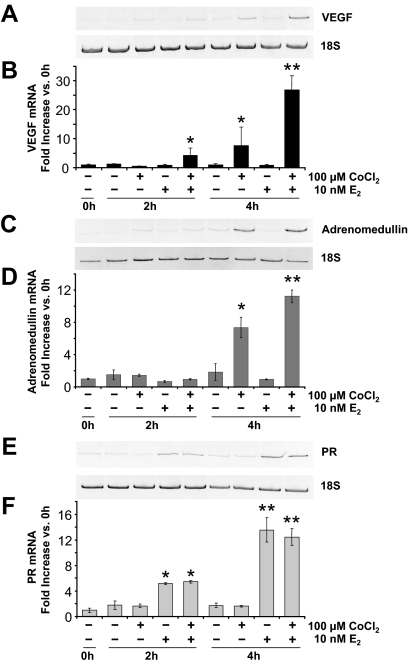
We next determined whether E2 would induce VEGF expression in ECC-1 cells if HIF-1α was present. As shown in Fig. 4, A and B, treatment with E2 alone produced no increase in VEGF mRNA above the very low level present in vehicle-treated cells at either 2 or 4 h. Treatment with CoCl2 alone had no detectable effect on VEGF expression at 2 h but did induce a significant increase by 4 h. This indicates that when the level of HIF-1α increases sufficiently, HIF-1 alone will drive increased VEGF expression. Cotreatment with E2, however, induced a significant increase in VEGF mRNA at 2 h, and an even greater increase, and one larger than that induced by CoCl2 alone at 4 h. This pattern exactly parallels that of HIF-1α protein expression (Fig. 2C), indicating that HIF-1α is an essential mediator of E2 induction of VEGF gene expression.
Figure 4.
Effect of CoCl2 and E2 on VEGF, adrenomedullin, and PR mRNA expression. Cells were treated as described in Fig. 2C and VEGF, adrenomedullin, PR, and 18S mRNA levels measured by RT-PCR. A and B, VEGF mRNA expression. A, Conventional RT-PCR representative gels. B, Real-time RT-PCR results (mean ± sem, n = 3 replicate cultures/group; *, P < 0.05, **, P < 0.0001 vs. all other groups). C and D, Adrenomedullin mRNA expression. C, Conventional RT-PCR representative gels. D, Real-time RT-PCR results (mean ± sem, n = 3 replicate cultures/group; *, P < 0.001, **, P < 0.0001 vs. all other groups). E and F, PR mRNA expression. E, Conventional RT-PCR representative gels. F, Real-time RT-PCR results (mean ± sem, n = 3 replicate cultures/group; *, P < 0.01, **, P < 0.0001 vs. all other groups).
Adrenomedullin is another gene induced by E2 in the uterus (33,34,35) and one that is also known to be controlled by HIF-1 (36). As shown in Fig. 4, C and D, adrenomedullin mRNA levels were induced about 7-fold by CoCl2 alone but about 11-fold (P < 0.0005 vs. CoCl2 alone) by the combination of HIF-1 and E2 at 4 h. E2 alone had no effect at all at 4 h. This confirms that HIF-1’s role in E2 action is not limited to the induction of VEGF but extends to other genes with HRE-containing promoters.
To determine whether the expression of other E2-induced genes would be affected by CoCl2, or the resulting increase in HIF-1α, expression of the PR and trefoil factor 1 (TFF1, also known as pS2) genes were examined. Because the expression of these genes is induced by E2 in cultured cancer cells and is not known to require HIF-1, one would predict that their induction by E2 would be unaffected by CoCl2. As expected, CoCl2 alone had no effect on basal PR mRNA levels and did not alter the E2 induction of PR expression at either 2 or 4 h (Fig. 4, E and F). Similar results were obtained for TFF1 mRNA expression (data not shown). These results are consistent with the ability of E2 to induce PR expression in breast and endometrial cancer cells in vitro in the absence of HIF-1 (Fig. 1) and indicate that neither CoCl2 nor HIF-1α interferes with the E2-induced expression of non-HIF-1 dependent genes.
E2 induces ERα recruitment to the VEGF gene promoter only when HIF-1α is present
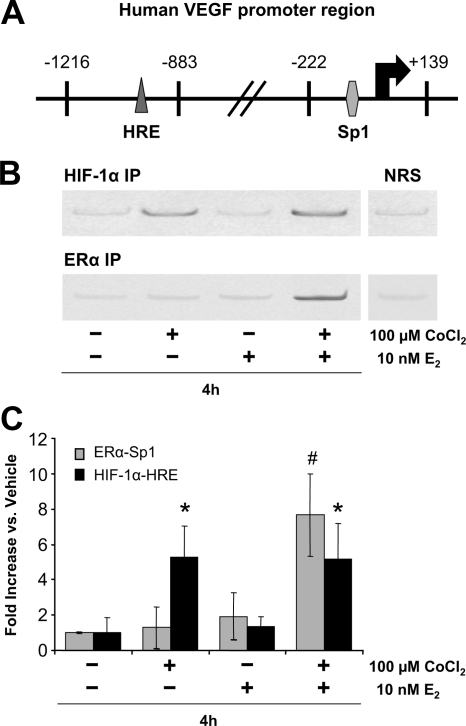
We next performed ChIP analysis of ERα and HIF-1α binding to the VEGF promoter in ECC-1 cells. We have previously shown that ERα binds to the VEGF promoter on proximal Sp1 sites (6). Interestingly, E2 induced recruitment of ERα to that region only when CoCl2 was also present (Fig. 5, B and C). This suggests that HIF-1α must be present for ERα to bind and that a priming or cooperative mechanism may be involved.
Figure 5.
Effect of CoCl2 and E2 on recruitment of HIF-1α and ERα to the VEGF gene promoter. ChIPs were carried out using normal rabbit serum (NRS) or specific antibodies for ERα or HIF-1α (see Materials and Methods). A, Primers for the −1216 to −883 region of the human VEGF promoter, which contains the HRE site to which HIF-1α binds, and the −222 to +139 region of the human VEGF promoter, which contains the Sp1 sites to which ERα binds, were used for PCR. B, Representative conventional PCR gels. C, Real-time PCR results (mean ± sem, n = 6 replicate cultures/group; *, P < 0.05, #, P < 0.01 vs. other treatment groups). IP, Immunoprecipitation.
CoCl2 alone is sufficient to induce HIF-1α binding to the VEGF promoter
As shown in Fig. 5, B and C, HIF-1α binding to the HRE of the VEGF promoter was induced to a similar degree by CoCl2 alone and CoCl2 plus E2. Because VEGF expression was much greater with CoCl2 plus E2 compared with CoCl2 alone (Fig. 4), we conclude that maximal expression of VEGF expression in ECC-1 cells is dependent on the recruitment of both ERα and HIF-1 to the VEGF promoter.
Discussion
These results reaffirm that cells grown in high oxygen completely lack detectable HIF-1α and express no VEGF in response to E2. Furthermore, they show that restoring HIF-1α restores the ability of E2 to strongly induce VEGF expression, as it does in the uterus in vivo (6,7). This further confirms that HIF-1α plays a critical role in E2-induced VEGF gene expression. They also suggest that HIF-1α may play a more extensive role in E2 action. The absence of HIF-1α correlated with an absence of E2-induced binding of ERα to the VEGF promoter. This is also consistent with our previous in vivo findings, which showed that blocking HIF-1α recruitment to the VEGF promoter, by blocking the upstream PI3K/Akt pathway, also blocked ERα recruitment (7). This suggests that HIF-1 may define a subset of E2-responsive genes, most likely ones containing an HRE, that become accessible to ligand-activated ERα only on HIF-1 binding, similar to the role proposed for Forkhead box-A1 in E2 action (37,38). HIF-1α is a master controller of gene transcription, regulating more than 100 genes involved in cell survival, cell proliferation, angiogenesis, glycolysis, erythropoiesis, and a wide range of other vital processes (39,40). Many of these same genes are also known to be regulated by E2 in the uterus. These include adrenomedullin (33,34,35), erythropoietin (41), inducible nitric oxide synthase (42), glucose transporter-1 (43), and several glycolytic enzymes (44). Many of the same genes required for cell survival and adaptation during hypoxic stress are apparently also required to handle the metabolic stress imposed by the rapid cell growth and tissue remodeling triggered by E2 in the endometrium. As with VEGF, E2 only induced the expression of adrenomedullin, the promoter of which contains an HRE and binds HIF-1 (45), when HIF-1α was present (Fig. 4). Interestingly, it has been reported that E2 has no effect on adrenomedullin expression in ovarian cancer cells in vitro (46), even though its expression in ovarian tumors correlates with ERα expression (47). Our results suggest that, as with VEGF, the difference is due to an absence of HIF-1α. We predict that HIF-1-dependent E2 induction of VEGF expression will be found to be the archetype for that of many other genes as well.
These results also confirm our in vivo observation that in addition to transcriptionally activating HIF-1α, E2 subsequently induces its expression (6,8). This only became apparent in vitro when the abnormally high rate of HIF-1α degradation induced by 20% oxygen was reduced by CoCl2 to a level below the rate of E2-stimulated HIF-1α synthesis. In the uterus, this increase follows the E2-induced increase in VEGF expression, showing that the low basal level of HIF-1α normally present in epithelial cells is sufficient for that increase (6,8). This is consistent with our earlier observation that the E2 induction of VEGF in the uterus does not require new protein synthesis (4). The subsequent increase in the level of HIF-1α suggests that it plays roles in E2 effects beyond the rapid stimulation of VEGF expression, which is not surprising, given the large number of other genes known to be regulated by HIF-1 and its central role in cell metabolism. The stimulation of HIF-1α synthesis by E2 also suggests that it, along with hypoxia, may contribute to the high levels of HIF-1α found in tumors of the endometrium and breast (30,31). It might be the major driving force in less hypoxic regions of tumors.
There is mounting evidence that VEGF plays an essential role in the normal effects of E2 on its major target tissues (3,48,49). It is important therefore to know whether its production is regulated by E2 in cancer cells whose growth is E2 dependent. Whereas it is well established that E2 rapidly induces robust increases in VEGF mRNA in the uterus in vivo (4,5), it does not have a comparable effect on cultured breast or endometrial cancer cells. Specifically, E2 has been reported to minimally up-regulate (2-fold or less) (13,16), inhibit (17), or have no effect at all (12,14,15,20) on VEGF expression in vitro. Studies using cells transfected with VEGF promoter-reporter gene constructs yield similar results, namely modest or no induction by E2 (18,19). A few studies reported somewhat stronger inductions in vitro, in two cases with MCF-7 cells (50,51) and in another with ZR-75 cells (52). We were unable to replicate this (Fig. 1). These variant results are likely due to differences in culture conditions between laboratories that affect HIF-1α expression. For example, anything restricting oxygen exposure, thereby reducing HIF-1α degradation, or inducing HIF-1α expression [e.g. the use of growth factors, high cell density, or hyperactivated cytoplasmic signaling pathways (53)] might result in sufficient HIF-1α to support E2-induced VEGF expression. Our findings provide for the first time a likely explanation both for why the majority of studies of E2’s effects on VEGF expression have shown little or no effect and why a small number have. None of these studies determined whether HIF-1α was present in their cells.
In the presence of oxygen, HIF-1α is hydroxylated on critical proline residues by iron-dependent HIF prolyl 4-hydroxylases. Hydroxylation leads to HIF-1α binding of the Von Hippel-Lindau tumor suppressor protein, which causes its ubiquitination and rapid proteosomal degradation (54). In vivo, tissue oxygen levels are on the order of 3–5%, which maintains a low equilibrium level of HIF-1α in cells, but the 20% oxygen experienced by cultured cells tips the balance toward complete degradation. CoCl2 inhibits this degradation by inhibiting prolyl 4-hydroxylases (55,56). Concentrations of CoCl2 up to 500 μm have been used in studies of HIF-1α (57,58) although 100 μm is sufficient to increase HIF-1α in cells while retaining additional E2 effects (Fig. 2) (59). Clearly this dose of CoCl2 did not interfere with normal transcription or translation. More work on identifying the optimal dose of CoCl2 (i.e. one that maintains a low equilibrium level of HIF-1α over time), as well as a comparison to the effects of other inhibitory agents such as nickel and zinc salts or the iron chelator desferrioxamine, are needed. A minor difference between previous in vivo studies and these results is that maximal VEGF expression occurs 1–2 h after E2 treatment in vivo (6,7,8) and returns to baseline levels by 4 h, whereas in cultured cells VEGF expression peaks at 4 h. Expression in vitro may lag because sufficient HIF-1α must first accumulate. Pretreating cells with CoCl2 might restore the more rapid response to E2. Finally, it should be noted that whereas CoCl2 is often referred to as a hypoxia mimetic, that is something of a misnomer because cells are still exposed to 20% oxygen and any other effects this high concentration may have.
There are alternative ways to restore HIF-1α in cells, such as transfecting in the HIF-1α gene (60). That approach, however, would make an already artificial system even less physiological and likely result in abnormally high HIF-1α expression. By comparison, CoCl2 simply reverses the artifactually high level of degradation induced by 20% oxygen, restoring the normal condition: the presence of a recruitable pool of endogenous HIF-1α within the cell. This approach also allowed us to observe an additional effect of E2: induction of HIF-1α expression, as occurs in the rat uterus in vivo (6).
The HIF-1α induced by CoCl2 was almost entirely present in the nucleus. It is often implied that translocation from the cytoplasm to the nucleus is a key event in the transcriptional activation HIF-1α, although there seems to be little evidence that this is in fact a regulated activation event as opposed to a constitutive process. The complete nuclear localization after inhibition of degradation argues for the latter, although CoCl2 may have a transcriptional activation effect as well. It has also been reported that CoCl2, in addition to blocking degradation, can act through the PI3K/Akt pathway to induce HIF-1α expression (57,61). In our experiments, however, E2 clearly stimulated further synthesis.
These results highlight the huge impact that culture conditions can have on results of studies of the effects of E2 and other hormones on cells in vitro. As stated previously, HIF-1α is a master regulator of gene transcription, including that of other transcription factors (39). It is surprising, therefore, how many studies of E2-induced VEGF expression and microarray analyses of global E2-induced gene expression have been done using cancer cells that likely lacked this key transcription factor. This probably explains in part the results of a recent study by Horwitz and colleagues (22), which showed just an 11% overlap in genes induced by E2 in breast cancer cells in vitro and in the same cells grown as tumors in vivo. These results also have major implications for studies of E2-induced cancer cell proliferation, which still remains poorly understood but clearly involves circulating growth factors (62,63,64,65,66,67). We have proposed that VEGF, through its effects on microvascular permeability, exposes epithelial cells to plasma growth factors (supplemental Fig. S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). The lack of HIF-1α in cultured cells therefore has likely obscured recognition of the central role of VEGF in E2-induced tumor cell proliferation.
Supplementary Material
Footnotes
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (NIH) through Cooperative Agreement U54 HD36207 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, NIH Grants R21 ES013061 and RO1 HD047275. K.H.M. was supported by National Heart, Lung, and Blood Institute/NIH Institutional Training Grant HL72751.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 9, 2009
Abbreviations: ChIP, Chromatin immunoprecipitation; E2, 17β-estradiol; ER, estrogen receptor; ERE, estrogen response element; HIF, hypoxia-inducible factor; HRE, hypoxia response element; PI3K, phosphatidylinositol 3-kinase; PR, progesterone receptor; TFF1, trefoil factor 1; VEGF, vascular endothelial growth factor.
References
- Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF 2008 Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis 11:109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N 2004 Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25:581–611 [DOI] [PubMed] [Google Scholar]
- Rockwell LC, Pillai S, Olson CE, Koos RD 2002 Inhibition of vascular endothelial growth factor/vascular permeability factor action blocks estrogen-induced uterine edema and implantation in rodents. Biol Reprod 67:1804–1810 [DOI] [PubMed] [Google Scholar]
- Cullinan-Bove K, Koos RD 1993 Vascular endothelial growth factor/vascular permeability factor expression in the rat uterus: rapid stimulation by estrogen correlates with estrogen-induced increases in uterine capillary permeability and growth. Endocrinology 133:829–837 [DOI] [PubMed] [Google Scholar]
- Hyder SM, Stancel GM, Chiappetta C, Murthy L, Boettger-Tong HL, Makela S 1996 Uterine expression of vascular endothelial growth factor is increased by estradiol and tamoxifen. Cancer Res 56:3954–3960 [PubMed] [Google Scholar]
- Kazi AA, Jones JM, Koos RD 2005 Chromatin immunoprecipitation analysis of gene expression in the rat uterus in vivo: estrogen-induced recruitment of both estrogen receptor α and hypoxia-inducible factor 1 to the vascular endothelial growth factor promoter. Mol Endocrinol 19:2006–2019 [DOI] [PubMed] [Google Scholar]
- Kazi AA, Koos RD 2007 Estrogen-induced activation of hypoxia-inducible factor-1α, vascular endothelial growth factor expression, and edema in the uterus are mediated by the phosphatidylinositol 3-kinase/Akt pathway. Endocrinology 148:2363–2374 [DOI] [PubMed] [Google Scholar]
- Kazi AA, Molitoris KH, Koos RD 2009 Estrogen rapidly activates the PI3K/Akt pathway and hypoxia-inducible factor 1 and induces vascular endothelial growth factor expression in luminal epithelial cells of the rat uterus. Biol Reprod 2009 81:378–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL 2006 Regulation of physiological responses to continuous and intermittent hypoxia by hypoxia-inducible factor 1. Exp Physiol 91:803–806 [DOI] [PubMed] [Google Scholar]
- Boutin AT, Johnson RS 2007 Waiting to inhale: HIF-1 modulates aerobic respiration. Cell 129:29–30 [DOI] [PubMed] [Google Scholar]
- Gordan JD, Simon MC 2007 Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev 17:71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder SM, Murthy L, Stancel GM 1998 Progestin regulation of vascular endothelial growth factor in human breast cancer cells. Cancer Res 58:392–395 [PubMed] [Google Scholar]
- Maity A, Sall W, Koch CJ, Oprysko PR, Evans SM 2001 Low pO2 and β-estradiol induce VEGF in MCF-7 and MCF-7–5C cells: relationship to in vivo hypoxia. Breast Cancer Res Treat 67:51–60 [DOI] [PubMed] [Google Scholar]
- Bogin L, Degani H 2002 Hormonal regulation of VEGF in orthotopic MCF7 human breast cancer. Cancer Res 62:1948–1951 [PubMed] [Google Scholar]
- Scott PA, Gleadle JM, Bicknell R, Harris AL 1998 Role of the hypoxia sensing system, acidity and reproductive hormones in the variability of vascular endothelial growth factor induction in human breast carcinoma cell lines. Int J Cancer 75:706–712 [DOI] [PubMed] [Google Scholar]
- Buteau-Lozano H, Ancelin M, Lardeux B, Milanini J, Perrot- Applanat M 2002 Transcriptional regulation of vascular endothelial growth factor by estradiol and tamoxifen in breast cancer cells: a complex interplay between estrogen receptors α and β. Cancer Res 62:4977–4984 [PubMed] [Google Scholar]
- Stoner M, Wang F, Wormke M, Nguyen T, Samudio I, Vyhlidal C, Marme D, Finkenzeller G, Safe S 2000 Inhibition of vascular endothelial growth factor expression in HEC1A endometrial cancer cells through interactions of estrogen receptor α and Sp3 proteins. J Biol Chem 275:22769–22779 [DOI] [PubMed] [Google Scholar]
- Mueller MD, Vigne JL, Minchenko A, Lebovic DI, Leitman DC, Taylor RN 2000 Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors α and β. Proc Natl Acad Sci USA 97:10972–10977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermont L, Lamielle F, Lorchel F, Fauconnet S, Esumi H, Weisz A, Adessi GL 2001 Insulin up-regulates vascular endothelial growth factor and stabilizes its messengers in endometrial adenocarcinoma cells. J Clin Endocrinol Metab 86:363–368 [DOI] [PubMed] [Google Scholar]
- Mirkin S, Wong BC, Archer DF 2005 Effect of 17β-estradiol, progesterone, synthetic progestins, tibolone, and tibolone metabolites on vascular endothelial growth factor mRNA in breast cancer cells. Fertil Steril 84:485–491 [DOI] [PubMed] [Google Scholar]
- Nakamura J, Savinov A, Lu Q, Brodie A 1996 Estrogen regulates vascular endothelial growth/permeability factor expression in 7,12-dimethylbenz (a) anthracene-induced rat mammary tumors. Endocrinology 137:5589–5596 [DOI] [PubMed] [Google Scholar]
- Harvell DM, Richer JK, Allred DC, Sartorius CA, Horwitz KB 2006 Estradiol regulates different genes in human breast tumor xenografts compared with the identical cells in culture. Endocrinology 147:700–713 [DOI] [PubMed] [Google Scholar]
- Cicatiello L, Scafoglio C, Altucci L, Cancemi M, Natoli G, Facchiano A, Iazzetti G, Calogero R, Biglia N, De Bortoli M, Sfiligoi C, Sismondi P, Bresciani F, Weisz A 2004 A genomic view of estrogen actions in human breast cancer cells by expression profiling of the hormone-responsive transcriptome. J Mol Endocrinol 32:719–775 [DOI] [PubMed] [Google Scholar]
- Lin CY, Ström A, Vega VB, Kong SL, Yeo AL, Thomsen JS, Chan WC, Doray B, Bangarusamy DK, Ramasamy A, Vergara LA, Tang S, Chong A, Bajic VB, Miller LD, Gustafsson JA, Liu ET 2004 Discovery of estrogen receptor α target genes and response elements in breast tumor cells. Genome Biol 5:R66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DY, Fulthorpe R, Liss SN, Edwards EA 2004 Identification of estrogen-responsive genes by complementary deoxyribonucleic acid microarray and characterization of a novel early estrogen-induced gene: EEIG1. Mol Endocrinol 18:402–411 [DOI] [PubMed] [Google Scholar]
- Ochsner SA, Steffen DL, Hilsenbeck SG, Chen ES, Watkins C, McKenna NJ 2009 GEMS (Gene Expression MetaSignatures), a Web resource for querying meta-analysis of expression microarray datasets: 17β-estradiol in MCF-7 cells. Cancer Res 69:23–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovic Z 2009 Hypoxia or in situ normoxia: the stem cell paradigm. J Cell Physiol 219:271–275 [DOI] [PubMed] [Google Scholar]
- Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C, Gassmann M, Candinas D 2001 HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J 15:2445–2453 [DOI] [PubMed] [Google Scholar]
- Wiener CM, Booth G, Semenza GL 1996 In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem Biophys Res Commun 225:485–488 [DOI] [PubMed] [Google Scholar]
- Quintero M, Mackenzie N, Brennan PA 2004 Hypoxia-inducible factor 1 (HIF-1) in cancer. Eur J Surg Oncol 30:465–468 [DOI] [PubMed] [Google Scholar]
- Kimbro KS, Simons JW 2006 Hypoxia-inducible factor-1 in human breast and prostate cancer. Endocr Relat Cancer 13:739–749 [DOI] [PubMed] [Google Scholar]
- Yuan Y, Hilliard G, Ferguson T, Millhorn DE 2003 Cobalt inhibits the interaction between hypoxia-inducible factor-α and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-α. J Biol Chem 278:15911–15916 [DOI] [PubMed] [Google Scholar]
- Cameron VA, Autelitano DJ, Evans JJ, Ellmers LJ, Espiner EA, Nicholls MG, Richards AM 2002 Adrenomedullin expression in rat uterus is correlated with plasma estradiol. Am J Physiol Endocrinol Metab 282:E139–E146 [DOI] [PubMed] [Google Scholar]
- Ikeda K, Arao Y, Otsuka H, Kikuchi A, Kayama F 2004 Estrogen and phytoestrogen regulate the mRNA expression of adrenomedullin and adrenomedullin receptor components in the rat uterus. Mol Cell Endocrinol 223:27–34 [DOI] [PubMed] [Google Scholar]
- Watanabe H, Takahashi E, Kobayashi M, Goto M, Krust A, Chambon P, Iguchi T 2006 The estrogen-responsive adrenomedullin and receptor-modifying protein 3 gene identified by DNA microarray analysis are directly regulated by estrogen receptor. J Mol Endocrinol 36:81–89 [DOI] [PubMed] [Google Scholar]
- Zudaire E, Martínez A, Cuttitta F 2003 Adrenomedullin and cancer. Regul Pept 112:175–183 [DOI] [PubMed] [Google Scholar]
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M 2005 Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122:33–43 [DOI] [PubMed] [Google Scholar]
- Laganière J, Deblois G, Lefebvre C, Bataille AR, Robert F, Giguère V 2005 From the cover: location analysis of estrogen receptor alpha target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc Natl Acad Sci USA 102:11651–11656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL 2003 Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med 5417–5428 [DOI] [PubMed] [Google Scholar]
- Yee Koh M, Spivak-Kroizman TR, Powis G 2008 HIF-1 regulation: not so easy come, easy go. Trends Biochem Sci 33:526–534 [DOI] [PubMed] [Google Scholar]
- Yokomizo R, Matsuzaki S, Uehara S, Murakami T, Yaegashi N, Okamura K 2002 Erythropoietin and erythropoietin receptor expression in human endometrium throughout the menstrual cycle. Mol Hum Reprod 8:441–446 [DOI] [PubMed] [Google Scholar]
- Cameron IT, Campbell S 1998 Nitric oxide in the endometrium. Hum Reprod Update 4:565–569 [DOI] [PubMed] [Google Scholar]
- Welch RD, Gorski J 1999 Regulation of glucose transporters by estradiol in the immature rat uterus. Endocrinology 140:3602–3608 [DOI] [PubMed] [Google Scholar]
- Reiss NA 1988 Ontogeny and estrogen responsiveness of creatine kinase and glycolytic enzymes in brain and uterus of rat. Neurosci Lett 84:197–202 [DOI] [PubMed] [Google Scholar]
- Leonard MO, Cottell DC, Godson C, Brady HR, Taylor CT 2003 The role of HIF-1α in transcriptional regulation of the proximal tubular epithelial cell response to hypoxia. J Biol Chem 278:40296–40304 [DOI] [PubMed] [Google Scholar]
- Giacalone PL, Daurés JP, Ouafik L, Martin PM, Laffargue F, Maudelonde T 2003 Steroids and adrenomedullin growth patterns in human ovarian cancer cells: estrogenic-regulation assay. Gynecol Oncol 91:651–656 [DOI] [PubMed] [Google Scholar]
- Giacalone PL, Vuaroqueaux V, Daurés JP, Houafic L, Martin PM, Laffargue F, Maudelonde T 2003 Expression of adrenomedullin in human ovaries, ovarian cysts and cancers. Correlation with estrogens receptor status. Eur J Obstet Gynecol Reprod Biol 110:224–229 [DOI] [PubMed] [Google Scholar]
- Rossiter H, Barresi C, Ghannadan M, Gruber F, Mildner M, Födinger D, Tschachler E 2007 Inactivation of VEGF in mammary gland epithelium severely compromises mammary gland development and function. FASEB J 21:3994–4004 [DOI] [PubMed] [Google Scholar]
- Qiu Y, Bevan H, Weeraperuma S, Wratting D, Murphy D, Neal CR, Bates DO, Harper SJ 2008 Mammary alveolar development during lactation is inhibited by the endogenous antiangiogenic growth factor isoform, VEGF165b. FASEB J 22:1104–1112 [DOI] [PubMed] [Google Scholar]
- Ruohola JK, Valve EM, Karkkainen MJ, Joukov V, Alitalo K, Härkönen PL 1999 Vascular endothelial growth factors are differentially regulated by steroid hormones and antiestrogens in breast cancer cells. Mol Cell Endocrinol 149:29–40 [DOI] [PubMed] [Google Scholar]
- Sengupta K, Banerjee S, Saxena N, Banerjee SK 2003 Estradiol-induced vascular endothelial growth factor-A expression in breast tumor cells is biphasic and regulated by estrogen receptor-α dependent pathway. Int J Oncol 22:609–614 [PubMed] [Google Scholar]
- Stoner M, Wormke M, Saville B, Samudio I, Qin C, Abdelrahim M, Safe S 2004 Estrogen regulation of vascular endothelial growth factor gene expression in ZR-75 breast cancer cells through interaction of estrogen receptor α and SP proteins. Oncogene 23:1052–1063 [DOI] [PubMed] [Google Scholar]
- Bilton RL, Booker GW 2003 The subtle side to hypoxia inducible factor (HIFα) regulation. Eur J Biochem 270:791–798 [DOI] [PubMed] [Google Scholar]
- Semenza G 2002 Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol 64:993–998 [DOI] [PubMed] [Google Scholar]
- Wang GL, Semenza GL 1993 General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA 90:4304–4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coradini D, Pellizzaro C, Speranza A, Daidone MG 2004 Hypoxia and estrogen receptor profile influence the responsiveness of human breast cancer cells to estradiol and antiestrogens. Cell Mol Life Sci 61:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardyanto TD, Osaki M, Tokuyasu N, Nagahama Y, Ito H 2006 CoCl2-induced HIF-1α expression correlates with proliferation and apoptosis in MKN-1 cells: a possible role for the PI3K/Akt pathway. Int J Oncol 29:549–555 [PubMed] [Google Scholar]
- Cho J, Kim D, Lee S, Lee Y 2005 Cobalt chloride-induced estrogen receptor α down-regulation involves hypoxia-inducible factor-1α in MCF-7 human breast cancer cells. Mol Endocrinol 19:1191–1199 [DOI] [PubMed] [Google Scholar]
- Jung JY, Roh KH, Jeong YJ, Kim SH, Lee EJ, Kim MS, Oh WM, Oh HK, Kim WJ 2008 Estradiol protects PC12 cells against CoCl2-induced apoptosis. Brain Res Bull 76:579–585 [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL 1996 Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16:4604–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachami G, Simos G, Hatziefthimiou A, Bonanou S, Molyvdas PA, Paraskeva E 2004 Cobalt induces hypoxia-inducible factor-1α expression in airway smooth muscle cells by a reactive oxygen species- and PI3K-dependent mechanism. Am J Respir Cell Mol Biol 31:544–551 [DOI] [PubMed] [Google Scholar]
- Dickson RB, Lippman ME 1995 Growth factors in breast cancer. Endocr Rev 16:559–589 [DOI] [PubMed] [Google Scholar]
- Hamelers IH, Steenbergh PH 2003 Interactions between estrogen and insulin-like growth factor signaling pathways in human breast tumor cells. Endocr Relat Cancer 10:331–345 [DOI] [PubMed] [Google Scholar]
- Anderson E, Clarke RB 2004 Steroid receptors and cell cycle in normal mammary epithelium. J Mammary Gland Biol Neoplasia 9:3–13 [DOI] [PubMed] [Google Scholar]
- Lann D, LeRoith D 2008 The role of endocrine insulin-like growth factor-I and insulin in breast cancer. J Mammary Gland Biol Neoplasia 13:371–379 [DOI] [PubMed] [Google Scholar]
- Santen RJ, Song RX, Masamura S, Yue W, Fan P, Sogon T, Hayashi S, Nakachi K, Eguchi H 2008 Adaptation to estradiol deprivation causes up-regulation of growth factor pathways and hypersensitivity to estradiol in breast cancer cells. Adv Exp Med Biol 630:19–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Pollard JW 2007 Estradiol-17β regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling. Proc Natl Acad Sci USA 104:15847–15851 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.