Abstract
Forebrain ventricular delivery of melanocortin receptor (MC3/4R) agonist increases energy expenditure and decreases food intake (FI). Because forebrain ventricular delivery provides ligand to various anatomically distributed MC3/4R-bearing nuclei, it is unclear which of the receptor subpopulations contributes to the feeding suppression and the sympathetic-thermogenic effects observed. The literature indicates that reexpression of MC4R in the paraventricular nucleus (PVH) affects the feeding but not the energetic phenotype of the MC4R knockout, suggesting that divergent MC4R populations mediate energy expenditure (hindbrain) and FI (hypothalamus) effects of stimulation. Not consistent with this view are data indicating that PVH sympathetic projection neurons express MC4Rs and that feeding effects are induced from hindbrain MC4R sites. Therefore, we hypothesize an opposing perspective: that stimulation of anatomically diverse MC3/4R-bearing nuclei triggers energetic as well as feeding effects. To test this hypothesis, ventricle subthreshold doses of MC3/4R agonist (5 and 10 pmol) were applied in separate experiments to six hindbrain and hypothalamic sites; core temperature (Tc), heart rate (HR), spontaneous activity (SPA), and FI were measured in behaving rats. Nucleus tractus solitarius and PVH stimulation increased Tc, HR, and SPA and decreased FI. Rostral ventrolateral medulla, parabrachial nucleus, and retrochiasmatic area stimulation increased Tc, HR, but not SPA, and decreased FI. The response profile differed to some extent for each nucleus tested, suggesting differential output circuitries for the measured parameters. Data are consistent with the view that energetic and feeding responses are not controlled by regionally divergent MC3/4Rs and can be elicited from multiple, anatomically distributed MC3/4R populations.
Food intake and energy expenditure effects of central melanocortin stimulation are driven by anatomically distributed melanocortin receptor expressing neurons.
Humans with melanocortin receptor-4 (MC4R) mutation are hyperphagic and obese (1,2). They also exhibit lower energy expenditure (3) and decreased cardiovascular parameters (heart rate and blood pressure) (4). MC4R knockout mice show a similar energy balance profile. These results, and others, support the view that the central melanocortin system is an important element of the central nervous system (CNS) circuits controlling food intake and energy expenditure. Ventricular pharmacological stimulation of central melanocortin receptors (MCRs; MC3Rs and MC4Rs) increases energy expenditure and cardiovascular parameters and decreases food intake (5,6,7). It is unclear, however, which of the anatomically distributed MCR subpopulations contribute to the food intake and to the sympathetic-energetic effects observed.
Despite the broad anatomic distribution of CNS MCRs, especially MC4R, MCR-expressing neurons in the hypothalamus are viewed as the principal site of action for the food intake as well as the energy expenditure effects of melanocortin ligand stimulation. This view, especially pertaining to food intake, is supported by data indicating that third ventricular injections of MC3/4R agonist (MTII) as well as intraparenchymal injections into the paraventricular hypothalamic nucleus (PVH) reduces food intake (5,8). Additional support for this anatomical perspective comes from the results showing that reexpression of MC4Rs in the PVH (and in some amygdala and hypothalamic neurons) in obese MC4R knockout mice reverses their hyperphagia to a level of food intake seen in wild-type control mice (9).
The obesity of these selective MC4R-expressing mice is not, however, fully reversed as their energy expenditure (measured by oxygen consumption) is not affected by the treatment, in contrast to their feeding (9). This result is surprising because it is clear that MC4R-expressing parvocellular PVH neurons are sympathetic premotor neurons whose output contributes to the control of brown adipose tissue (BAT) thermogenesis (10,11,12). Nevertheless, the observed contrast in the pattern of energy balance effects led to a new hypothesis (9) about the control of energy balance function exerted by anatomically distinct MCRs: that hypothalamic/PVH MC4R-expressing neurons contribute to control of food intake and MC4R-expressing sites elsewhere, likely in the caudal brain stem, contribute to the energy expenditure effects of melanocortins.
Consistent with this organizational perspective several caudal brain stem nuclei contain sympathetic premotor neurons that express MC4R and contribute to the control of BAT thermogenesis including the raphe pallidus, rostral ventrolateral medulla (RVLM), nucleus tractus solitarius (NTS) and the pontine parabrachial nucleus (PBN) (11). Hindbrain ventricular or raphe pallidus injection of the MC3/4R agonist MTII increases energy expenditure (elevating core temperature and BAT activity) and heart rate (7,13). Although these caudal brain stem MCR-driven energetic effects are consistent with the idea of hypothalamic-caudal brain stem divergence of function for feeding vs. energy expenditure other published findings are not. Application of picomolar doses of MTII and antagonist (SHU-9119) to the NTS results respectively in robust food intake inhibition or hyperphagia (14,15,16); low-dose agonist stimulation of raphe pallidus inhibits feeding (7). These findings are inconsistent with the notion that the feeding effects of CNS MCR stimulation are localized to hypothalamic MCR stimulation. Rather, these results appear to indicate that the energy balance effects of melanocortins are anatomically distributed and not regionally segregated.
To better distinguish between the applicability of these two competing views of the relation between MCR location and the control of energy intake and sympathetic-energy expenditure responses, it is necessary to compare the range of energy balance responses obtained by stimulating a number of distinct MC4R-expressing nuclei at the level of the hypothalamus and the caudal brain stem. Here we pursue such studies. Using ventricle subthreshold, low, picomolar doses of the MC3/4R agonist MTII experiments assessed the energy balance response profiles observed with direct parenchymal stimulation of six different MC4R-bearing nuclei, two within hypothalamus [PVH and retrochiasmatic area (RCh)] and three within the caudal brain stem (NTS, RVLM, and PBN). For each nucleus included in this study MCR expression and connections to sympathetic outflow were a prerequisite (11,12). The three caudal brain stem sites are anatomically segregated such that stimulation of one would not indirectly stimulate the other. Given that two hypothalamic sites are relatively close to each other a third site, the anterior hypothalamic area (AHA), was included as an anatomical control for the spread of injected ligand.
Stimulation of all but one of the sites tested led to an increase in core temperature and heart rate, a decrease in food intake and body weight, and in some cases increases in activity. These results are consistent with the hypothesis that energy balance effects of melanocortins are distributed across many anatomically distinct brain regions including the hypothalamus and caudal brain stem.
Materials and Methods
Subjects
Male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA), weighing 300–400 g at surgery and housed individually in plastic bins maintained on a 12-h light, 12-h dark cycle (0800 h lights on), participated in the experiments described below. Pelleted food (Purina 5001; St. Louis, MO) and water were available ad libitum unless otherwise noted. All protocols and procedures were approved by the institutional care and use committee (University of Pennsylvania).
Surgery
Rats were anesthetized with ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) delivered im.
Cannula implantation
All rats received a guide cannula (22 gauge; Plastics One, Inc., Roanoke, VA) with its tip stereotaxically positioned 1.0 (AHA), 3.0 mm (RVLM), or 2.0 mm above the target parenchymal site. The following coordinates were used: 1) NTS: 0.7 mm from the midline, on the occipital suture, and 6.9 mm ventral to the skull, with injector aimed 8.9 mm ventral from skull; 2) RVLM: 2 mm from the midline, 1.8 mm rostral to occipital suture, and 7.9 mm ventral to the skull, with injector aimed 10.9 mm ventral to skull; 3) PBN: 2.0 mm lateral to midline, 9.5 mm posterior to bregma and 4.5 mm ventral to the dura, with injector aimed 6.5 mm ventral to dura; 4) PVH: 0.5 mm lateral to midline, 1.8 mm posterior to bregma, and 5.7 mm ventral to the dura, with injector aimed 7.7 mm ventral to dura; 5) AHA: 0.5 mm lateral to midline, 1.8 mm posterior to bregma, and 7.7 mm ventral to the dura, with injector aimed 8.7 mm ventral to dura; and 6) RCh: 0.5 mm lateral to midline, 1.8 mm posterior to bregma, and 7.7 mm ventral to the dura, with injector aimed 9.7 mm ventral to dura. Cannulas were attached to the skull with dental acrylic and jeweler’s screws and closed with an obturator. Cannula placement was verified histologically after the experiment by examining the anatomical placement of an injection of pontamine sky blue (100 nl volume matched drug delivery in the experiments) made at the time the animals were killed. Only rats whose dye injection site was found within the intended parenchymal site were included in the physiological data analysis. For the NTS group, an additional functional verification was applied. The placement was assessed 7 d after the surgery by measurement of the sympathoadrenal-mediated glycemic response to an injection of 5-thio-d-glucose (24 μg in 100 nl artificial cerebral spinal fluid) (17). A postinjection elevation in baseline plasma glucose level of at least 100% was required for subject inclusion using this verification criterion.
Telemetric transponder surgery
Under anesthesia telemetric transponders (HRC 4000 VitalView; Mini Mitter/Respironics, Bend, OR) for recording core temperature (TC), heart rate (HR), and spontaneous physical activity (SPA) were inserted into the abdominal cavity, with the leads positioned sc and secured to the chest muscles on either side of the heart with sutures as described in (7).
Experimental procedures
Habituation training
Before the start of experimental testing, rats were habituated to the handling and injection procedures to be used during formal testing.
Testing days
All rats received vehicle injections (100 nl artificial cerebral spinal fluid) counterbalanced with one (RCh, AHA; 10 pmol MTII) or two doses (NTS, RVLM, PBN, PVH; 5 and 10 pmol MTII) of MTII. TC, HR, and SPA were recorded telemetrically for 1 h before injections and 6 h after injections; the frequency of measurement was every 5 min (TC, SPA) or 1 min (HR). Food was removed just before injection time (early in the light cycle, between 0930 and 1000 h) and returned 6 h later, late in the light phase. Thereby food was not available during the period of energetic-cardiovascular response measurement. Food intake and body weight measurements were performed 24 h after the injection of drug. Given this design, all noted differences in food intake reflect longer latency effects of MTII (from 6 to 24 h after injection). Food was always available during the dark cycle. A minimum of a 48-h period intervened between experimental testing.
Statistical analysis
TC, HR, and SPA were analyzed by ANOVA on postinjection 6-h average values of each parameter and followed by Student’s t test or Tukey test as appropriate. Twenty-four-hour food intake and body weight were analyzed by ANOVA and followed by t test or Tukey test as appropriate.
Results
NTS
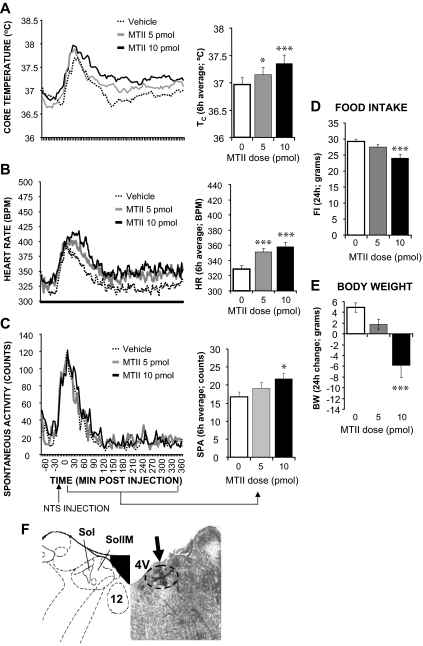
Microscopic assessment of the brains of rats in this group (n = 23) indicated that the center of the dye injection was located within the NTS at the level of fourth ventricle; the dye also reached the dorsal motor nucleus of the vagus nerve (DMX) in some cases. Figure 1 shows that MTII injection significantly increased Tc (5 pmol: P < 0.05; 10 pmol: P < 0.0005), HR (5 pmol: P < 0.0005; 10 pmol: P < 0.0005), and spontaneous activity (10 pmol: P < 0.05). Injection procedures/handling induced a short (30 min) spike for all measured energy expenditure parameters and all treatment conditions/locations; this was likely due to waking the rats in their sleep cycle as well as a small anxiety response to procedures despite the fact that all rats were habituated to all procedures multiple times. Overnight food intake and body weight were reduced by 10 pmol MTII treatment (P < 0.0005, P < 0.0005, respectively). The 5 pmol MTII dose did not yield significant changes in activity, food intake, or body weight.
Figure 1.
Effect of MTII stimulation of NTS MCRs on TC (A), HR (B), and spontaneous activity (C) in rats. Line graphs represent across-rat average parameter measurements through the recording period. The bracketed time period on the line graph x-axis indicates the periods used in the adjacent histograms, which provide 6-h postinjection averages ± sem for each parameter at each dose. Effect of NTS MTII injection on 24-h food intake (FI; D) and 24 h change in body weight (BW; E) in awake, nonanesthetized rats. Food was made available to animals 6 h after injections and through the 12-h period of the dark cycle. Histograms represent means ± sem. *, P < 0.05; **, P < 0.005; ***, P < 0.0005. F, Representative NTS injection site (arrow). 4V, Fourth ventricle; Sol, nucleus of the solitary tract; SolIM, intermedial nucleus of the solitary tract. All following figures will have a similar format; the principal variable is the site of parenchymal site of injection of MTII.
RVLM
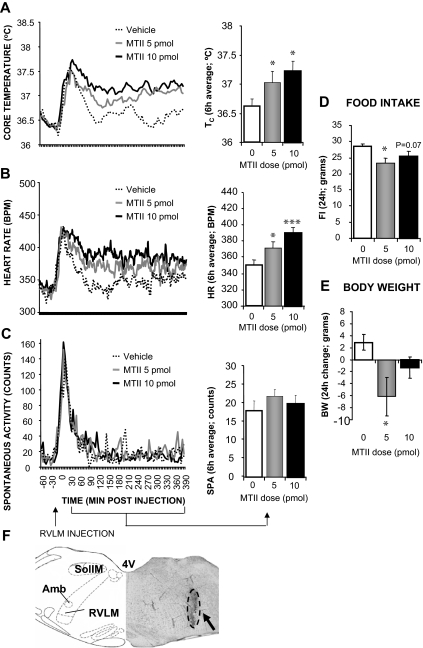
Microscopic analysis indicated that the center of dye injection for these rats (n = 12) were located within the RVLM, with small amounts of ink in some cases reaching near the nucleus ambiguous. Figure 2 shows that MTII stimulation significantly increased Tc (5 pmol: P < 0.05; 10 pmol: P < 0.05) and HR (5 pmol: P < 0.05; 10 pmol: P < 0.0005). No significant spontaneous activity changes were noted after MTII application to the RVLM. Overnight food intake and body weight were reduced by 5 pmol MTII treatment (P < 0.005, P < 0.05, respectively).
Figure 2.
Effect of MTII stimulation of RVLM MCRs on TC (A), HR (B), spontaneous activity (C), 24-h food intake (FI; D), and 24-h change in body weight (BW; E). F, Representative RVLM injection site (arrow). 4V, Fourth ventricle; SolIM, intermedial nucleus of the solitary tract; Amb, nucleus ambiguus.
PBN
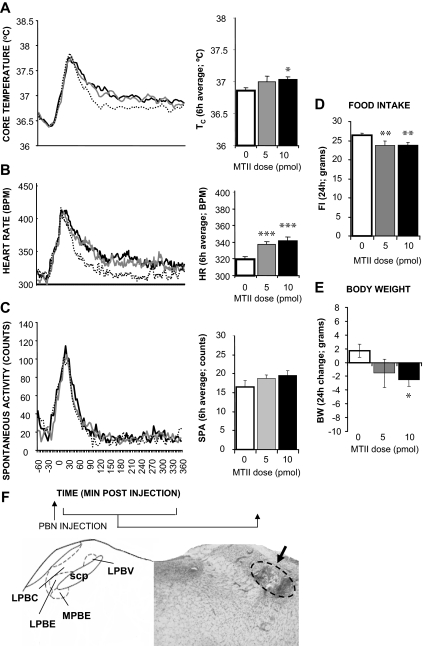
The dye injection for this group (n = 37) was centered at the external lateral division of the PBN with trace amounts of ink reaching other PBN nuclei. Figure 3 shows that MTII stimulation significantly increased Tc (5 pmol: P = 0.13; 10 pmol: P < 0.05), HR (5 pmol: P < 0.0005; 10 pmol: P < 0.0005), but not spontaneous activity. Overnight food intake and body weight were reduced by the MTII treatment (5 pmol: P < 0.005; 10 pmol: P < 0.005; 5 pmol: P = 0.055; 10 pmol: P < 0.05, respectively).
Figure 3.
Effect of MTII stimulation of PBN MCRs on TC (A), HR (B), spontaneous activity (C), 24-h food intake (FI; D), and 24-h change in body weight (BW; E). F, Representative PBN injection site (arrow). LPBV, Lateral parabrachial nucleus ventral; LPBE, lateral parabrachial nucleus external; LPBC, lateral parabrachial nucleus central; MPBE, medial parabrachial nucleus external; scp, superior cerebellar peduncle.
PVH
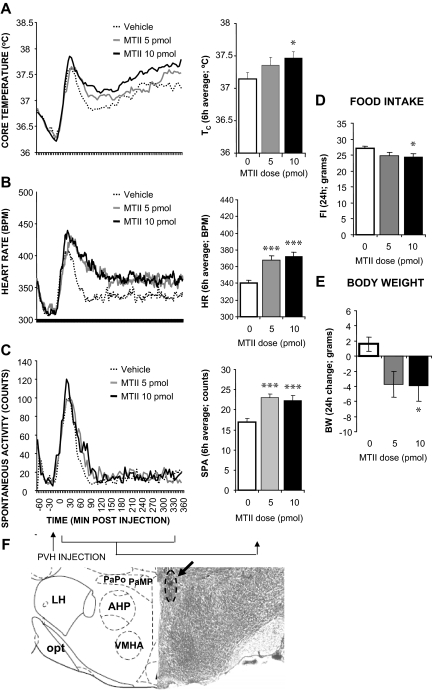
The dye injection for rats in this group (n = 40) was centered at the dorsal or medial parvicellular PVH. Figure 4 shows that MTII stimulation significantly increased Tc (5 pmol: P = 0.23; 10 pmol: P < 0.05), HR (5 pmol: P < 0.0005; 10 pmol: P < 0.0005), and spontaneous activity (5 pmol: P < 0.0005; 10 pmol: P < 0.0005). Overnight food intake and body weight were reduced by the MTII treatment (5 pmol: P = 0.076; 10 pmol: P < 0.05; 5 pmol: P < 0.05; 10 pmol: P < 0.05, respectively).
Figure 4.
Effect of MTII stimulation of PVH MCRs on TC (A), HR (B), spontaneous activity (C), 24-h food intake (FI; D), and 24-h change in body weight (BW; E). F, Representative PVH injection site (arrow). PaPo, Paraventricular nucleus posterior part; PaMP, paraventricular nucleus medial parvicellular; LH, lateral hypothalamus; VMHA, ventromedial hypothalamus anterior; opt, optic tract.
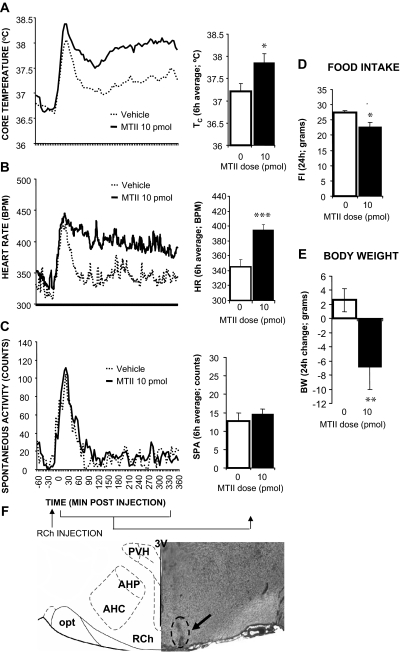
RCh
Microscopic evaluation determined that in all rats (n = 12) in this group, the dye injection was centered in the RCh. Figure 5 shows that MTII stimulation significantly increased Tc (10 pmol: P < 0.01) and HR (10 pmol: P < 0.0005) but had no effect on spontaneous activity (10 pmol: P = 0.42). Overnight food intake and body weight were reduced by the MTII treatment (10 pmol: P < 0.01; 10 pmol: P < 0.005, respectively).
Figure 5.
Effect of MTII stimulation of RCh MCRs on TC (A), HR (B), spontaneous activity (C), 24-h food intake (D), and 24-h change in body weight (E). F, Representative RCh injection site (arrow). AHC, Anterior hypothalamic area central; AHP, anterior hypothalamic area posterior; opt, optic tract; 3V, third ventricle.
AHA
To show that the doses and volume chosen were indeed selective for the targeted nuclei, this site was selected as an anatomical control placement based on its adjacency to the PVH and the RCh targets. MTII injected into the AHA did not produce any significant changes in any of the measured parameters (n = 12; supplemental Fig. S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). The location of the AHA directly adjacent to the two hypothalamic targets that yielded a positive effect (just dorsally to RCh and just ventrally to PVH) and the lack of observed effects confirm that this target served as a negative control area.
Discussion
Each of the sites targeted in these studies, PBN, NTS, RVLM, PVH, and RCh, was selected for its expression of MC4Rs and its established connection to the sympathetic outflow (11,12,18,19). This phenotypic profile provided the foundation for the hypothesis that direct melanocortinergic stimulation of these different hypothalamic and hindbrain neurons could drive energetic and cardiovascular responses. The results obtained are consistent with that hypothesis: direct administration of low, picomolar doses of MC3/4R agonist into these areas triggered hyperthermia, tachycardia, and in some cases hyperactivity. Also noteworthy, stimulation of these sites induced anorexia and body weight loss. The lack of an effect on any measured parameter from melanocortinergic stimulation of the AHA site, intermediate between the PVH and RCh sites, supports the interpretation that the observed effects arise from ligand application to the targeted neurons. To our knowledge these are the first reports of the effects of direct melanocortinergic stimulation in the PBN and RCh and the first assessment of the energetic effects of PVH and RVLM MCR stimulation in a nonanesthetized rat. Collectively, the data presented indicate that the energy intake and energy expenditure effects of CNS MCR stimulation are mediated by an anatomically distributed network of MCR-bearing neurons. These data are not consistent with an anatomical organization of MCR-stimulated energy balance response in which the control of feeding and energetic-sympathetic responses is regionally segregated.
Hyperthermia
Stimulation of MCR-expressing neurons in the NTS, PBN, RCh, and PVH increased core temperature significantly (RVLM and hyperthermia is discussed later in the paragraph). BAT thermogenesis driven by sympathetic outflows is a likely contributor to the observed hyperthermia as both MC4R expression and neural connections to BAT (retrograde viral tracing from BAT) are present in these nuclei (11). BAT temperature and uncoupling protein-1 gene expression was not measured in the current study; however, fourth ventricular delivery of MTII gives rise to a profile of response that includes hyperthermia and elevations in BAT temperature (7) and BAT uncoupling protein-1 mRNA (12,13). Direct MTII injection into the caudal raphe (10 pmol, lowest effective dose) increases BAT temperature (7). Elevation in BAT temperature is also observed after PVH MTII stimulation (50 pmol, lowest effective dose) in the hamster (12). Interestingly, unlike the thermoregulatory effect of MCR stimulation of PVH shown here in a rat and by others for a hamster (12), MC4R reexpression in the PVH of the MC4-R knockout mouse model does not result in increased energy expenditure (9).
This study is the first to show thermal effects of direct PBN melanocortin stimulation. A contribution of PBN to the neural control of thermoregulation has been suggested by the work of others. Electrical stimulation of PBN produces BAT hyperthermia and increases oxygen consumption and bilateral PBN lesion disrupts the thermogenic response triggered by environmental cooling (20). Lateral PBN neurons are activated by skin cooling and may influence BAT thermogenesis via their rostral projections to neurons of the hypothalamic medial preoptic area (21). The contribution of PBN output to BAT thermogenesis could also involve descending PBN projections (22) to the raphe pallidus, a region critical for the neural control of BAT thermogenesis (23). Although not a focus of study for its effects on temperature, the connection between RCh neurons and BAT (viral tracing studies) and the MC4R expression in these neurons (24) suggests that the hyperthermia triggered by MCR stimulation of RCh involves BAT thermogenesis.
Other mechanisms may also contribute to the observed hyperthermia. Neither skin temperature nor vascular resistance changes were measured here, therefore vasoconstriction remains a possible mechanism contributing to the elevation in TC induced by central melanocortinergic stimulation. In fact, studies using neuroexcitation (chemical and electrical) of the RVLM show that thermoregulatory effects are associated with changes in sympathetic activity to vasculature (specifically tail vein) rather than to changes in BAT thermogenesis (25,26). Here we showed that MCR stimulation of RVLM increased core temperature. Therefore, it will be important to consider whether a given effect of MCR stimulation, e.g. hyperthermia, can arise from distinct MC4R-bearing nuclei via separable and distinct physiological effectors. We noted above that the hyperthermic effect observed for NTS stimulation may result from a BAT-mediated output, whereas the hyperthermic effect after RVLM stimulation may be mediated by sympathetic nervous system (SNS) control of the vasculature. Consistent with this view are data showing that SNS output to certain tissues can be differential with potentially different output circuitries reaching different tissues (26,27,28,29).
Tachycardia
MTII stimulation of both hindbrain and hypothalamic nuclei elicited a marked and long-lasting tachycardia. The 5-pmol MTII dose was effective in triggering this response from the PVH, PBN, RVLM, and NTS sites. The same dose was of varying effectiveness in inducing thermogenesis, possibly implicating a higher sensitivity of the tachycardic response. All MTII-stimulated tachycardic-responsive areas in this study are directly or indirectly connected to autonomic outflow to the heart (30,31). A role for RVLM in cardiovascular control is well established; sympathoexitatory RVLM neurons project directly to intermediolateral cell column (32,33). MCR stimulated tachycardia from RVLM is likely mediated by the increased sympathetic nerve activity. Support for this interpretation comes from a study showing that RVLM injection of ACTH (with affinity for MC1–5R; greatest affinity for MC2R) in anesthetized rats increases sympathetic nerve activity, blood pressure, and HR (34). The greater magnitude (2- to 3-fold larger) and duration (10 times longer; 20 min vs. 6 h) of the HR effect in our study is likely attributable to the greater affinity of the agonist used (MTII vs. ACTH) and the behavioral state of rats. The tachycardic effect we report for NTS stimulation is not consistent with the direction of the effect, bradycardia, reported for ACTH injection into NTS at the area postrema level of anesthetized rats (35). The same group of investigators however, showed that injections of SHU 9119 (MC3/4R antagonist) also produces bradycardia in anesthetized rats, potentially blocking the effects of endogenously released melanocortin agonist. A role for the PBN in cardiovascular regulation is well established. Electrical or chemical stimulation of PBN elicits tachycardia via RVLM projections and subsequent SNS premotor neuron activation (36,37,38). Melanocortinergic stimulation of the PBN induced a small magnitude but statistically significant tachycardia; this is the first report of a direct effect on HR of melanocortins in PBN.
Spontaneous locomotor activity
Activity can be a contributing factor to the pathogenesis of obesity. Decreases in spontaneous activity are strongly correlated with obesity development and fat pad deposition (39). Whereas little is known about the neural basis of this response, available evidence implicates many of the neuropeptides associated with food intake in the control of physical activity (40). Central (ventricular) MTII stimulation increases and antagonist (agouti gene related peptide) application decreases spontaneous locomotor activity in rats (41,42,43). Our study suggests that MCR-bearing neurons of the PVH and PBN contribute to this effect of MTII because low, picomolar doses of MTII in these nuclei increased physical activity.
Food intake
The NTS, RCh, PVH, and PBN are among the nuclei most prominently implicated in the neural control of food intake. The anorexia shown here after MTII delivery to NTS and PVH is consistent with the results of other publications (8,14,44). The anorexia induced by picomolar MTII dose delivered to PBN and RCh is the first such report. The size of the anorexic effect from these sites (∼5 g over the18 h measurement) is comparable with that observed after PVH and NTS stimulation. It is important to note that the experimental design used here, however, only captures food intake effects of a longer term nature (6–24 h after drug injections). It is entirely possible that the magnitude and temporal pattern of the effect on food intake would differ across these sites if food had been available and intake measured during the initial postinjection period (1–6 h after drug).
The energy balance effects reported here arise from the exogenous stimulation of a number of anatomically disparate sites and lead naturally to a consideration of the endogenous melanocortin system and its role in normal physiology. One important but unresolved question is the source of the endogenous agonist for the hypothalamic and hindbrain sites investigated. Two populations of proopiomelanocortin (POMC) neurons, one in hypothalamic arcuate nucleus (ARC) and the other in hindbrain NTS, are the sources of endogenous α-MSH. Some MCR-bearing nuclei are innervated by one of those populations, but others might have dual innervation. Until recently this problem has not been adequately explored. Berthoud et al. (45) showed that MC4R-bearing NTS neurons receive a majority (∼70%) of their α-MSH input from the hypothalamic arcuate POMC population but some (∼30%) originate locally from the NTS POMC neurons (45). This result is consistent with a suggestion by Palkovits et al. (46) that hypothalamic α-MSH fibers might provide a majority of the innervation for the midline hindbrain nuclei like NTS and medullary raphe, whereas the more lateral MCR-expressing hindbrain regions (e.g. RVLM) might receive a greater percentage of their innervation from NTS α-MSH fibers. The PVH and RCh receive projections from ARC POMC neurons (47) but NTS α-MSH projections to these areas have not been extensively studied and cannot be ruled out (see (48,49). The PBN receives input from the commissural NTS (48,49) and the ARC (47); however, more remains to be done in determining the neurochemical phenotype of the PBN-projecting ARC and NTS neurons. Given that ARC and NTS POMC neurons might receive a different set of inputs, the MCR-bearing target nuclei that receive projections from both POMC sources would logically integrate a wider range of information.
It remains to be determined how the melanocortinergic tone (i.e. activation of POMC neurons leading to α-MSH ligand release onto target nuclei) may be altered under specific environmental changes, e.g. fasting, high-fat diet maintenance, cold challenge, infection, etc. One approach to addressing these questions would be to block endogenous melanocortin signaling in a given nucleus through the use of site-specific receptor antagonist or via viral knockdown of a given MRC. Garza et al. (50) used RNAi technology to show that MC4R signaling in PVH is necessary for the regulation of food intake and body weight regulation under unique circumstances (energy expenditure not measured). The hyperphagia and obesity was observed only when rats were maintained on high-fat food; PVH MC4R knockdown rats maintained on chow were normophagic. The results suggest that the high-fat feeding alters endogenous melanocortinergic tone where normal MC4R function keeps high-fat-induced hyperphagia in check.
It will be useful to determine the neurochemical phenotype of the MTII-stimulated neurons. Such detail could provide insight into the downstream pathways and help to better define the neurocircuitry mediating melanocortinergic effects. For example, in considering MCR function for NTS neurons, it has become apparent that some MC4Rs are located presynaptically on vagal afferent terminals, indicating that melanocortin signals are likely interacting with the vagal transmission to influence the excitability of NTS recipient neurons (51). This modulation could indicate a potential mechanism for the anorexic effect of NTS MTII stimulation in which vagal signals arising from, for example, gastric distention or cholecystokinin stimulation could be enhanced by MC4R activation, leading to a more potent decrease of food intake than that induced by vagal activation alone (51). In the PVH, Mountjoy et al. (18) reported that high concentrations of the MC4R mRNA are found in both the parvocellular and magnocellular neurons, indicating the potential of central (e.g. sympathetic) as well as neuroendocrine mediation of MC4R stimulation effects in this nucleus. PVH TRH-expressing neurons are densely innervated by melanocortin fibers and central intake, thermogenic and cardiovascular effects of TRH are similar to those of MC4R stimulation (52), making this neuropeptide a potential mediator of some of the observed effects of melanocortin signaling. Oxytocin is also expressed in the parvocellular PVH neurons that influence BAT function (24,53); however, interaction of melanocortin with oxytocin has been mainly indicated at the magnocellular division of PVH (54), leaving mediation of effects by oxytocin-expressing neurons unclear. In PBN MC4R are expressed on neurons that are activated (expression of c-fos mRNA) by lipopolysaccharide or lithium chloride treatment and potentially project from the external lateral PBN to the amygdala, relaying to the forebrain information of relevance to certain aspects of sickness behavior (55). Therefore, the anorexic effects of MC4R stimulation in PBN obtained here might be of relevance to acute immune response anorexia and could represent an integral part of this pathway because central blockade of MCRs attenuates acute immune response anorexia (56).
The current data are consistent with the hypothesis that different aspects of energy balance control can be elicited by melanocortin ligand stimulation of a single MCR-bearing nucleus. It remains to be determined, however, whether the same neurons within each nucleus relay signals pertaining to all relevant parameters, e.g. intake, thermogenesis and tachycardia or whether different subpopulations of neurons relay information pertaining to each output parameter separately.
Our results show that there is a certain degree of redundancy in the organization of the central melanocortin system because the same responses can be obtained by stimulation of anatomically disparate MCR-expressing neurons. This idea of redundancy (57) is strengthened by the fact that rats with electrolytic PVH lesions show melanocortin-induced anorexia identical with that of controls (58), indicating that other MCR populations can be used when one is rendered dysfunctional. Although focal PVH MCR stimulation (as shown in the current data) induced energy balance effects, it is not necessary for the physiological MCR output as shown in the PVH lesion result just noted. Redundancy of function can have interesting implications for our understanding of the CNS control of energy balance. For example, characterizing changes in MCR expression in one area of the brain is no longer sufficient for indicating a physiological change in behavior, given the presence of other brain regions that could still mediate the MCR effect with the end result of unaltered physiological outcome (59,60,61). Future studies should therefore benefit from extending their focus to a more distributed melanocortin system when evaluating control of energy balance.
Supplementary Material
Acknowledgments
We thank Amber Alhadeff, Theresa Leichner, Anita Deshpande, Hannah MacAyeal, and Holly Greenwald for their technical assistance and Matt Hayes for his editorial comments.
Footnotes
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Research Grant DK21397 (to H.J.G.) and National Research Service Award NS059254 (to K.P.S.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 23, 2009
For editorial see page 5195
Abbreviations: AHA, Anterior hypothalamic area; ARC, arcuate nucleus; BAT, brown adipose tissue; CNS, central nervous system; HR, heart rate; MCR, melanocortin receptor; MTII, MC3/4R agonist; NTS, nucleus tractus solitarius; PBN, parabrachial nucleus; POMC, proopiomelanocortin; PVH, paraventricular hypothalamic nucleus; RCh, retrochiasmatic area; RVLM, rostral ventrolateral medulla; SNS, sympathetic nervous system; SPA, spontaneous physical activity; TC, core temperature.
References
- Ma L, Tataranni PA, Bogardus C, Baier LJ 2004 Melanocortin 4 receptor gene variation is associated with severe obesity in Pima Indians. Diabetes 53:2696–2699 [DOI] [PubMed] [Google Scholar]
- Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O'Rahilly S 1998 A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet 20:111–112 [DOI] [PubMed] [Google Scholar]
- Cai G, Cole SA, Butte N, Bacino C, Diego V, Tan K, Goring HH, O'Rahilly S, Farooqi IS, Comuzzie AG 2006 A quantitative trait locus on chromosome 18q for physical activity and dietary intake in Hispanic children. Obesity (Silver Spring) 14:1596–1604 [DOI] [PubMed] [Google Scholar]
- Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O'Rahilly S, Farooqi IS 2009 Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med 360:44–52 [DOI] [PubMed] [Google Scholar]
- Murphy B, Nunes CN, Ronan JJ, Hanaway M, Fairhurst AM, Mellin TN 2000 Centrally administered MTII affects feeding, drinking, temperature, and activity in the Sprague-Dawley rat. J Appl Physiol 89:273–282 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM 1998 Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J Neurosci 18:10128–10135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibicka KP, Grill HJ 2008 Energetic responses are triggered by caudal brainstem melanocortin receptor stimulation and mediated by local sympathetic effector circuits. Endocrinology 149:3605–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth MM, Olszewski PK, Yu C, Levine AS, Giraudo SQ 2001 Paraventricular hypothalamic α-melanocyte-stimulating hormone and MTII reduce feeding without causing aversive effects. Peptides 22:129–134 [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB 2005 Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123:493–505 [DOI] [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF 2003 Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol 460:303–326 [DOI] [PubMed] [Google Scholar]
- Voss-Andreae A, Murphy JG, Ellacott KL, Stuart RC, Nillni EA, Cone RD, Fan W 2007 Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology 148:1550–1560 [DOI] [PubMed] [Google Scholar]
- Song CK, Vaughan CH, Keen-Rhinehart E, Harris RB, Richard D, Bartness TJ 2008 Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: neuroanatomical and functional evidence. Am J Physiol Regul Integr Comp Physiol 295:R417–R428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Bowers RR, Bartness TJ, Kaplan JM, Grill HJ 2003 Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology 144:4692–4697 [DOI] [PubMed] [Google Scholar]
- Williams DL, Kaplan JM, Grill HJ 2000 The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology 141:1332–1337 [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Phifer CB, Berthoud HR 2005 Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol 289:R247–R258 [DOI] [PubMed] [Google Scholar]
- Li G, Zhang Y, Rodrigues E, Zheng D, Matheny M, Cheng KY, Scarpace PJ 2007 Melanocortin activation of nucleus of the solitary tract avoids anorectic tachyphylaxis and induces prolonged weight loss. Am J Physiol Endocrinol Metab 293:E252–E258 [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT, Zhang Y 2000 Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res 856:37–47 [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD 1994 Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol 8:1298–1308 [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Wild JM 1998 Melanocortin-4 receptor mRNA expression in the developing autonomic and central nervous systems. Brain Res Dev Brain Res 107:309–314 [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Osaka T 2003 Involvement of the parabrachial nucleus in thermogenesis induced by environmental cooling in the rat. Pflugers Arch 446:760–765 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF 2007 Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 292:R127–R136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M 1997 Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars α demonstrated by iontophoretic application of choleratoxin (subunit b). J Chem Neuroanat 13:1–21 [DOI] [PubMed] [Google Scholar]
- Morrison SF 2003 Raphe pallidus neurons mediate prostaglandin E2-evoked increases in brown adipose tissue thermogenesis. Neuroscience 121:17–24 [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ 2002 The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience 110:515–526 [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, McAllen RM 2005 Interactive drives from two brain stem premotor nuclei are essential to support rat tail sympathetic activity. Am J Physiol Regul Integr Comp Physiol 289:R1107–R1115 [DOI] [PubMed] [Google Scholar]
- Morrison SF 1999 RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. Am J Physiol 276:R962–R973 [DOI] [PubMed] [Google Scholar]
- Rathner JA, McAllen RM 1999 Differential control of sympathetic drive to the rat tail artery and kidney by medullary premotor cell groups. Brain Res 834:196–199 [DOI] [PubMed] [Google Scholar]
- Morrison SF 2001 Differential control of sympathetic outflow. Am J Physiol Regul Integr Comp Physiol 281:R683–R698 [DOI] [PubMed] [Google Scholar]
- Morrison SF 2001 Differential regulation of sympathetic outflows to vasoconstrictor and thermoregulatory effectors. Ann NY Acad Sci 940:286–298 [DOI] [PubMed] [Google Scholar]
- Ter Horst GJ, Postema F 1997 Forebrain parasympathetic control of heart activity: retrograde transneuronal viral labeling in rats. Am J Physiol 273:H2926–H2930 [DOI] [PubMed] [Google Scholar]
- Ter Horst GJ, Hautvast RW, De Jongste MJ, Korf J 1996 Neuroanatomy of cardiac activity-regulating circuitry: a transneuronal retrograde viral labelling study in the rat. Eur J Neurosci 8:2029–2041 [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Koshiya N, Huangfu D, Baraban SC, Stornetta RL, Li YW 1996 Role of medulla oblongata in generation of sympathetic and vagal outflows. Prog Brain Res 107:127–144 [DOI] [PubMed] [Google Scholar]
- Brown DL, Guyenet PG 1985 Electrophysiological study of cardiovascular neurons in the rostral ventrolateral medulla in rats. Circ Res 56:359–369 [DOI] [PubMed] [Google Scholar]
- Kawabe T, Chitravanshi VC, Kawabe K, Sapru HN 2006 Cardiovascular effects of adrenocorticotropin microinjections into the rostral ventrolateral medullary pressor area of the rat. Brain Res 1102:117–126 [DOI] [PubMed] [Google Scholar]
- Brown S, Chitravanshi VC, Sapru HN 2006 Cardiovascular actions of adrenocorticotropin microinjections into the nucleus tractus solitarius of the rat. Neuroscience 143:863–874 [DOI] [PubMed] [Google Scholar]
- Agarwal SK, Calaresu FR 1993 Supramedullary inputs to cardiovascular neurons of rostral ventrolateral medulla in rats. Am J Physiol 265:R111–R116 [DOI] [PubMed] [Google Scholar]
- Miura M, Takayama K 1991 Circulatory and respiratory responses to glutamate stimulation of the lateral parabrachial nucleus of the cat. J Auton Nerv Syst 32:121–133 [DOI] [PubMed] [Google Scholar]
- Mraovitch S, Kumada M, Reis DJ 1982 Role of the nucleus parabrachialis in cardiovascular regulation in cat. Brain Res 232:57–75 [DOI] [PubMed] [Google Scholar]
- Castañeda TR, Jürgens H, Wiedmer P, Pfluger P, Diano S, Horvath TL, Tang-Christensen M, Tschöp MH 2005 Obesity and the neuroendocrine control of energy homeostasis: the role of spontaneous locomotor activity. J Nutr 135:1314–1319 [DOI] [PubMed] [Google Scholar]
- Kotz CM, Teske JA, Billington CJ 2008 Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am J Physiol Regul Integr Comp Physiol 294:R699–R710 [DOI] [PubMed] [Google Scholar]
- Koo BB, Feng P, Dostal J, Strohl KP 2008 α-Melanocyte stimulating hormone and adrenocorticotropic hormone: an alternative approach when thinking about restless legs syndrome? Mov Disord 23:1234–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa JJ, Ghibaudi L, Gao J, Parker EM 2001 Central melanocortin system modulates energy intake and expenditure of obese and lean Zucker rats. Am J Physiol Regul Integr Comp Physiol 281:R444–R451 [DOI] [PubMed] [Google Scholar]
- Tang-Christensen M, Vrang N, Ortmann S, Bidlingmaier M, Horvath TL, Tschöp M 2004 Central administration of ghrelin and agouti-related protein (83–132) increases food intake and decreases spontaneous locomotor activity in rats. Endocrinology 145:4645–4652 [DOI] [PubMed] [Google Scholar]
- Giraudo SQ, Billington CJ, Levine AS 1998 Feeding effects of hypothalamic injection of melanocortin 4 receptor ligands. Brain Res 809:302–306 [DOI] [PubMed] [Google Scholar]
- Berthoud HR BK, Wan S, Patterson LM, Babic T, Townsend RL, Sutton G, Skibicka KP, Butler A, Grill HJ, Travagli RA, Zheng H Brainstem melanocortin signaling in the control of food intake and energy balance. Keystone Symposia, 2008, Banff, Canada p169 (Abstract P110) [Google Scholar]
- Palkovits M, Mezey E, Eskay RL 1987 Pro-opiomelanocortin-derived peptides (ACTH/β-endorphin/α-MSH) in brainstem baroreceptor areas of the rat. Brain Res 436:323–338 [DOI] [PubMed] [Google Scholar]
- Joseph SA, Michael GJ 1988 Efferent ACTH-IR opiocortin projections from nucleus tractus solitarius: a hypothalamic deafferentation study. Peptides 9:193–201 [DOI] [PubMed] [Google Scholar]
- Sim LJ, Joseph SA 1994 Efferents of the opiocortin-containing region of the commissural nucleus tractus solitarius. Peptides 15:169–174 [DOI] [PubMed] [Google Scholar]
- Sim LJ, Joseph SA 1994 Connectivity between POMC systems and brainstem nuclei Regulatory. Peptides 53:S135–S136 [Google Scholar]
- Garza JC, Kim CS, Liu J, Zhang W, Lu XY 2008 Adeno-associated virus-mediated knockdown of melanocortin-4 receptor in the paraventricular nucleus of the hypothalamus promotes high-fat diet-induced hyperphagia and obesity. J Endocrinol 197:471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S, Browning KN, Coleman FH, Sutton G, Zheng H, Butler A, Berthoud HR, Travagli RA 2008 Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J Neurosci 28:4957–4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechan RM, Fekete C 2006 Role of melanocortin signaling in the regulation of the hypothalamic-pituitary-thyroid (HPT) axis. Peptides 27:310–325 [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW 1982 Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol 205:260–272 [DOI] [PubMed] [Google Scholar]
- Caquineau C, Leng G, Guan XM, Jiang M, Van der Ploeg L, Douglas AJ 2006 Effects of α-melanocyte-stimulating hormone on magnocellular oxytocin neurones and their activation at intromission in male rats. J Neuroendocrinol 18:685–691 [DOI] [PubMed] [Google Scholar]
- Paues J, Mackerlova L, Blomqvist A 2006 Expression of melanocortin-4 receptor by rat parabrachial neurons responsive to immune and aversive stimuli. Neuroscience 141:287–297 [DOI] [PubMed] [Google Scholar]
- Huang QH, Hruby VJ, Tatro JB 1999 Role of central melanocortins in endotoxin-induced anorexia. Am J Physiol 276:R864–R871 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Kaplan JM 2002 The neuroanatomical axis for control of energy balance. Front Neuroendocrinol 23:2–40 [DOI] [PubMed] [Google Scholar]
- Dube MG, Kalra SP, Kalra PS 2006 The hypothalamic paraventricular nucleus is not essential for orexigenic NPY or anorexigenic melanocortin action. Peptides 27:2239–2248 [DOI] [PubMed] [Google Scholar]
- Münzberg H, Flier JS, Bjørbaek C 2004 Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145:4880–4889 [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Grill HJ 2009 Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology 150:1705–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münzberg H, Björnholm M, Bates SH, Myers Jr MG 2005 Leptin receptor action and mechanisms of leptin resistance. Cell Mol Life Sci 62:642–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.