Abstract
Transfection of human peripheral blood lymphocytes with DNA from mouse L929 cytoplasts induced proliferation of lymphocytes and the formation of B and T cell-derived cell lines with apparently unlimited growth potential. The cell lines could be grown in serum-containing media as well as in chemically defined serum-free media, have a nearly normal human karyotype, did not form colonies in soft-agar medium, and were not tumorigenic after injection into nude mice. For immortalization of human lymphocytes, DNA from L929 cytoplasts was 100-fold more efficient than L929 nuclear DNA. The ability of cytoplast DNA to immortalize lymphocytes could be consecutively transferred by using total cellular DNA from primary or secondary transfectants. Circular or linear mitochondrial DNA of L929 cells did not lead to immortalization of human lymphocytes. Since DNA with immortalizing activity could be isolated from cytoplasts, the Hirt supernatant, and a mitochondria-depleted cytoplasmic fraction of L929 cells, we conclude that the immortalizing DNA is located extramitochondrially in the cytoplasm of L929 cells.
Full text
PDF
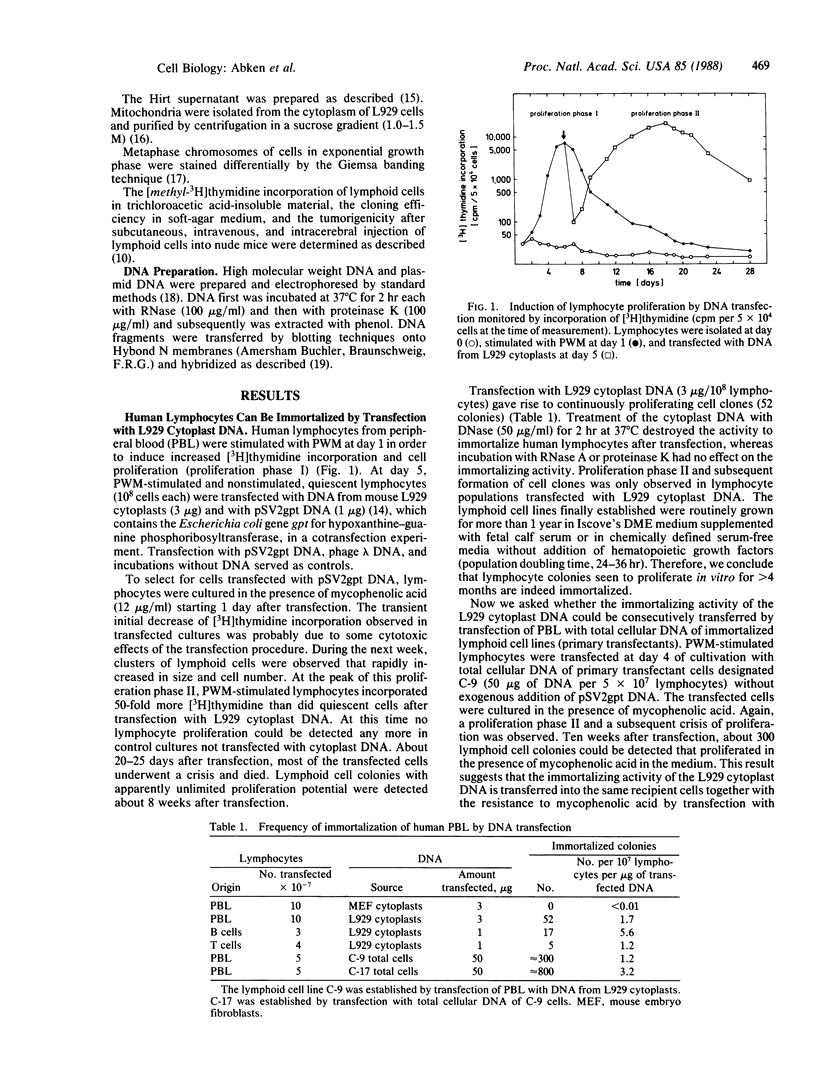
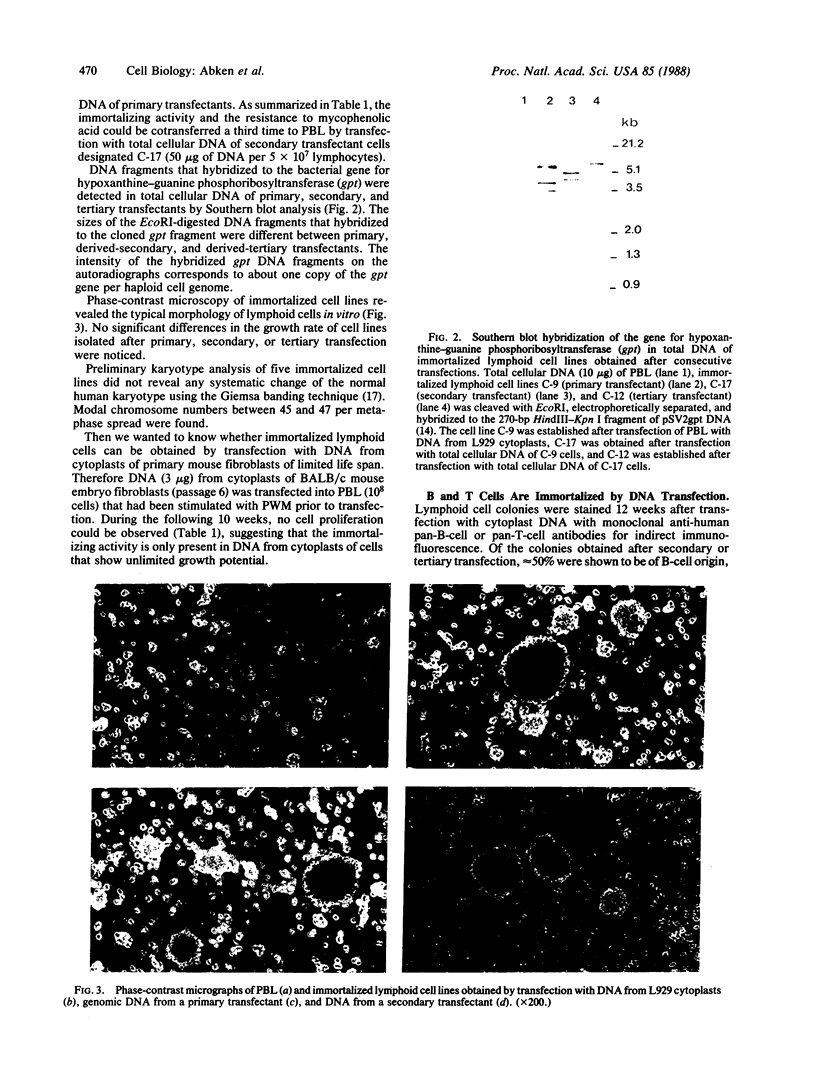


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abken H., Bützler C., Willecke K. Adenovirus type 5 persisting in human lymphocytes is unlikely to be involved in immortalization of lymphoid cells by fusion with cytoplasts or by transfection with DNA of mouse L cells. Anticancer Res. 1987 Jul-Aug;7(4A):553–558. [PubMed] [Google Scholar]
- Abken H., Jungfer H., Albert W. H., Willecke K. Immortalization of human lymphocytes by fusion with cytoplasts of transformed mouse L cells. J Cell Biol. 1986 Sep;103(3):795–805. doi: 10.1083/jcb.103.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A., Kühn B., Fischer J. An unusual accumulation of repetitive sequences in the rat genome. Gene. 1983 Dec;26(2-3):303–306. doi: 10.1016/0378-1119(83)90202-0. [DOI] [PubMed] [Google Scholar]
- Augenlicht L. H., Kobrin D., Pavlovec A., Royston M. E. Elevated expression of an endogenous retroviral long terminal repeat in a mouse colon tumor. J Biol Chem. 1984 Feb 10;259(3):1842–1847. [PubMed] [Google Scholar]
- Bennett K. L., Hastie N. D. Looking for relationships between the most repeated dispersed DNA sequences in the mouse: small R elements are found associated consistently with long MIF repeats. EMBO J. 1984 Feb;3(2):467–472. doi: 10.1002/j.1460-2075.1984.tb01829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D., Clayton D. A. The number of mitochondrial deoxyribonucleic acid genomes in mouse L and human HeLa cells. Quantitative isolation of mitochondrial deoxyribonucleic acid. J Biol Chem. 1974 Dec 25;249(24):7991–7995. [PubMed] [Google Scholar]
- Böyum A. A one-stage procedure for isolation of granulocytes and lymphocytes from human blood. General sedimentation properties of white blood cells in a 1g gravity field. Scand J Clin Lab Invest Suppl. 1968;97:51–76. [PubMed] [Google Scholar]
- Gopal T. V. Gene transfer method for transient gene expression, stable transformation, and cotransformation of suspension cell cultures. Mol Cell Biol. 1985 May;5(5):1188–1190. doi: 10.1128/mcb.5.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jenkins J. R., Rudge K., Currie G. A. Cellular immortalization by a cDNA clone encoding the transformation-associated phosphoprotein p53. Nature. 1984 Dec 13;312(5995):651–654. doi: 10.1038/312651a0. [DOI] [PubMed] [Google Scholar]
- Jenuwein T., Müller R. Structure-function analysis of fos protein: a single amino acid change activates the immortalizing potential of v-fos. Cell. 1987 Feb 27;48(4):647–657. doi: 10.1016/0092-8674(87)90243-1. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Smith L. A., Lueders K. K. Intracisternal A-particle genes in Mus musculus: a conserved family of retrovirus-like elements. Mol Cell Biol. 1981 Mar;1(3):216–227. doi: 10.1128/mcb.1.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lichtman A. H., Reynolds D. S., Faller D. V., Abbas A. K. Mature murine B lymphocytes immortalized by Kirsten sarcoma virus. Nature. 1986 Dec 4;324(6096):489–491. doi: 10.1038/324489a0. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold R. F., Overell R. W. Fibroblast immortality is a prerequisite for transformation by EJ c-Ha-ras oncogene. Nature. 1983 Aug 18;304(5927):648–651. doi: 10.1038/304648a0. [DOI] [PubMed] [Google Scholar]
- Rein A., Keller J., Schultz A. M., Holmes K. L., Medicus R., Ihle J. N. Infection of immune mast cells by Harvey sarcoma virus: immortalization without loss of requirement for interleukin-3. Mol Cell Biol. 1985 Sep;5(9):2257–2264. doi: 10.1128/mcb.5.9.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Wirth T., Kröger B., Horak I. Structure and genomic organization of a new family of murine retrovirus-related DNA sequences (MuRRS). Nucleic Acids Res. 1985 May 24;13(10):3461–3470. doi: 10.1093/nar/13.10.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandidos D. A., Wilkie N. M. Malignant transformation of early passage rodent cells by a single mutated human oncogene. Nature. 1984 Aug 9;310(5977):469–475. doi: 10.1038/310469a0. [DOI] [PubMed] [Google Scholar]
- Stevenson M., Volsky D. J. Activated v-myc and v-ras oncogenes do not transform normal human lymphocytes. Mol Cell Biol. 1986 Oct;6(10):3410–3417. doi: 10.1128/mcb.6.10.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worton R. G., Duff C. Karyotyping. Methods Enzymol. 1979;58:322–344. doi: 10.1016/s0076-6879(79)58148-8. [DOI] [PubMed] [Google Scholar]
- van den Elsen P., de Pater S., Houweling A., van der Veer J., van der Eb A. The relationship between region E1a and E1b of human adenoviruses in cell transformation. Gene. 1982 May;18(2):175–185. doi: 10.1016/0378-1119(82)90115-9. [DOI] [PubMed] [Google Scholar]