Abstract
Elevated levels of cholesterol/low-density lipoprotein (LDL) are a risk factor for the development of nonalcoholic steatohepatitis and its associated hepatic fibrosis. However, underlying mechanisms remain elusive. We previously reported that curcumin induced gene expression of peroxisome proliferator-activated receptor (PPAR)-γ and stimulated its activity, leading to the inhibition of the activation of hepatic stellate cells (HSCs), the major effector cells during hepatic fibrogenesis. We recently showed that curcumin suppressed gene expression of LDL receptor in activated HSCs in vitro by repressing gene expression of the transcription factor sterol regulatory element binding protein-2 (SREBP-2), leading to the reduction in the level of intracellular cholesterol in HSCs and to the attenuation of the stimulatory effects of LDL on HSCs activation. The current study aimed at exploring molecular mechanisms by which curcumin inhibits srebp-2 expression in HSCs. Promoter deletion assays, mutagenesis assays, and EMSAs localize a specificity protein-1 (SP-1) binding GC-box in the srebp-2 promoter, which is responsible for enhancing the promoter activity and responding to curcumin in HSCs. Curcumin suppresses gene expression of SP-1 and reduces its trans-activation activity, which are mediated by the activation of PPARγ. The inhibitory effect of curcumin on SP-1 binding to the GC-box is confirmed by chromatin immuno-precipitation. In summary, our results demonstrate that curcumin inhibits srebp-2 expression in cultured HSCs by activating PPARγ and reducing the SP-1 activity, leading to the repression of ldlr expression. These results provide novel insights into molecular mechanisms by which curcumin inhibits LDL-induced HSC activation.
The activation of PPARγ by curcumin results in the suppression of gene expression of SP-1 and the reduction in its trans-activation activity, leading to the repression of gene expression of sterol regulatory element binding protein-2 in activated hepatic stellate cells.
Obesity is associated with a variety of medical conditions including diabetes, cardiovascular disease, and nonalcoholic fatty liver diseases (1). Nonalcoholic steatohepatitis (NASH) is an advanced form of nonalcoholic fatty liver diseases. Approximately 15–40% of NASH patients develop hepatic fibrosis. Elevated levels of cholesterol are frequently observed in subjects with obesity. However, the role of elevated levels of cholesterol in pathogenesis of NASH-associated hepatic fibrosis remains elusive (2,3,4) .
In circulation, cholesterol is mainly transported by low-density lipoproteins (LDL), and transported into cells via endocytosis mediated by LDL receptor (LDLR). The liver is a major organ that undertakes the turnover of LDLR, most of which are in hepatocytes (5). Sterol regulatory element binding protein (SREBP)-2 is the major transcription factor that controls cellular cholesterol homeostasis by either regulating LDLR gene expression or regulating expression of genes involved in cholesterol biosynthesis. The active form of SREBP-2 is derived from proteolytical cleavage of its membrane-bound precursor upon depletion of cellular sterol. Active SREBP-2 is trafficked into the nucleus and binds to sterol regulatory elements in promoter regions of target genes, thus facilitating transcription of the target genes, including ldlr (6).
Hepatic stellate cells (HSCs) are the major effector cells involved in hepatic fibrogenesis. Upon liver injury, HSCs become active, undergoing phenotypic change from quiescent cells to myofibroblast-like cells. Activated HSCs are characterized by enhanced cell growth and overproduction of extracellular matrix components. Culturing quiescent HSCs on plastic plates causes spontaneous activation, mimicking the process seen in vivo, which provides a good model for elucidating underlying mechanisms of HSC activation and for studying possible therapeutic intervention of the process (7,8).
Curcumin, the yellow pigment in curry from turmeric, has caught much attention as a promising remedy for treatment of multiple disorders (9,10). In animal models, curcumin ameliorates diet-induced hypercholesterolemia and CCl4-induced hepatic fibrosis (11,12). We previously reported that curcumin induced gene expression of peroxisome proliferator-activated receptor (PPAR)-γ in activated HSCs, leading to the inhibition of HSC activation (13,14,15). We recently showed that curcumin suppressed expression of LDLR gene (i.e. ldlr) in activated HSCs in vitro by activating PPARγ and suppressing expression of SREBP-2 gene (i.e. srebp-2), leading to the reduction in the level of cellular cholesterol and to the attenuation of the stimulatory effects of LDL on HSC activation (16). Our results also revealed that the activation of PPARγ mediated the inhibitory effect of curcumin on regulating srebp-2 expression (16). However, how curcumin suppressed srebp-2 expression in activated HSCs remained to be defined. The current study aimed at exploring the underlying mechanisms. This report demonstrated that the activation of PPARγ by curcumin resulted in the suppression of gene expression of the transcription factor specificity protein-1 (SP-1) and the reduction in its trans-activation activity, leading to the repression of gene expression of SREBP-2 in HSCs.
Materials and Methods
Isolation and culture of rodent HSCs and chemicals
Rat HSCs were isolated from male Sprague Dawley rats (200–250 g) as we described previously (13). Similarly, mouse HSCs were isolated from male C57BL/6 mice (20–25 g). Passaged HSCs were grown in DMEM supplemented with 10% (vol/vol) of fetal bovine serum (FBS). Cultured HSCs were used at passage 4–9. In some experiments, cells were cultured in serum-depleted DMEM, containing 0.5% (vol/vol) of FBS for 24 h before treatment. The process of serum starvation made HSCs more sensitive to stimuli. Curcumin (purity >94%) and 15-deoxy-Δ12,14-prostaglandin (PG) J2 were purchased from Sigma (St. Louis, MO).
Western blotting analyses
The experiments were conduced as we previously described (13). The primary rabbit polyclonal anti-SP-1 antibodies (1:300 dilution in use) and secondary antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Densities of bands in Western blotting analyses were normalized with the internal invariable control. Levels of target protein bands were densitometrically determined by using Quantity One 4.4.1 (Bio-Rad Laboratories, Hercules, CA). Variations in the density were expressed as fold changes compared with the control in the blot.
Plasmids, transient transfections, and luciferase activity assays
The mouse srebp-2 promoter luciferase reporter plasmids p-4316-Luc, p-2053-Luc, and p-1019-Luc, respectively, containing 4316, 2053, and 1019 bp nucleotides of the 5′-flanking promoter region of the SREBP-2 gene were generous gifts from Dr. Timothy F. Osborne (Department of Molecular Biology and Biochemistry, University of California, Irvine, Irvine, CA) (17). The SP-1 cDNA expression plasmid and the SP-1 activity luciferase reporter plasmid pAldGCB4-Luc were kindly provided by Dr. Gerald Thiel (Department of Medical Biochemistry and Molecular Biology, University of Saarland Medical Center) (18). The PPARγ cDNA expression plasmid pPPARγ, containing a full size of PPARγ cDNA, was a gift from Dr. Reed Graves (Department of Medicine, University of Chicago, Chicago, IL). The dominant-negative PPARγ cDNA expression plasmid pdn-PPARγ was generously provided by Dr. V. Krishna K. Chatterjee (Department of Medicine, University of Cambridge, Cambridge, UK) (19). Transient transfection and luciferase activity assays were previously described (13). Each treatment had triplicates in every experiment. Each experiment was repeated at least three times. Transfection efficiency was determined by cotransfection of a β-galactosidase reporter, pSV-β-gal (Promega Corp., Madison, WI). Luciferase activities were expressed as relative unit after normalization with ß-galactosidase activities. Results were combined from at least three independent experiments.
Promoter deletion and site-directed mutagenesis
The srebp-2 promoter luciferase reporter plasmid p-1019-Luc was used as a PCR template to generate constructs with various lengths of the gene promoter. The primers used for PCR were the following: the common reverse primer, 5′-GC CTC GAG GCT TGG CGC AGA GTC CCC CG-3′; the forward primers for p-400, 5′-GC GAG CTC GCC AGA AGA AGG CTG GGA CGA GG-3′; p-200, 5′-GC GAG CTC GTG GGC GCG GCC CGG GGC GG-3′; p-150, 5′-GCC GAG CTC GGC GCA GCT CAA ACA TGG-3′; p-100, 5′-GC GAG CTC GGT GGG GGC TGT CGG GTG TCA TGG-3′. The forward primers were tailed with a SacI site (underlined) and the reverse primer was tailed with a XhoI site (underlined). PCR products were subcloned into the vector pGL3(basic) (Promega) at SacI/XhoI sites. The site-directed mutageneses were carried out using the GeneTailor site-directed mutagenesis kit (Invitrogen, Carlsbad, CA) according to the manufacture’s instruction. The sequence of 5′-TTC CGT GGA GTC GGG GCG CAG CTC AAA CAT GG-3′ (−151 to −120), containing the putative SP-1 binding GC-box in the plasmid p-1019-Luc, was mutated to 5′-TTC CGT GGA GTC GTT TTT CAG CTC AAA CAT GG-3′ in the plasmid p-1019-mut-Luc. The mutations (bold) were verified by DNA sequencing.
RNA isolation and real-time PCR
The experiments were conduced as we previously described (20,21). mRNA fold changes of target genes relative to the control of endogenous glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were calculated as suggested by Schmittgen et al. (22). Primers for SP-1 used in real-time PCR were: forward, 5′-AGG TGC ACC AGC TTC CAG GCC TG-3′ and reverse, 5′-CCA GGT CCA TGA AGG CCA AGT TG-3′. Primers for GAPDH were previously described (14).
EMSAs
Single-stranded oligonucleotide probes were biotin-labeled using the biotin 3′ end DNA labeling kit (Pierce Co., Rockford, IL) and annealed to form oligonucleotide duplex after labeling. EMSAs were conducted using LightShift chemiluminescent EMSA kit, following the protocol provided by the manufacturer (Pierce). The sequences of the probes used for EMSAs were presented in the following. P(sp1-wt) contained the putative SP-1 binding GC-box: 5′-TTC CGT GGA GTC GGG GCG CAG CTC AAA CAT GG-3′ (−151/−120); P(sp1-mut) contained the SP-1 binding GC-box with site-directed mutations: 5′-TTC CGT GGA GTC GTT TTT CAG CTC AAA CAT GG-3′. Nuclear extracts were prepared from cultured rat HSCs with or without curcumin treatment (0–30 μm) for 24 h, as we previously described (23). Three micrograms of nuclear extracts of each sample were used in EMSAs.
Chromatin immunoprecipitation (ChIP)
ChIP assays were conducted by following the protocol described by Bonofiglio et al. (24). Briefly, cultured mouse HSCs were cross-linked with 1% formaldehyde in DMEM for 10 min at 37 C. Cells were harvested in Lysis buffer [1% sodium dodecyl sulfate, 10 mm EDTA, 50 mm Tris-HCl (pH 8.0) with protease inhibitor cocktail]. Chromatin in extracts was sheared to fragments at about 500-1000 bp by sonication. Samples with an equal amount of total DNA were analyzed by PCR using the following primers as input controls. The extracts were pretreated with salmon sperm DNA (Sigma), normal rabbit IgG, and protein A/G-conjugated beads (Santa Cruz Biotechnology) at 4 C for 2 h with gentle agitation. The beads were pelleted and discarded. Cross-linked chromatin fragments were immunoprecipitated by using normal rabbit IgG or rabbit anti-SP-1 antibodies (Santa Cruz Biotechnology) and protein A/G-conjugated beads at 4 C overnight. After reversing cross-links by heating at 65 C for 6 h, DNA was isolated and analyzed by PCR. Primers (forward, 5′-GCT GCT GAT CGA TGA CGC ACC ATC-3′ and reverse, 5′-GCT TGG CGC AGA GTC CCC CG-3′) were used to amplify a fragment (312 bp) of the srebp-2 promoter containing the GC-box. PCR products were detected by 2% agarose gel electrophoresis.
Statistical analyses
Differences between means were evaluated by an unpaired two-sided Student’s t test (P < 0.05 considered as significant). Where appropriate, comparisons of multiple treatment conditions with controls were analyzed by ANOVA with the Dunnett’s test for post hoc analysis.
Results
Localization and identification of curcumin response element(s) in the srebp-2 promoter in HSCs
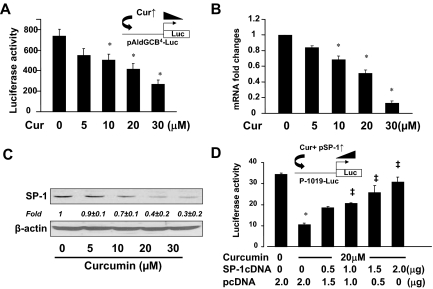
To elucidate underlying mechanisms by which curcumin suppressed the expression of srebp-2, HSCs were transfected with a group of luciferase reporter constructs, respectively, containing 4316, 2053, and 1019 bp nucleotides of the 5′-flanking region of the srebp-2 promoter. After overnight recovery, cells were treated with or without curcumin at 20 μm for 24 h. Luciferase activity assays in Fig. 1A indicated that cells transfected with the three plasmids responded to curcumin by reducing luciferase activities by approximately 80–90%, suggesting that the curcumin response element(s) might be located within the 1019-bp nucleotides of the 5′-flanking promoter region. The srebp-2 promoter region of −1019 bp was further truncated into fragments with −400, −200, −150, and −100 bp nucleotides and subcloned into the vector pGL3(basic). HSCs were transiently transfected with these plasmids and treated with or without curcumin at 20 μm for 24 h. Luciferase activity assays in Fig. 1B showed that compared with no treatment controls, curcumin significantly reduced luciferase activities in most of these cells by approximately 80%, except the cells transfected with the plasmid p-100-Luc as well as the control vector pGL3(basic), which lacked eukaryotic promoter and enhancer sequences. On the other hand, compared with cells transfected with pGL3(basic), cells with p-100-Luc still showed higher luciferase activities at approximately 3.5 × 100 arbitrary units, indicating the presence of the basal promoter activity in the plasmid. However, they did not respond to curcumin. These results suggested that the curcumin response element(s) might be localized within the 5′-flanking promoter region between −150- and −100-bp nucleotides.
Figure 1.
Localization and identification of curcumin (Cur) response element(s) in the srebp-2 promoter in HSCs. Semiconfluent rat HSCs were transiently transfected with a group of luciferase (LUC) reporter plasmids with indicated sizes of the srebp-2 promoter or the vector pGL3(basic). Cells were treated with or without curcumin at 20 μm after transfection. Luciferase activities were expressed as relative units after β-galactosidase normalization (means ± sd; n ≥ 6). The percentages indicated the reduction in luciferase activities in cells treated with curcumin, compared with those in corresponding cells with no treatment. A and B, Luciferase activity assays of cells transfected with plasmids with indicated lengths of the srebp-2 promoter. C, Luciferase activity assays of cells transfected with p-1019 or the mutant (mut) p-1019-mut. Ctr, Control; WT, wild type.
Computer-aided analyses found a GC-box sequence, i.e. GGGGCG, localized at −139 to −134 bp within the promoter. GC-boxes are preferentially bound by the transcription factor SP-1 (25,26,27). To evaluate the role of the putative SP-1 binding GC-box in the curcumin-caused inhibition of the srebp-2 promoter activity, the plasmid p-1019-mut-Luc with site-directed mutations in the GC-box was generated from the parental plasmid p-1019-Luc. HSCs were respectively transfected with the plasmids p-1019-Luc and p-1019-mut-Luc and treated with or without curcumin at 20 μm for 24 h. Luciferase activity assays in Fig. 1C demonstrated that compared with cells transfected with p-1019-Luc, cells transfected with the mutant p-1019-mut-Luc showed a significant reduction in the luciferase activity, even without the curcumin treatment. In addition, cells transfected with p-1019-mut-Luc showed no apparent response to curcumin. However, it is noteworthy that compared with pGL-3(basic), neither the deletion of the GC-box in p-100-Luc nor site-directed mutation of the GC-box in p-1019-mut-Luc could eliminate the basal promoter activity of the gene (Fig. 1, B and C). Cells transfected with these plasmids still showed luciferase activities at approximately 3.5 × 100 arbitrary units (Fig. 1, B and C). However, both of the plasmids did not respond to curcumin. These results suggested that the GC-box in the srebp-2 promoter might be responsible for enhancing the promoter activity as well as responding to the curcumin treatment. Taken together, our results suggested that the SP-1 binding GC-box located at −139 to −134 bp within the srebp-2 promoter was a major curcumin response element and played a critical role in enhancing the activity of the srebp-2 promoter in activated HSCs.
Curcumin reduces the SP-1 trans-activation activity by suppressing the gene expression of SP-1 in HSCs
To evaluate the effect of curcumin on the SP-1 trans-activation activity, rat HSCs were transfected with the SP-1 activity luciferase reporter plasmid pAldGCB4-Luc, which contained four SP-1 binding GC-boxes derived from the aldolase C promoter (18). After recovery, cells were treated with curcumin at various concentrations (0–30 μm) for 24 h. As shown in Fig. 2A by luciferase activity assays, curcumin caused a dose-dependent reduction in luciferase activities, suggesting that curcumin reduced the SP-1 trans-activation activity in HSCs.
Figure 2.
Curcumin reduces the SP-1 trans-activation activity by suppressing the gene expression of SP-1 in HSCs. A, Luciferase activity assays of cells transfected with the SP-1 activity luciferase reporter plasmid pAldGCB4-Luc and treated with curcumin (Cur) for 24 h. The floating schema denoted the luciferase reporter construct pAldGCB4-Luc in use and the application of curcumin to the system. *, P < 0.05 vs. the untreated control (first column). B, Real-time PCR analyses of the steady-state levels of SP-1 mRNA in cells treated with curcumin (n = 3). GAPDH was used as an invariant control for calculating mRNA fold changes. *, P < 0.05 vs. the untreated control (first column). C, Western blotting analyses of SP-1 in cells treated with curcumin at indicated concentrations. β-Actin was used as an invariant control for equal loading. Representative was shown from three independent experiments. Italic numbers beneath the blot were fold changes (mean ± sd) in the densities of the bands compared with the untreated control in the blot (n = 3) after normalization with β-actin. D, Luciferase (Luc) activity assays of cells cotransfected with the srebp-2 promoter luciferase reporter plasmid p-1019-Luc and the SP-1 cDNA expression plasmid pSP-1 at indicated doses. The empty vector pcDNA3 was added to ensure an equal amount of total DNA in each transfection. Cells were treated with or without curcumin at 20 μm for 24 h. Values in all panels were expressed as means ± sd (n ≥ 3). *, P < 0.05 vs. the untreated control (first column); ‡, P < 0.05 vs. cells treated with curcumin only (second column).
To elucidate the underlying mechanisms, we assumed that curcumin might suppress the gene expression of SP-1 in cultured HSCs. To test the assumption, semiconfluent rat HSCs were treated with curcumin at various concentrations (0–30 μm) for 24 h. Total RNA and whole-cell extracts were prepared for real-time PCR and Western blotting analyses. As shown in Fig. 2, B and C, curcumin dose-dependently reduced mRNA levels and the protein contents of SP-1 in the cells. To further validate the role of SP-1 in mediating the inhibitory effect of curcumin on the srebp-2 promoter, HSCs were cotransfected with the srebp-2 promoter luciferase reporter plasmid p-1019-Luc and the SP-1 cDNA expression plasmid pSP-1. A total of 4.5 μg of plasmid DNA per well was used for cotransfection of HSCs in six-well culture plates. It included 2 μg of p-1019-Luc, 0.5 μg of pSV-ß-gal, and 2.0 μg of pSP-1 at indicated doses plus the empty vector pcDNA3. The latter was used to ensure an equal amount of total DNA in transfection assays. After recovery, cells were treated with or without curcumin at 20 μm. Luciferase activity assays in Fig. 2D indicated that compared with the untreated control (the first column), curcumin, as expected, remarkably reduced the luciferase activity (the second well). Cotransfection of pSP-1 dose-dependently eliminated the inhibitory effect of curcumin and increased luciferase activities, suggesting that forced expression of SP-1 cDNA abrogated the inhibitory effect of curcumin on the srebp-2 promoter activity in HSCs. Taken together, these results indicated that curcumin reduced the SP-1 trans-activation activity by suppressing the gene expression of SP-1 in HSCs.
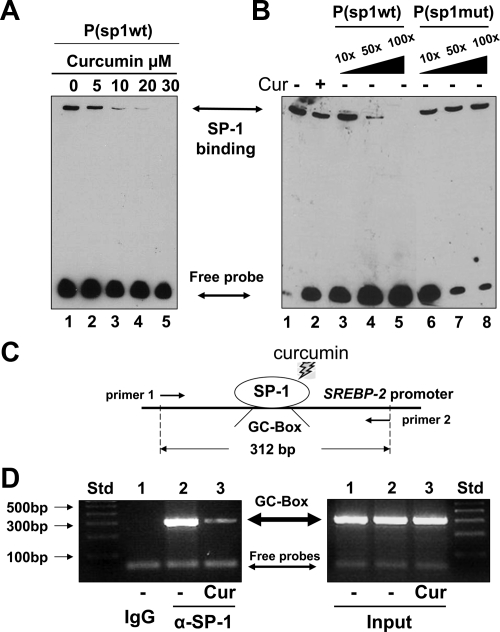
Curcumin significantly reduces the contents of SP-1 binding to the GC-box found in the srebp-2 promoter in intact HSCs
Additional experiments were designed to verify the effect of curcumin on the DNA binding activity of SP-1 to the GC-box found in the srebp-2 promoter. Nuclear extracts were prepared from passaged rat HSCs treated with or without curcumin at indicated concentrations (0–20 μm) for 24 h. EMSAs were conducted using the biotin-labeled oligonucleotide probe P(sp1-wt) containing the SP-1 binding GC-box from the srebp-2 promoter. As shown in Fig. 3A, a clear and strong protein-probe complex was observed in nuclear extracts from HSCs with no treatment (lane 1). The density of the protein-probe complex was dose-dependently reduced by curcumin (lanes 2–5). To examine the DNA binding specificity of the protein to the GC-box-containing probe, competition assays were performed using the biotin-labeled probe P(sp1-wt) and a 10-, 50-, or 100-fold excess of the unlabeled probe P(sp1-wt), or the unlabeled mutant probe P(sp1-mut), containing site-directed mutations in the GC-box. As shown in Fig. 3B, compared with the control (lane 1), curcumin reduced, as expected, the DNA binding activity of the protein to the GC-box-containing probe P(sp1-wt) (lane 2). The increase in the content of the unlabeled probe P(sp1-wt) dose-dependently competed with the labeled P(sp1-wt) for the protein, leading to the reduction in the density of the protein-probe complex (lanes 3–5). However, the same increase in the content of the unlabeled mutant probe P(sp1-mut) showed no competition with the labeled P(sp1-wt) for the DNA binding protein (lanes 6–8). These results collectively indicated that the DNA binding protein specifically bound to the GC-box found in the srebp-2 promoter, which was eliminated by curcumin in HSCs in vitro.
Figure 3.
Curcumin significantly reduces the contents of SP-1 binding to the GC-box found in the srebp-2 promoter in intact HSCs. A and B, Gel shift assays (i.e. EMSAs). Passaged rat HSCs were treated with or without curcumin (Cur) at indicated concentrations for 24 h. EMSAs were conducted by using the biotin-labeled probe P(sp1-wt) containing the GC-box found in the srebp-2 promoter. Representatives were shown from three independent experiments. A, EMSAs of nuclear extracts from cells treated with various concentrations of curcumin. B, Competition EMSAs using a 10-, 50-, or 100-fold excess of the unlabeled P(sp1-wt) (lanes 3–5) or the unlabeled mutant probe P(sp1-mut) (lanes 6–8). C and D, ChIP assays. Cultured mouse HSCs were treated with or without curcumin (20 μm) for 24 h. Cross-linked chromatin fragments were immunoprecipitated by using normal IgG, or anti-SP-1 antibodies (α-SP-1). C, A diagram of the ChIP assays. D, Detection of the GC-box DNA fragment (312 bp) by PCR and 2% agarose gel electrophoresis. Inputs indicated equal DNA contents in samples before immunoprecipitation. A representative of ChIP assays was presented from three independent experiments. Std, Standard.
It is noteworthy that the aforesaid transfection assays and EMSAs of mouse srebp-2 gene expression were conducted in rat HSCs. To make sure what we observed in rat HSCs in vitro could be confirmed in mouse HSCs in vivo and to clarify the DNA binding protein, ChIP assays were performed, as illustrated in Fig. 3C, using intact mouse HSCs. Cultured mouse HSCs were treated with or without curcumin (20 μm) in DMEM with FBS (10%) for 24 h. The same amount of cells was used in ChIP assays, which was normalized by DNA contents and confirmed by PCR, as demonstrated by inputs (Fig. 3D, right panel). Results from ChIP assays revealed that immunoprecipitation of samples from untreated cells with normal IgG did not show the presence of the GC-box-containing DNA fragment (312 bp) (Fig. 3D, left panel, first well). However, immunoprecipitation of the same samples with anti-SP-1 antibodies (α-SP-1) found the DNA fragment at 312 bp (second well). However, α-SP-1 immunoprecipitated a much less amount of the DNA fragment from cells treated with curcumin (third well). These results collectively demonstrated that curcumin significantly reduced the contents of SP-1 binding to the GC-box found in the srebp-2 promoter in intact HSCs.
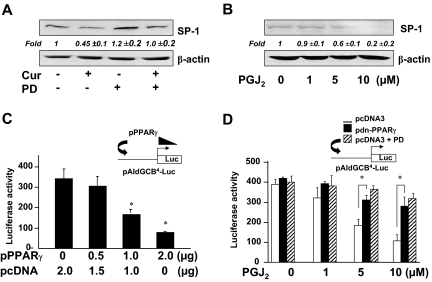
Activation of PPARγ results in the suppression of the gene expression and the activity of SP-1 in activated HSCs
We recently reported that the activation of PPARγ was required for curcumin to inhibit the gene expression of SREBP-2 in cultured HSCs (16). We postulated that the activation of PPARγ might mediate the curcumin-caused repression of the gene expression of SP-1 and its activity, leading to the reduction in the srebp-2 promoter activity and the suppression of srebp-2 expression in activated HSCs. To test the postulation, rat HSCs were pretreated with or without the PPARγ antagonist PD68235 (20 μm) for 30 min before the addition of curcumin (20 μm) for an additional 24 h. Whole-cell extracts were prepared from the cells. As shown in Fig. 4A by Western blotting analyses, compared with the untreated control (first well), curcumin significantly reduced, as expected, the abundance of SP-1 (second well). The blockade of PPARγ activation by the PPARγ antagonist PD68235 apparently attenuated the inhibitory effect of curcumin on the SP-1 abundance in activated HSCs (fourth well), suggesting the requirement of PPARγ activation in the process.
Figure 4.
The activation of PPARγ by curcumin results in the suppression of gene expression and the activity of SP-1 in activated HSCs in vitro. A and B, Western blotting analyses of SP-1. Representatives were from three independent experiments. Italic numbers beneath the blots were fold changes (mean ± sd) in the densities of the bands compared with the untreated control in the blot (n = 3), after normalization with β-actin. A, Passaged rat HSCs were pretreated with PD68235 (PD; 20 μm) for 30 min before the addition of curcumin (Cur; 20 μm) for 24 h. B, Serum-starved rat HSCs were treated with PGJ2 at indicated doses for 24 h. C and D, Luciferase (Luc) activity assays. Values were expressed as means ± sd (n ≥ 6). The insets denoted plasmid constructs cotransfect to the system. C, Rat HSCs were cotransfected with pAldGCB4-Luc and the cDNA expression plasmid pPPARγ at indicated doses. The empty vector pcDNA3 were used to ensure an equal amount of total DNA in each transfection. *, P < 0.05 vs. the control cells (first column). D, Rat HSCs were cotransfected with pAldGCB4-Luc (2 μg) and the dominant-negative PPARγ cDNA expression plasmid pdn-PPARγ (2 μg) or pcDNA3 (2 μg). After recovery, cells were pretreated with or without PD68235 (PD; 20 μm) for 30 min before the addition of PGJ2 at indicated doses for 24 h. *, P < 0.05 vs. corresponding cells transfected with pcDNA3.
To evaluate the role of PPARγ activation in regulating SP-1 expression, rat HSCs were serum starved for 24 h before the stimulation with the natural PPARγ agonist 15-deoxy-Δ12,14-PGJ2 in serum-depleted media for additional 24 h. Although significantly reduced, PPARγ was still detectable in cultured HSCs and responded to PGJ2 (13,28). Serum starvation rendered cells more sensitive to subsequent stimulation (16). Culturing cells in serum-depleted media eliminated the interference from factors in sera. Western blotting analyses in Fig. 4B revealed that PGJ2 dose-dependently reduced the abundance of SP-1 in the cells, suggesting an inhibitory role of PPARγ activation in regulating SP-1 expression.
To assess the inhibitory effect of PPARγ on the SP-1 trans-activation activity, rat HSCs were cotransfected with the SP-1 activity luciferase reporter plasmid pAldGCB4-Luc and the PPARγ cDNA expression plasmid pPPARγ. A total of 4.5 μg of plasmid DNA per well was used for cotransfection of HSCs in six-well culture plates. It included 2 μg of pAldGCB4-Luc, 0.5 μg of pSV-ß-gal, and 2.0 μg of pPPARγ at indicated doses plus the empty vector pcDNA3. The latter was used to ensure an equal amount of total DNA in transfection assays. After recovery, cells were cultured in DMEM with FBS (10%) for 24 h. Prior experiments suggested that 10% of FBS in the media contains enough agonists to activate PPARγ in HSCs (13,14,29). Luciferase activity assays in Fig. 4C indicated that forced expression of PPARγ cDNA dose-dependently reduced the SP-1 trans-activation activity in rat HSCs.
To further evaluate the effect of PPARγ activation by PGJ2 on the SP-1 trans-activation activity, rat HSCs were cotransfected with pAldGCB4-Luc (2 μg) plus the dominant-negative PPARγ cDNA expression plasmid pdn-PPARγ (2 μg) or the empty vector pcDNA3 (2 μg). After recovery, cells were pretreated with or without the PPARγ antagonist PD68235 (20 μm) for 30 min before the addition of PGJ2 (0–10 μm) in serum-depleted media for 24 h. Luciferase activity assays in Fig. 4D indicated that PGJ2 dose-dependently reduced luciferase activities in the cells cotransfected with pcDNA3 (Fig. 4D, white columns), which was eliminated by the PPARγ antagonist PD68235 (Fig. 4D, hatched columns). Additional experiments revealed that cotransfection with pdn-PPARγ dramatically and significantly attenuated the inhibitory effects of PGJ2 on luciferase activities in these cells (Fig. 4D, black columns), compared with those in cells cotransfected with pcDNA3 (Fig. 4D, white columns). These results demonstrated the necessity of functional PPARγ and the involvement of PPARγ pathway in the PGJ2-caused inhibition of the SP-1 trans-activation activity. Results in Fig. 4, C and D, collectively indicated the inhibitory role of PPARγ activation in the SP-1 trans-activation activity in activated HSCs in vitro. Taken together, the results in Fig. 4 revealed that activation of PPARγ resulted in the suppression of gene expression and the trans-activation activity of SP-1 in activated HSCs.
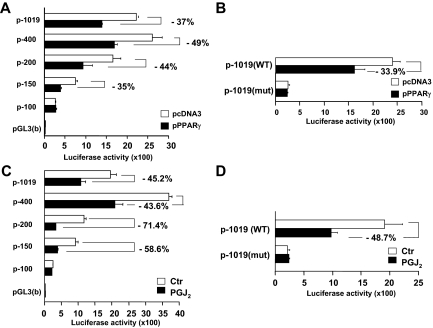
The activation of PPARγ reduces the srebp-2 promoter activity in HSCs likely through the SP-1 binding GC-box in the srebp-2 promoter
Additional experiments were conducted to further test our aforesaid assumption that the activation of PPARγ might mediate the curcumin-caused repression of the gene expression of SP-1 and its activity, leading to the reduction in the srebp-2 promoter activity and to the suppression of srebp-2 expression in activated HSCs. Rat HSCs were cotransfected with the series of luciferase reporter plasmids with the truncated srebp-2 promoter (2 μg) plus the PPARγ cDNA expression plasmid pPPARγ (2 μg) or the empty plasmid pcDNA3 as a control (2 μg). After recovery, cells were cultured in DMEM with FBS (10%) for 24 h. Luciferase activity assays in Fig. 5A demonstrated that compared with cells transfected with pcDNA3, transfection with pPPARγ significantly reduced luciferase activities in cells cotransfected with the plasmids containing −1019, −400, −200, and −150 bp of the promoter region. However, cells cotransfected with the plasmid p-100-Luc lost its response to PPARγ activation. Compared with cells transfected with pGL3(basic), cells with p-100-Luc still showed relatively higher luciferase activities, indicating the presence of the basal promoter activity in the plasmid. These results revealed that the activation of PPARγ by forced expression of PPARγ cDNA inhibited the srebp-2 promoter activity and that its response element(s) might be located within −150 and −100 bp nucleotides of the srebp-2 promoter region, in which the SP-1 binding GC-box was located (Figs. 1–3).
Figure 5.
The activation of PPARγ reduces the srebp-2 promoter activity likely through the SP-1 binding GC-box in HSCs. Rat HSCs were transfected with luciferase reporter plasmids with various sizes of the srebp-2 promoter or the vector pGL3(basic). The percentages indicated the reduction in luciferase activities in cells, compared with those in corresponding control cells. Luciferase activities were expressed as relative units after β-galactosidase normalization (means ± sd; n ≥ 6). A and B, Luciferase activity assays of cells cotransfected with pPPARγ or the empty vector pcDNA3. C and D, Luciferase activity assays of cells treated with or without PGJ2 at 20 μm for 24 h. Ctr, Control; WT, wild type; mut, mutation.
To evaluate the role of the SP-1 binding GC-box in the PPARγ-caused suppression of the promoter activity, HSCs were cotransfected with pPPARγ (2 μg) or pcDNA3 and p-1019-Luc or p-1019-mut-Luc (2 μg), which contained a mutated GC-box. Luciferase activity assays in Fig. 5B showed that compared with cells transfected with pcDNA3, forced expression of PPARγ cDNA significantly reduced luciferase activities in cells cotransfected with the wild-type p-1019-Luc, as expected. However, forced expression of PPARγ cDNA did not show an apparent difference in luciferase activities in cells transfected with the mutant p-1019-mut-Luc. Compared with cells transfected with pGL3(basic), cells transfected with p-1019-mut-Luc still showed the basic promoter activity of the plasmid. The similar results were verified in HSCs transfected with the same set of the srebp-2 luciferase reporter plasmids and subsequently treated with or without PGJ2 (5 μm) in serum-depleted media for 24 h (Fig. 5, C and D). Taken together, these results suggested that the activation of PPARγ inhibited the srebp-2 promoter activity likely through the SP-1 binding GC-box in the srebp-2 promoter in HSCs.
The activation of PPARγ inhibits the interaction between SP-1 and the GC-box in the srebp-2 promoter in intact HSCs
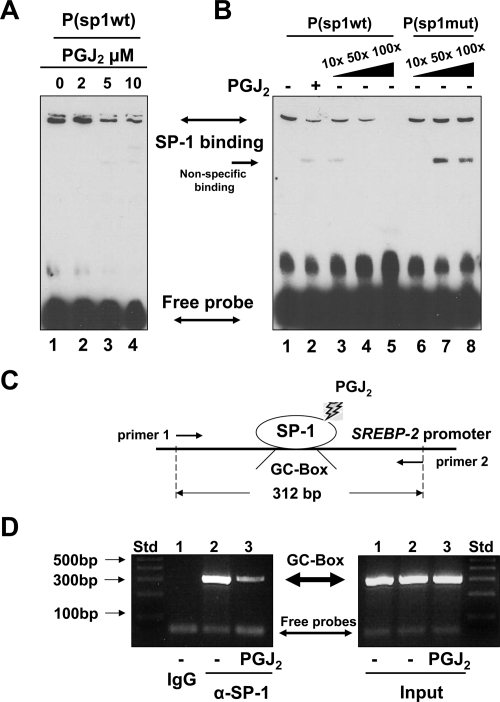
Additional EMSAs were conducted to evaluate the effect of PPARγ activation on the DNA binding activity of SP-1 to the GC-box. Cultured rat HSCs were treated with or without PGJ2 at indicated concentrations (0–10 μm) in serum-depleted media for 24 h. Nuclear protein extracts were prepared for EMSAs using the biotin-labeled P(sp1-wt). As shown in Fig. 6A, PGJ2 dose-dependently reduced the density of the protein-probe complex in EMSAs. Competition assays in Fig. 6B revealed that the density of the protein-probe complex was competitively reduced in a dose-dependent manner by an excess of the unlabeled probe P(sp1-wt) (lanes 3–5) but not by the unlabeled mutant probe P(sp1-mut) (lanes 6–8), suggesting the binding specificity of the protein to the wild-type GC-box-containing probe. These results indicated that the activation of PPARγ reduced the DNA binding activity of the protein to the GC-box found in the srebp-2 promoter.
Figure 6.
The activation of PPARγ inhibits the interaction between SP-1 and the GC-box in intact HSCs. A and B, Gel shift assays (i.e. EMSAs). Passaged rat HSCs were treated with or without PGJ2 at indicated concentrations for 24 h. EMSAs were conducted using the biotin-labeled probe P(sp1-wt). Representatives were shown from three independent experiments. A, EMSAs of nuclear extracts from cells treated with various concentrations of PGJ2. B, Competition EMSAs using a 10-, 50-, or 100-fold excess of the unlabeled P(sp1-wt) (lanes 3–5) or the unlabeled mutant probe P(sp1-mut) (lanes 6–8). C and D, ChIP assays. Cultured mouse HSCs were serum starved for 24 h before the stimulation with or without PGJ2 (5 μm) in serum-free media for an additional 24 h. Cross-linked chromatin fragments were immunoprecipitated by using normal IgG or α-SP-1. C, A diagram of the ChIP assays D, Detection of the GC-box DNA fragment (312 bp) by PCR and 2% agarose gel electrophoresis. Inputs indicated equal DNA contents in samples before immunoprecipitation. A representative of ChIP assays was presented from three independent experiments. Std, Standard.
To verify the effects of PPARγ activation on the srebp-2 promoter in intact HSCs and clarify the DNA binding protein, ChIP assays were conducted as diagrammed in Fig. 6C. Cultured mouse HSCs were serum starved for 24 h before the stimulation with or without PGJ2 (5 μm) in serum-free media for an additional 24 h. The same amount of chromatin DNA in each group of cells was used and evaluated, as inputs, by PCR before immunoprecipitation (Fig. 6D, right panel). ChIP results in the left panel in Fig. 6D demonstrated that immunoprecipitation of samples from untreated cells with normal IgG did not show the presence of the GC-box-containing DNA fragment (312 bp) (Fig. 3D, left panel, first well). However, immunoprecipitation of the same samples with anti-SP-1 antibodies (α-SP-1) found the DNA fragment at 312 bp (second well). However, α-SP-1 immunoprecipitated a much less amount of the DNA fragment from cells treated with curcumin (third well). These results collectively demonstrated that the activation of PPARγ inhibited the interaction between SP-1 and the GC-box found the mouse srebp-2 promoter in intact mouse HSCs.
Discussion
We previously reported that curcumin suppresses LDLR gene expression in activated HSCs in vitro by activating PPARγ and repressing the gene expression of SREBP-2, leading to the reduction in the level of cellular cholesterol and to the attenuation of the stimulatory effects of LDL on the activation of HSCs (16). The current study aimed at exploring the underlying mechanism by which curcumin inhibited the gene expression of SREBP-2. Our results demonstrated that the activation of PPARγ by curcumin resulted in the suppression of the gene expression of SP-1 and the reduction in its trans-activation activity, leading to the repression of srebp-2 expression in activated HSCs.
SREBP-2 plays a critical role in regulating cellular cholesterol homeostasis by regulating expression of a group of genes involved in cholesterol uptake and biosynthesis (6,30,31). Cell surface LDLR is responsible for the uptake of extracellular cholesterol. Its expression is tightly regulated by SREBP-2. Liver LDLR, making up 70% of total body LDLR, plays an essential role in regulating the whole-body cholesterol homeostasis (32). While conducing experiments for this project, a major concern was raised that if the curcumin-caused inhibition of srebp-2 expression in activated HSCs also occurred in other cells, including hepatocytes, it would deteriorate the state of hypercholesterolemia in vivo. Accumulating evidence has indicated that curcumin exhibits distinct regulatory effects on ldlr expression, depending on cell types. In contrast to the inhibition of ldlr expression in cultured HSCs (16), curcumin up-regulated ldlr expression in human hepatoma cell line HepG2 (33), Xenopus laevis oocytes (34), bovine vascular smooth muscle cells (35), and mouse macrophages (36) by inducing SREBP-2 activity. In addition, other studies demonstrated that curcumin has hypocholesterolemic effects by altering expression of genes involved in cholesterol homeostasis. These results suggest that curcumin might exhibit distinct regulatory effects on srebp-2 expression, depending on cell types. Our current studies demonstrated that curcumin suppressed srebp-2 expression by activating PPARγ and reducing SP-1 gene expression, suggesting a novel mechanism of curcumin in regulating the expression of LDLR gene in HSCs.
The transcription factor SP-1, preferably binding to GC-box or GT-box elements, is ubiquitously expressed and regulates expression of target genes during organism development (25,26,27). On the other hand, GC-box could also be bound by other factors, in addition to SP-1 (37,38). EMSA results in Figs. 3 and 6 revealed the inhibitory effects of curcumin and PPARγ activation on the DNA binding activity of a protein to the GC-box found in the srebp-2 promoter. However, additional experiments were required to clarify the DNA binding protein and demonstrate the specific interaction of the protein with the GC-box in intact HSCs. ChIP results in Figs. 3D and 6D demonstrated the specificity of SP-1 to the GC-box and confirmed the inhibitory impact of curcumin and PPARγ activation on the DNA binding activity of SP-1 to the GC-box in intact HSCs. Our results in Figs. 3 and 6 collectively demonstrated the inhibitory role of curcumin in regulating the DNA binding activity of SP-1 and confirmed the specific protein-DNA interaction between SP-1 and the GC-box in intact HSCs.
The current study observed that SP-1 positively regulated the transcription of SREBP-2 gene in cultured HSCs, whereas curcumin repressed the gene expression of SP-1, leading to the down-regulation of srebp-2 expression. Our observations were consistent with others. For instance, curcumin was reported to reduce the gene expression of SP-1 in bladder cancer cells (39). SP-1 was shown to regulate cholesterol homeostasis by directly targeting the LDLR gene promoter and coactivating SREBPs (40,41,42). Several lines of evidence has indicated that SP-1 is a profibrogenic transcription factor and enhances expression of fibrogenic genes in HSCs (43,44). Results in Figs. 3 and 6 showed that curcumin and the activation of PPARγ by PGJ2 apparently resulted in a reduction in the level of SP-1 binding to the GC-box. The reduction might result from the suppression of SP-1 gene expression and/or the PPARγ-SP-1 interaction. We observed that curcumin reduced the SP-1 trans-activation activity by inhibiting the gene expression of SP-1 in activated HSCs in vitro (Fig. 2). The process was mediated by the activation of PPARγ (Fig. 4). On the other hand, PPARγ could cross talk with multiple transcription factors and signaling pathways to regulate biological processes (45,46). It has been reported that PPARγ could directly interact with SP-1 and reduce its DNA binding activity to the promoters of target genes, leading to the negative regulation of its trans-activation activity (47,48,49).
Curcumin is one of the best-studied natural compounds (50,51). However, the underlying mechanisms are largely to be defined. The level of PPARγ is significantly reduced in activated HSCs (29,52,53). We previously reported that curcumin induces expression of endogenous PPARγ in activated HSCs in vitro by interrupting TGF-β signaling (23). There exists an antagonistic relationship between PPARγ activation and TGF-β signaling in HSCs (23). The curcumin-induced expression of PPARγ is likely activated by its agonists present in the media with 10% of FBS (13,14,29). No evidence has indicated curcumin to be a PPARγ agonist. A curcumin receptor in cells has not been reported.
Our results collectively revealed that curcumin suppressed srebp-2 expression in activated HSCs by activating PPARγ and reducing the SP-1 trans-activation activity, leading to the repression of ldlr expression and the elimination of the stimulatory effect of LDL on the activation of HSCs. It bears emphasis that the underlying mechanisms are certainly more complex than what is described here. In addition, our results do not exclude possible involvement of any other mechanisms in the curcumin-caused suppression of srebp-2 expression. Our study provides novel insights into molecular mechanisms by which curcumin inhibits the gene expression of SREBP-2 and LDLR in activated HSCs.
Footnotes
This work was supported by Grant RO1 DK 047995 from National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (to A.C.).
Disclosure Summary: The authors have nothing to declare.
First Published Online October 6, 2009
Abbreviations: ChIP, Chromatin immunoprecipitation; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HSC, hepatic stellate cell; LDL, low-density lipoprotein; LDLR, LDL receptor; NASH, nonalcoholic steatohepatitis; PG, prostaglandin; PPAR, peroxisome proliferator-activated receptor; SP-1, specificity protein-1; SREBP, sterol regulatory element binding protein.
References
- O'Brien PE, Dixon JB 2002 The extent of the problem of obesity. Am J Surg 184:4S–8S [DOI] [PubMed] [Google Scholar]
- Cave M, Deaciuc I, Mendez C, Song Z, Joshi-Barve S, Barve S, McClain C 2007 Nonalcoholic fatty liver disease: predisposing factors and the role of nutrition. J Nutr Biochem 18:184–195 [DOI] [PubMed] [Google Scholar]
- Clark JM 2006 The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol 40(Suppl 1):S5–S10 [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA 2001 Fatty liver and nonalcoholic steatohepatitis. Clin Cornerstone 3:47–57 (Review) [DOI] [PubMed] [Google Scholar]
- Bilheimer DW, Goldstein JL, Grundy SM, Starzl TE, Brown MS 1984 Liver transplantation to provide low-density-lipoprotein receptors and lower plasma cholesterol in a child with homozygous familial hypercholesterolemia. N Engl J Med 311:1658–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL 1997 The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89:331–340 [DOI] [PubMed] [Google Scholar]
- Kisseleva T, Brenner DA 2006 Hepatic stellate cells and the reversal of fibrosis. J Gastroenterol Hepatol 21(Suppl 3):S84–S87 [DOI] [PubMed] [Google Scholar]
- Friedman SL 2008 Mechanisms of hepatic fibrogenesis. Gastroenterology 134:1655–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell MA, Rushworth SA 2008 Curcumin: potential for hepatic fibrosis therapy? Br J Pharmacol 153:403–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishodia S, Sethi G, Aggarwal BB 2005 Curcumin: getting back to the roots. Ann NY Acad Sci 1056:206–217 [DOI] [PubMed] [Google Scholar]
- Arafa HM 2005 Curcumin attenuates diet-induced hypercholesterolemia in rats. Med Sci Monit 11:BR228–BR234 [PubMed] [Google Scholar]
- Fu Y, Zheng S, Lin J, Ryerse J, Chen A 2008 Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol 73:399–409 [DOI] [PubMed] [Google Scholar]
- Xu J, Fu Y, Chen A 2003 Activation of peroxisome proliferator-activated receptor-γ contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am J Physiol Gastrointest Liver Physiol 285:G20–G30 [DOI] [PubMed] [Google Scholar]
- Zheng S, Chen A 2004 Activation of PPARγ is required for curcumin to induce apoptosis and to inhibit the expression of extracellular matrix genes in hepatic stellate cells in vitro. Biochem J 384:149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Chen A 2006 Curcumin suppresses the expression of extracellular matrix genes in activated hepatic stellate cells by inhibiting gene expression of connective tissue growth factor. Am J Physiol Gastrointest Liver Physiol 290:G883–G893 [DOI] [PubMed] [Google Scholar]
- Kang Q, Chen A 2009 Curcumin suppresses expression of low-density lipoprotein (LDL) receptor, leading to the inhibition of LDL-induced activation of hepatic stellate cells. Br J Pharmacol 157:1354–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DJ, Osborne TF 2003 Thyroid hormone regulation and cholesterol metabolism are connected through sterol regulatory element-binding protein-2 (SREBP-2). J Biol Chem 278:34114–34118 [DOI] [PubMed] [Google Scholar]
- Al-Sarraj A, Day RM, Thiel G 2005 Specificity of transcriptional regulation by the zinc finger transcription factors Sp1, Sp3, and Egr-1. J Cell Biochem 94:153–167 [DOI] [PubMed] [Google Scholar]
- Gurnell M, Wentworth JM, Agostini M, Adams M, Collingwood TN, Provenzano C, Browne PO, Rajanayagam O, Burris TP, Schwabe JW, Lazar MA, Chatterjee VK 2000 A dominant-negative peroxisome proliferator-activated receptor γ (PPARγ) mutant is a constitutive repressor and inhibits PPARγ-mediated adipogenesis. J Biol Chem 275:5754–5759 [DOI] [PubMed] [Google Scholar]
- Chen A, Zhang L, Xu J, Tang J 2002 The antioxidant (-)-epigallocatechin-3-gallate inhibits activated hepatic stellate cell growth and suppresses acetaldehyde-induced gene expression. Biochem J 368:695–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumei F, Zhou Y, Zheng S, Chen A 2006 The antifibrogenic effect of (−)-epigallocatechin gallate results from the induction of de novo synthesis of glutathione in passaged rat hepatic stellate cells. Lab Invest 86:697–709 [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW 2000 Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem 285:194–204 [DOI] [PubMed] [Google Scholar]
- Zheng S, Chen A 2007 Disruption of transforming growth factor-β signaling by curcumin induces gene expression of peroxisome proliferator-activated receptor-γ in rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 292:G113–G123 [DOI] [PubMed] [Google Scholar]
- Bonofiglio D, Aquila S, Catalano S, Gabriele S, Belmonte M, Middea E, Qi H, Morelli C, Gentile M, Maggiolini M, Andò S 2006 Peroxisome proliferator-activated receptor-γ activates p53 gene promoter binding to the nuclear factor-κB sequence in human MCF7 breast cancer cells. Mol Endocrinol 20:3083–3092 [DOI] [PubMed] [Google Scholar]
- Hagen G, Müller S, Beato M, Suske G 1992 Cloning by recognition site screening of two novel GT box binding proteins: a family of Sp1 related genes. Nucleic Acids Res 20:5519–5525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga JT, Carner KR, Masiarz FR, Tjian R 1987 Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell 51:1079–1090 [DOI] [PubMed] [Google Scholar]
- Kingsley C, Winoto A 1992 Cloning of GT box-binding proteins: a novel Sp1 multigene family regulating T-cell receptor gene expression. Mol Cell Biol 12:4251–4261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zheng S, Lin J, Zhang QJ, Chen A 2007 The interruption of the PDGF and EGF signaling pathways by curcumin stimulates gene expression of PPARγ in rat activated hepatic stellate cell in vitro. Lab Invest 87:488–498 [DOI] [PubMed] [Google Scholar]
- Miyahara T, Schrum L, Rippe R, Xiong S, Yee Jr HF, Motomura K, Anania FA, Willson TM, Tsukamoto H 2000 Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J Biol Chem 275:35715–35722 [DOI] [PubMed] [Google Scholar]
- Goldstein JL, DeBose-Boyd RA, Brown MS 2006 Protein sensors for membrane sterols. Cell 124:35–46 [DOI] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS 2002 SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 109:1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt NB 1995 Cholesterol homeostasis: role of the LDL receptor. FASEB J 9:1378–1381 [DOI] [PubMed] [Google Scholar]
- Peschel D, Koerting R, Nass N 2007 Curcumin induces changes in expression of genes involved in cholesterol homeostasis. J Nutr Biochem 18:113–119 [DOI] [PubMed] [Google Scholar]
- Fan CL, Qian Y, Wo XD, Yan J, Gao LP 2005 [Effect of curcumin on the gene expression of low density lipoprotein receptors.]. Chin J Integr Med 11:201–204 (Chinese) [DOI] [PubMed] [Google Scholar]
- Liu Y, Hong XQ 2006 [Effect of three different curcumin pigments on the proliferation of vascular smooth muscle cells by ox-LDL and the expression of LDL-R]. Zhongguo Zhong Yao Za Zhi 31:500–503 (Chinese) [PubMed] [Google Scholar]
- Fan C, Wo X, Qian Y, Yin J, Gao L 2006 Effect of curcumin on the expression of LDL receptor in mouse macrophages. J Ethnopharmacol 105:251–254 [DOI] [PubMed] [Google Scholar]
- Philipsen S, Suske G 1999 A tale of three fingers: the family of mammalian Sp/XKLF transcription factors. Nucleic Acids Res 27:2991–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan NY, Khachigian LM 2009 Sp1 Phosphorylation and its Regulation of Gene Transcription. Mol Cell Biol 29:2483–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadalapaka G, Jutooru I, Chintharlapalli S, Papineni S, Smith 3rd R, Li X, Safe S 2008 Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res 68:5345–5354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanikar JN, Sanchez HB, Osborne TF 1997 Promoter selective transcriptional synergy mediated by sterol regulatory element binding protein and Sp1: a critical role for the Btd domain of Sp1. Mol Cell Biol 17:5193–5200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Ngo TT, Athanikar JN, Rosenfeld JM, Osborne TF 1999 Co-stimulation of promoter for low density lipoprotein receptor gene by sterol regulatory element-binding protein and Sp1 is specifically disrupted by the yin yang 1 protein. J Biol Chem 274:13025–13032 [DOI] [PubMed] [Google Scholar]
- Sanchez HB, Yieh L, Osborne TF 1995 Cooperation by sterol regulatory element-binding protein and Sp1 in sterol regulation of low density lipoprotein receptor gene. J Biol Chem 270:1161–1169 [DOI] [PubMed] [Google Scholar]
- García-Ruiz I, de la Torre P, Díaz T, Esteban E, Fernández I, Muñoz-Yagüe T, Solís-Herruzo JA 2002 Sp1 and Sp3 transcription factors mediate malondialdehyde-induced collagen alpha 1(I) gene expression in cultured hepatic stellate cells. J Biol Chem 277:30551–30558 [DOI] [PubMed] [Google Scholar]
- Inagaki Y, Nemoto T, Nakao A, Dijke Pt, Kobayashi K, Takehara K, Greenwel P 2001 Interaction between GC box binding factors and Smad proteins modulates cell lineage-specific α2(I) collagen gene transcription. J Biol Chem 276:16573–16579 [DOI] [PubMed] [Google Scholar]
- Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, Feng D, Zhuo D, Stoeckert Jr CJ, Liu XS, Lazar MA 2008 PPARγ and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev 22:2941–2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrke M, Pascual G, Glass CK, Lazar MA 2005 Gaining weight: the Keystone Symposium on PPAR and LXR. Genes Dev 19:1737–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Necela BM, Su W, Thompson EA 2008 Peroxisome proliferator-activated receptor γ down-regulates follistatin in intestinal epithelial cells through SP1. J Biol Chem 283:29784–29794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara A, Takeuchi K, Uruno A, Ikeda Y, Arima S, Kudo M, Sato K, Taniyama Y, Ito S 2001 Transcriptional suppression of type 1 angiotensin II receptor gene expression by peroxisome proliferator-activated receptor-γ in vascular smooth muscle cells. Endocrinology 142:3125–3134 [DOI] [PubMed] [Google Scholar]
- Sugawara A, Uruno A, Kudo M, Ikeda Y, Sato K, Taniyama Y, Ito S, Takeuchi K 2002 Transcription suppression of thromboxane receptor gene by peroxisome proliferator-activated receptor-γ via an interaction with Sp1 in vascular smooth muscle cells. J Biol Chem 277:9676–9683 [DOI] [PubMed] [Google Scholar]
- Joe B, Vijaykumar M, Lokesh BR 2004 Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr 44:97–111 [DOI] [PubMed] [Google Scholar]
- Singh S, Khar A 2006 Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med Chem 6:259–270 [DOI] [PubMed] [Google Scholar]
- Galli A, Crabb D, Price D, Ceni E, Salzano R, Surrenti C, Casini A 2000 Peroxisome proliferator-activated receptor γ transcriptional regulation is involved in platelet-derived growth factor-induced proliferation of human hepatic stellate cells. Hepatology 31:101–108 [DOI] [PubMed] [Google Scholar]
- Marra F, Efsen E, Romanelli RG, Caligiuri A, Pastacaldi S, Batignani G, Bonacchi A, Caporale R, Laffi G, Pinzani M, Gentilini P 2000 Ligands of peroxisome proliferator-activated receptor γ modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology 119:466–478 [DOI] [PubMed] [Google Scholar]