Abstract
Aged men have a greater incidence of Parkinson’s disease (PD) than women. PD is a neurodegenerative condition associated with the loss of dopamine neurons in the nigrostriatal pathway. This study examined the neurotoxic effects of androgens in a dopaminergic cell line (N27 cells) and the downstream signaling pathways activated by androgens. Treatment of N27 cells with testosterone- and dihydrotestosterone-induced mitochondrial dysfunction, protein kinase C (PKC)-δ cleavage, and apoptosis in dopaminergic neuronal cells. Inhibition of caspase-3 prevented the cleavage of PKCδ from the full-length element to the catalytic fragment and apoptosis in N27 cells, suggesting that androgen-induced apoptosis is mediated by caspase-3-dependent activation of PKCδ. Androgen-induced apoptosis may be specific to dopamine neurons as evidenced by a lack of testosterone-induced apoptosis in GnRH neurons. These results support a neurotoxic consequence of testosterone on dopaminergic neurons and may provide insight into the gender bias found in PD.
Androgens induce apoptosis in a dopaminergic cell line via caspase-3-dependent activation of PKCδ.
Recent studies report sex differences concerning the prevalence of certain neurodegenerative disorders (1,2), such as Parkinson’s disease (PD). Aged men have a greater incidence of PD than aged women (3,4,5,6,7). Clinical characteristics of PD include motor disturbances, such as tremor rigidity and bradykinesia (8,9,10,11). The primary pathological feature of PD is the loss of dopamine neurons within the substantia nigra pars compacta of the midbrain (8,9,11,12,13).
Oxidative stress is involved in mediating this loss of dopamine neurons in PD (6,7,11,14,15). Furthermore, animal models of PD have shown that oxidative stress leads to initiation of the apoptotic pathway (16,17,18,19). Loss of dopamine receptors in the midbrain has been found in both animal models of PD (20) and animals treated with androgens (21). Therefore, it is possible that testosterone may play a role in the progression of PD. Previous reports have shown that testosterone can increase cellular apoptosis (22,23,24). However, the role of testosterone in dopamine neuron death occurring in PD has not been examined.
Apoptosis is a form of controlled cell death (25,26), characterized by cell shrinkage, DNA fragmentation, and chromatin condensation (27,28,29). Apoptosis is associated with the cellular activation of the caspase family of cysteine proteases (25,26). Caspase-3 mediates the execution phase of the apoptosis cycle (28,30,31,32,33). Upon caspase-3 activation, protein kinase C (PKC)-δ is proteolytically cleaved, resulting in its activation within the cytosol and subsequent cell death (32,34,35). Several studies report that PKCδ positively feeds back on caspase-3 activation, thus inducing further apoptosis (36,37,38). PKCδ has also been shown to be a mediator of apoptosis in PD animal models (36,39). Several studies report that PKCδ is responsive to both oxidative stress and testosterone (37,38,40,41). Increased apoptosis via caspase-3 activation in response to oxidative stressors, such as hydrogen peroxide, has been reported in the dopaminergic N27 cell line (7). Testosterone levels in the LNCaP prostate cells were positively correlated with PKCδ levels (41). The likelihood that testosterone directly regulates PKCδ expression is further supported by the existence of a hormone response element for testosterone located 4.7 kb upstream of the transcription start site of the PKCδ promoter region (41).
It is important to note that androgens and its effects on oxidative stress have not been studied within a dopaminergic cell line. Therefore, the purpose of this study was to investigate the role of androgens in PD, using N27 cells as a model to test the neurotoxic effects of these hormones on dopamine neurons. Furthermore, to determine the specificity of androgen-induced neurotoxicity, a nondopaminergic cell line, GT1-7, was included.
Materials and Methods
Reagents
RPMI 1640 medium was purchased from Life Technologies, Inc. (Gaithersburg, MD), and fetal bovine serum was from American Type Culture Collection (Manassas, VA). Testosterone propionate, dihydrotestosterone (DHT), estradiol, and flutamide were from Sigma (St. Louis, MO). Caspase-3/CPP32 inhibitor (Z-DEVD-FMK) was from BioVision Inc. (Mountain View, CA). The Vybrant apoptosis assay kit was from Invitrogen (Eugene, OR), the fluorescent thiol detection kit was from Cell Technology (Mountain View, CA), and the QuantiChrom peroxide assay kit was from Bioassay Systems (Hayward, CA). N-acetyl-cysteine (NAC; A9165) was from Sigma. The rabbit polyclonal PKCδ (sc-213), rabbit polyclonal androgen receptor (sc-815; C19), horseradish peroxidase-conjugated antimouse IgG secondary antibody, and antirabbit IgG secondary antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). The monoclonal mouse β-actin (A5441) antibody was from Sigma-Aldrich. The polyclonal rabbit peroxiredoxin-3 antiserum (ab16830) was from Abcam Inc. (Cambridge, MA).
Cell cultures
Two cell lines were used in this study: N27 and the GT1-7. Both the GT1-7 (42,43,44,45) and the N27 cell lines (Fig. 1A) express androgen receptors. The N27 cell line (a gift from Dr. Curt Freed, University of Colorado Health Science Center, Denver, CO) comprises a homogenous population of tyrosine hydroxylase immortalized cells derived from mesencephalic tissue of 12-d-old rat fetuses, which produce dopamine and its metabolites. These cells express the dopamine transporter, PKC isoforms, and adrenoreceptors (46). However, they do not express dopamine D1 receptors (46), which is consistent with mature substantia nigra dopamine neurons (47,48). Furthermore, grafted N27 cells have been shown to significantly reduce the rate of methamphetamine-induced rotations in unilateral 6-hydroxydopamine lesioned rats (46). This cell line has previously been used as a model to study neurotoxicity (36,49,50). Cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml); grown at 37 C in a 5% CO2 incubator; and subcultured weekly. All experiments were performed between passages 12 and 19 and at approximately 80% confluency in RPMI 1640 serum-free media to avoid confounding variables (hormonal content within serum). Removal of serum does not induce a fasting or deprivation state due to the medium being heavy in glucose, amino acids, and necessary nutrients (51). No significant differences in the percentage of dead cells were found between 1 and 48 h exposure to serum-free media (0.163 ± 0.083 and 0.121 ± 0.113%, respectively) using propidium iodide fluorescence, a measure of cell death. These cells continue to proliferate in a serum-free environment (Cunningham, R. L., unpublished observations).
Figure 1.
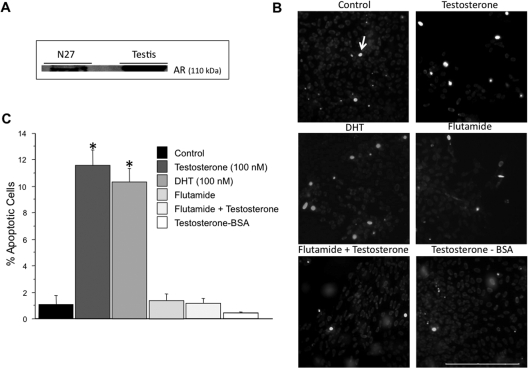
Androgen-induced apoptosis in N27 cells via the classical androgen receptor. N27 cells express androgen receptors. A testis homogenate was used as a positive control (A). Apoptosis was examined using Hoechst 33342. N27 cells were treated with vehicle control (n = 5), testosterone (100 nm, n = 4), flutamide (500 nm, n = 3), flutamide + testosterone (n = 3), DHT (100 nm, n = 4), or T-BSA (100 nm; n = 3). Nuclear staining was visualized by fluorescent microscopy. Apoptotic cells were distinguished by the presence of bright blue nuclei (B). Apoptotic nuclei were quantified from 10 random fields per chamber from each of the independent experiments. The data are expressed as the mean percent of apoptotic cells ± sem (C). Both testosterone and DHT significantly increased apoptosis in N27 cells compared with control. *, P < 0.05. T-BSA did not increase apoptosis. The androgen receptor antagonist, flutamide, inhibited testosterone-induced apoptosis. ANOVA was followed by Student’s PLSD post hoc test. Scale bar, 200 μm. Arrow indicates an apoptotic cell.
The hypothalamic GnRH GT1-7 cell line was a gift from Dr. Xin-Yun Lu (University of Texas Health Science Center, San Antonio, TX). This cell line was maintained in DMEM/F12 media supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) grown at 37 C in a 5% CO2 incubator and subcultured weekly. Experiments were performed in serum-free medium.
Treatments
N27 cells were exposed to physiologically relevant concentrations of androgens (10 or 100 nm), representing the low and high end of the normal range in human males (52,53,54,55,56). Cells were analyzed after 48 h of androgen exposure. The androgens used were the aromatizable androgen, testosterone propionate, the nonaromatizable androgen, DHT, and the nonmembrane permeable androgen, testosterone conjugated to BSA (T-BSA). Androgens were applied either individually or in combination with the androgen receptor antagonist flutamide (500 nm), the caspase-3/CPP32 inhibitor Z-DEVD-FMK (4 μm), or the antioxidant NAC (1 mm). The steroid hormones, testosterone and dihydrotestosterone, along with flutamide were from stock solutions made in ethanol (final concentration of ethanol <0.01%). T-BSA and Z-DEVD-FMK were dissolved in culture media. In this study we mainly used testosterone because this hormone is the most clinically relevant androgen measured in human male sera (57). In addition, aging has been shown to impact testosterone levels in males (58), without affecting DHT levels significantly (59,60). The same treatment paradigm was used with the hypothalamic cell line GT1-7 to determine whether androgens had similar effects in nondopaminergic neurons.
Apoptosis assay
Apoptosis was quantified using the Vybrant apoptosis assay kit no. 5 (Invitrogen) according to the manufacturer’s instructions. Briefly, equal volumes of Hoechst 33342 solution and propidium iodide (1:1000) were added to cells cultured in four-chamber slides, and the mixture was incubated for 30 min on ice. Fluorescence was measured under a fluorescence microscope with a ×20 objective, using filters for 4′,6′-diamino-2-phenylindole (DAPI) and Texas Red dye. The blue fluorescent dye Hoechst 33342 stains chromatin of apoptotic nuclei more vividly than nonapoptotic nuclei, whereas the red fluorescent dye propidium iodide is permeable only to the nuclei of dead cells that have lost plasma membrane integrity. Apoptotic and dead cells were quantified from 10 random fields per chamber in at least three independent experiments. Results are expressed as percentage of apoptotic cells (100 × Hoechst-positive cells/number of cells per field) and dead cells (100 × propidium iodide-positive cells/number of cells per field).
Detection of reduced thiols
The level of reduced thiols in cell lysate (1 × 105 cells) was determined by using the fluorescent thiol detection kit (Cell Technology) according to the manufacturer’s instructions in five independent experiments. This assay uses 2,4-dinitrobenzenesulfonyl fluorescein (dye) as a fluorescent probe for general reduced thiols. Fluorescence was excited at 405 nm and emitted at 525 nm.
Cell lysates
Cells were lysed in lysis buffer [50 mm Tris-HCL (pH 7.5), 100 mm NaCl, 0.1% Triton X-100, 0.1% sodium dodecyl sulfate, 1 mm EDTA] containing protease and phosphatase inhibitor cocktail [1:100 (P8340); Sigma] on ice, and the lysates were clarified by centrifugation at 4 C for 20 min at 13,000 rpm (16,000 × g) in a refrigerated microcentrifuge. Protein concentrations were determined by using the micro-BCA assay (Pierce, Rockford IL) according to the manufacturer’s instructions.
Peroxide assay
Quantichrom peroxide assay kit DIOX-250 (Bioassay System) was used to determine peroxide concentration according to manufacturer’s instructions. Equal amounts (45 μg) of protein were incubated at room temperature with 100 μl of either the working reagent (ammonium iron sulfate/sulfuric acid and xylenol orange) or the control reagent (sulfuric acid and xylenol orange) for 30 min in a 96-well plate. OD was measured using a microplate reader at 585 nm in at least three independent experiments.
Western blot analysis
Equal amounts (20 μg) of protein were separated in a 4–20% polyacrylamide gel, transferred to nitrocellulose membranes (0.2 μm pore size) at 4 C, and blocked for 1 h at room temperature with Tris-buffered saline containing 0.05% Tween 20 [TBST (pH 7.4)] and 5% nonfat dried milk. After washing with TBST, membranes were incubated with specific antibodies at 1:500 concentration overnight at 4 C. Membranes were then washed again with TBST and incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies at 1:2000 concentration for 1 h at room temperature. The protein bands were visualized with an enhanced chemiluminescence’s detection kit (GE Healthcare, Piscataway, NJ) according to the manufacturer’sinstructions. The intensity of the bands was quantified by densitometry using the National Institutes of Health Image J program (Bethesda, MD) in three independent experiments.
Statistical analysis
Statistical analysis was computed by the StatView 5.0 software (SAS Institute, Cary, NC). All data were expressed as mean ± sem and analyzed by a Student’s unpaired t test or an ANOVA for studies with more than two groups followed by Fisher’s protected least significant difference (PLSD) post hoc test. P ≤ 0.05 was designated as significant.
Results
Testosterone increases apoptosis through the classical intracellular androgen receptor
To determine whether androgen exposure elicits apoptosis in dopaminergic N27 neurons, these cells were chronically (48 h) treated with testosterone, the nonaromatizable androgen DHT, the androgen receptor antagonist flutamide, flutamide + testosterone, the nonmembrane permeable androgen T-BSA, or vehicle (control). After androgen treatment, an apoptosis assay that uses the dye Hoechst 33342 as a marker for apoptosis was performed (Fig. 1B). Both testosterone and DHT (100 nm) induced apoptosis, as evidenced by an increase in bright blue fluorescence-labeled cells compared controls (Fig. 1B), whereas testosterone at 10 nm had no effect (data not shown). On the other hand, T-BSA, an androgen that binds only to membrane androgen receptors (61,62), had no effect on apoptosis when used at the same concentration (100 nm) as testosterone (Fig. 1, B and C), as well as at 10 nm or 1 μm (data not shown). Androgen-induced apoptosis was blocked by flutamide (Fig. 1, B and C). Cell death, as expressed as the number of propidium iodide-positive cells per total number of cells per field, was significantly increased by testosterone or DHT (7.502 ± 0.668 and 8.238 ± 1.490%, respectively) compared with controls (0.986 ± 0.461%). The testosterone-induced cell death was reversed by flutamide (1.083 ± 0.142%), which had no effect on cell death when given alone (1.084 ± 0.308%). T-BSA also had no effect (0.473 ± 0.031%).
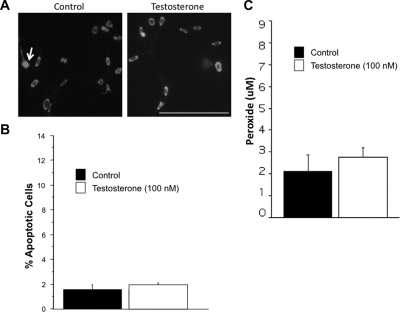
Testosterone increases mitochondrial dysfunction in N27 cells
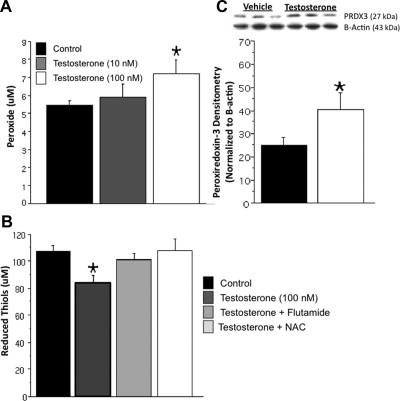
Previous studies suggested that mitochondrial dysfunction promotes neurotoxicity in dopamine cells (63,64,65). Mitochondrial dysfunction was examined by measuring reduced thiol levels and peroxide levels. Testosterone exposure significantly increased peroxide levels (Fig. 2A) and significantly decreased the amount of reduced thiols (Fig. 2B) in dopaminergic neurons compared with vehicle control. Protein expression of peroxiredoxin-3, a mitochondrial antioxidant specific for peroxides, was also significantly increased by testosterone exposure (Fig. 2C). The androgen receptor antagonist flutamide and the antioxidant NAC blocked androgen-induced oxidative stress (Fig. 2B).
Figure 2.
Testosterone-induced mitochondrial dysfunction. The levels of reduced thiols were assayed by using a fluorescent probe for general reduced thiols, and peroxides were assayed with a colorimetric kit in five independent experiments. N27 cells were treated with either testosterone or vehicle control for 48 h. Testosterone (100 nm) significantly increased peroxide concentration (*, P < 0.05), whereas 10 nm testosterone had no effect (A). Testosterone (100 nm) significantly decreased reduced thiols compared with control (*, P < 0.05). The antioxidant NAC significantly blocked testosterone’s effect on reduced thiols, as did the androgen receptor antagonist flutamide (B). To localize the increased peroxide radicals, Western blot analysis of the protein peroxiredoxin-3 (PRDX3), a mitochondrial antioxidant specific for peroxide free radicals, was analyzed in three independent experiments to localize the increased peroxide radicals. Testosterone significantly increased PRDX3 expression (*, P < 0.05) (C). ANOVA was followed by Student’s PLSD post hoc test.
Testosterone activates the PKCδ apoptotic signaling pathway
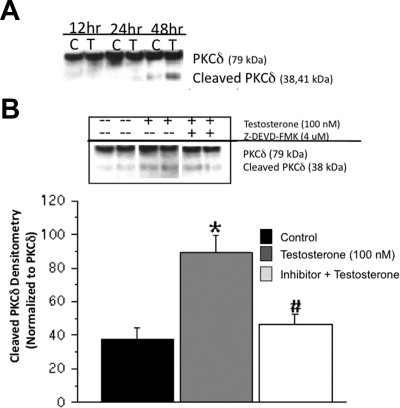
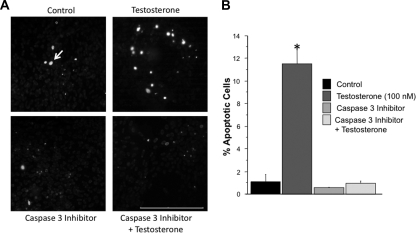
To determine the mechanism of androgen-induced apoptosis in N27 cells, proteolytic activation of PKCδ was measured at 12, 24, and 48 h after testosterone exposure. Testosterone treatment induced cleavage of the full-length PKCδ (72–74 kDa) into the active catalytic fragments (42 and 38 kDa) at 48 h after treatment (Fig. 3A). Because previous studies have shown that dopaminergic apoptosis is caspase-3 dependent (36,38), cells were cotreated with testosterone and the caspase-3-specific inhibitor Z-DEVD-FMK (4 μm). The androgen-induced PKCδ cleavage was significantly inhibited by the Z-DEVD-FMK (Fig. 3B). As shown in Fig. 4, A and B, caspase-3 inhibition also blocked androgen-induced apoptosis in dopamine neurons. Significantly fewer bright blue fluorescent-positive cells were found in the inhibitor + testosterone group compared with the testosterone group, whereas similar levels of blue fluorescent-labeled cells were observed in the control and inhibitor-only groups. Androgen-induced cell death, as measured by propidium iodide fluorescence, was suppressed by Z-DEVD-FMK (0.897 ± 0.227%) compared with controls (0.986 ± 0.461%).
Figure 3.
Testosterone-induced proteolytic cleavage of PKCδ via the caspase-3 pathway. Activation of PKCδ was assayed in N27 cells after testosterone treatment at 12, 24, and 48 h. Western blot analysis was used to determine the catalytic fragments of PKCδ. Testosterone induced PKCδ cleavage at 48 h after treatment (A). Inhibition of caspase-3 by Z-DEVD-FMK (4 μm) significantly blocked testosterone-induced PKCδ proteolysis (#, P < 0.05) (B). ANOVA was followed by Student’s PLSD post hoc test.
Figure 4.
Inhibition of testosterone-induced apoptosis by caspase-3 inhibition in N27 cells. Apoptosis was assessed by using Hoechst 33342. N27 cells were treated with vehicle control (n = 5), testosterone (100 nm, n = 4), the caspase-3 inhibitor Z-DEVD-FMK (4 μm, n = 3), or Z-DEVD-FMK + testosterone (n = 3). Nuclear staining was visualized by fluorescent microscopy. Apoptotic cells were identified by the presence of bright blue-labeled cells. Testosterone significantly increased apoptosis compared with control (*, P < 0.05) (A). Inhibition of caspase-3 blocked testosterone-induced apoptosis (B). ANOVA was followed by Student’s PLSD post hoc test. Scale bar, 200 μm. Arrow indicates an apoptotic cell.
Androgen-induced apoptosis is not a generalized neuronal response
To ascertain whether androgens induce apoptosis in other neuronal cell types, the nondopaminergic cell line GT1-7 was exposed to chronic (48 h) androgen treatment using the same paradigm as described for the N27 cells. Fig. 5A illustrates fluorescent micrographs of Hoechst-33342-labeled GT1-7 neurons. The testosterone and control groups had similar levels of bright blue fluorescent-labeled cells (Fig. 5B), and no differences were found with respect to cell death (testosterone, 1.738 ± 0.141%; control, 2.176 ± 0.107%). GT1-7 cells (2.111 ± 0.782) had lower basal levels of oxidative stress than N27 cells (5.447 ± 0.240) as measured by a peroxide assay. Nevertheless, testosterone did not increase oxidative stress (Fig. 5C).
Figure 5.
Testosterone does not induce apoptosis in the GT1-7 cells line. Androgen-induced apoptosis was examined in a nondopaminergic cell line (GT1-7) using Hoechst 33342 in four independent experiments. GT1-7 cells were treated with either vehicle control or testosterone (100 nm). No differences in bright blue fluorescent-labeled apoptotic cells were found between the testosterone and control groups (A and B). Testosterone did not increase peroxide levels in GT1-7 cells (C). Student’s unpaired t test. Scale bar, 200 μm. Arrow indicates an apoptotic cell.
Discussion
This study shows that physiologically relevant levels of androgens induce neurotoxicity in dopaminergic neurons. The key findings include: 1) testosterone increases apoptosis and cell death in N27 cells via the classical intracellular androgen receptor, 2) androgens increase oxidative stress-induced mitochondrial dysfunction, 3) testosterone induces caspase-3-dependent proteolytic activation of PKCδ, and 4) androgen-induced apoptosis is not a generalized neuronal event. These findings suggest that androgens may contribute to the gender disparities concerning the prevalence of PD (1,2).
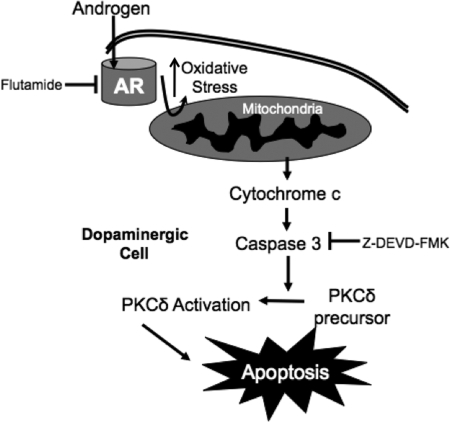
A model for androgen-induced apoptosis in dopaminergic neurons is presented in Fig. 6. This model proposes that androgens may promote dopaminergic cell damage through a cascade that involves compromised mitochondrial function and increased oxidative stress, leading to caspase-3-dependent cleavage (and thus activation) of PKCδ. Upon PKCδ cleavage, DNA fragmentation and apoptosis would result (66). This model suggests that an androgen receptor antagonist can reverse the apoptotic events triggered by androgens. On the other hand, pharmacological inhibition of caspase-3 activity can block the apoptotic effects of PKCδ activation.
Figure 6.
Putative model of androgen-induced apoptosis in N27 cells. Androgens enter the cell, bind to the classical intracellular androgen receptor (AR), and induce oxidative stress leading to mitochondrial dysfunction. Release of cytochrome c from the mitochondria activates the apoptotic caspase cascade, promoting PKCδ activation. Pharmacological inhibition of the androgen receptor by flutamide or of caspase-3 activity by Z-DEVD-FMK blocks androgen-induced apoptosis.
Oxidative stress has been described as an integral component of the pathogenesis of PD (67,68,69). In this study we examined the effects of chronic exposure to androgens on oxidative stress by measuring the levels of reduced thiols and peroxide free-radical species in N27 cells. Reduced thiols can be used as an indirect measure (or sensor) for reactive oxygenated species (ROS). Indeed, one cellular mechanism to counteract increased ROS is the production of the thiol-reducing glutathione and thioredoxin, which maintain the reduction-oxidation homeostasis of the cell (70,71). Thus, decreased reduced thiols are indicative of increased ROS in the cell. Our data indicate that testosterone decreased reduced thiol levels in N27 cells, suggestive of a testosterone-induced increase of ROS. This effect was blocked by the antioxidant NAC. Furthermore, testosterone increased peroxide free-radical species (Fig. 2), which are indicative of increased mitochondrial oxidative stress. These results have led to the formulation of our working model (Fig. 6), suggesting that chronic exposure to testosterone promotes mitochondrial dysfunction, thus further increasing the oxidative stress load of dopamine neurons. Such a cascade of events is consistent with prior studies implicating oxidative stress in the pathogenesis of PD (67,72).
The effects of physiological levels of androgens on dopamine neuron apoptosis have not been examined at time periods longer than 24 h, and only data using supraphysiological levels of testosterone are available (22). Previous studies have shown that 24 h exposure to testosterone at 10 and 100 nm had no effect on dopaminergic SH-SY5Y cell viability (22). These results are in agreement with our study, showing no effect of 24 h androgen exposure in N27 cells. However, longer (48 h) incubation induced apoptosis, suggesting that cell viability is dependent on the length of androgen exposure.
Androgens have been shown to have both neuroprotective (73,74,75) and toxic effects (41), depending on the system and concentration of androgens being studied (73,74). Furthermore, androgens can activate different intracellular pathways, such as the ERK/MAPK pathway that have been implicated in neuroprotection (73,75). On the other hand, physiological levels of androgens have been shown to increase apoptosis in prostate cells (41) and mixed cortical cells in the presence of an aromatase inhibitor (76). Different androgen metabolites or activation of either a putative membrane or the classical intracellular androgen receptor might explain these diverging effects. Testosterone can be converted to estradiol via aromatase, or reduced to DHT through the enzyme, 5α-reductase (77,78,79). Estrogen has consistently been shown to be neuroprotective (80,81,82), and increased dopaminergic apoptosis has been reported in aromatase knockout mice (83). These studies suggest that androgenic mechanisms contribute to dopaminergic apoptosis and estrogenic mechanisms mediate dopaminergic neuroprotection.
A recent study in primary cortical astrocytes showed that apoptosis as a result of androgen exposure might be linked to activation of membrane androgen receptors (23). Interestingly, the apoptotic effects of T-BSA, which does not permeate the plasma membrane, have been shown to decrease Akt phosphorylation in primary cortical astrocytes (signaling pathway involved in cellular protection) and to increase caspase activation (23). Thus, it is possible that the subtype or the location (i.e. membrane vs. nuclear) of the androgen receptor is crucial in determining the protective or toxic properties of testosterone.
Because membrane androgen receptors mediate apoptosis in glial cells, we examined the impact of these receptors on apoptosis in N27 cells. Interestingly, whereas testosterone promoted apoptosis, T-BSA did not, even when applied at equimolar concentrations. On the other hand, the nonaromatizable androgen, DHT, also increased apoptosis, supporting an androgen-mediated mechanism. Lastly, the androgen receptor antagonist, flutamide, blocked the effects of testosterone and DHT. Flutamide has been shown to preferentially block the classical intracellular androgen receptor rather than the membrane androgen receptor and further distinguishes the two receptor mechanisms (23). Therefore, these results show for the first time that androgen-induced apoptosis in dopaminergic neuronal cells occurs via the classical intracellular androgen receptor, rather than a putative membrane androgen receptor that is believed to be pharmacologically distinct.
PKCδ has been shown to be a mediator of apoptosis in animal models of PD (36,39). Previous studies showed that PKCδ is highly expressed in the striatum and substantia nigra (SN) of the brain (32,84). Dopamine neuron loss in the striatum and SN are one of the hallmark features of PD (6,7,11,14,15,20). Also, PKCδ expression increases with age (85), which is a risk factor for PD (3,4,5). Proteolytic activation of PKCδ is involved in the regulation of gene transcription in apoptosis (66). In keeping with these findings, we observed increased PKCδ cleavage in N27 cells after 48 h testosterone exposure, suggesting that the observed apoptosis is PKCδ-mediated.
PKCδ activation can occur through three different mechanisms: 1) membrane translocation of PKCδ, 2) tyrosine phosphorylation, and 3) caspase-3-dependent proteolytic activation (32). Mitochondrial dysfunction resulting from free radicals, increased ROS production, and subsequent cytochrome c release, can activate the caspase-3 pathway (86,87,88). In this study, we showed that testosterone increased oxidative stress and free-radical production and confirmed the involvement of the caspase-3 signaling pathway in these responses. Inhibition of caspase-3 by Z-DEVD-FMK blocked the testosterone-induced PKCδ cleavage and apoptosis in N27 cells, suggesting that androgen-induced apoptosis in these neurons occurs through caspase-3-dependent proteolytic activation of PKCδ. These results are consistent with previous reports of PKCδ-mediated apoptosis in animal models of PD (36,39).
The observation that testosterone did not induce apoptosis in the nondopaminergic GT1-7 cell line suggests that dopaminergic N27 cells are more sensitive to the neurotoxic effects of androgens. One inherent property of dopamine cells is a high degree of ROS formation and oxidative stress due to the catabolism of dopamine itself (89,90,91). Androgen-induced apoptosis in N27 cells may result from an additive effect of testosterone further increasing oxidative stress and free-radical species in a system that already possesses a high oxidative stress load.
Clinical studies show that aged males have a higher incidence of PD (1,2). The onset of clinical symptoms of PD occurs after approximately 60% loss of dopamine neurons within the SN pars compacta (SNc) (8). In rat SN, androgen receptors are mainly expressed in dopaminergic neurons of the SNc and are not expressed in the SN pars reticulata region (92), suggesting a possible role for androgens in rendering these cells more vulnerable than other subpopulations of dopamine neurons (such as those in the ventral tegmental area). Changes in androgen receptor levels along with reciprocal changes in testosterone levels have been found in the aged brain (cortex, hypothalamus, and hippocampus) (93,94,95), although no studies have systematically examined the impact of age and hormone levels on androgen receptor expression in dopamine neurons within the SNc. Nevertheless, age and gender have been shown to alter androgen receptor phosphorylation, thus affecting its function (96). Indeed, the phosphorylation levels of mouse cortical androgen receptors in response to testosterone are higher in aged males than young males and aged or young females (96). If similar changes occur in other brain regions, such as the SNc, phosphorylation (activation) of androgen receptors could contribute to further oxidative stress in dopamine neurons. Conversely, testosterone levels decrease with aging (95,97,98,99,100) and PD (101,102), but testosterone levels continue to remain higher in aged males than females (103). Thus, this persistent difference in testosterone levels could also result in higher oxidative stress in dopamine neurons, which over time, may contribute to the increased risk of PD in males. Further studies are required to confirm this hypothesis and determine whether the relationship between androgen receptor function and testosterone levels contributes to the pathogenesis of PD.
In conclusion, these results demonstrate that androgens increase apoptosis in dopaminergic neurons by inducing mitochondrial dysfunction through increased production of free-radical species and oxidative stress, leading to PKCδ activation.
Acknowledgments
We thank Dr. Meharvan Singh for his insightful comments and technical advice on the manuscript.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant AG-08538 and American Parkinson’s Disease Association (APDA) grant Roger Duvoisin Award (to J.L.R.), NIH GrantNS050401-05A1 (to A.G.), and NIH Grant F32NS061417-01 (to R.L.C.).
Disclosure Summary: A.G. and J.L.R. have nothing to declare. R.L.C. received grant support from 2008 to 2011 from the National Institutes of Health.
First Published Online October 16, 2009
Abbreviations: DHT, Dihydrotestosterone; NAC, N-acetyl-cysteine; PD, Parkinson’s disease; PKC, protein kinase C; PLSD, protected least significant difference; ROS, reactive oxygenated species; SN, substantia nigra; SNc, SN pars compacta; T-BSA, testosterone conjugated to BSA; TBST, Tris-buffered saline containing Tween 20.
References
- Gillies GE, Murray HE, Dexter D, McArthur S 2004 Sex dimorphisms in the neuroprotective effects of estrogen in an animal model of Parkinson’s disease. Pharmacol Biochem Behav 78:513–522 [DOI] [PubMed] [Google Scholar]
- Schrag A, Ben-Shlomo Y, Quinn NP 2000 Cross sectional prevalence survey of idiopathic Parkinson’s disease and Parkinsonism in London. BMJ 321:21–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux R, Marder K, Cote LJ, Denaro J, Hemenegildo N, Mejia H, Tang MX, Lantigua R, Wilder D, Gurland B, et al 1995 The frequency of idiopathic Parkinson’s disease by age, ethnic group, and sex in northern Manhattan, 1988–1993. Am J Epidemiol 142:820–827 [DOI] [PubMed] [Google Scholar]
- Hofman A, Collette HJ, Bartelds AI 1989 Incidence and risk factors of Parkinson’s disease in The Netherlands. Neuroepidemiology 8:296–299 [DOI] [PubMed] [Google Scholar]
- Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM 2003 Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol 157:1015–1022 [DOI] [PubMed] [Google Scholar]
- Hanrott K, Gudmunsen L, O'Neill MJ, Wonnacott S 2006 6-Hydroxydopamine-induced apoptosis is mediated via extracellular auto-oxidation and caspase 3-dependent activation of protein kinase CΔ. J Biol Chem 281:5373–5382 [DOI] [PubMed] [Google Scholar]
- Lotharius J, Brundin P 2002 Pathogenesis of Parkinson’s disease: dopamine, vesicles and α-synuclein. Nat Rev Neurosci 3:932–942 [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S 2003 Parkinson’s disease: mechanisms and models. Neuron 39:889–909 [DOI] [PubMed] [Google Scholar]
- Forno LS 1996 Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol 55:259–272 [DOI] [PubMed] [Google Scholar]
- Mouradian MM 2002 Recent advances in the genetics and pathogenesis of Parkinson disease. Neurology 58:179–185 [DOI] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL 2003 Molecular pathways of neurodegeneration in Parkinson’s disease. Science 302:819–822 [DOI] [PubMed] [Google Scholar]
- Dietz V, Quintern J, Berger W 1981 Electrophysiological studies of gait in spasticity and rigidity. Evidence that altered mechanical properties of muscle contribute to hypertonia. Brain 104:431–449 [DOI] [PubMed] [Google Scholar]
- Shastry BS 2003 Neurodegenerative disorders of protein aggregation. Neurochem Int 43:1–7 [DOI] [PubMed] [Google Scholar]
- Greenamyre JT, Hastings TG 2004 Biomedicine. Parkinson’s—divergent causes, convergent mechanisms. Science 304:1120–1122 [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Faucheux B, Damier P, Mouatt-Prigent A, Agid Y 1997 Neuronal vulnerability in Parkinson’s disease. J Neural Transm Suppl 50:79–88 [DOI] [PubMed] [Google Scholar]
- Heikkila R, Cohen G 1971 Inhibition of biogenic amine uptake by hydrogen peroxide: a mechanism for toxic effects of 6-hydroxydopamine. Science 172:1257–1258 [DOI] [PubMed] [Google Scholar]
- Kumar R, Agarwal AK, Seth PK 1995 Free radical-generated neurotoxicity of 6-hydroxydopamine. J Neurochem 64:1703–1707 [DOI] [PubMed] [Google Scholar]
- Oishi T, Hasegawa E, Murai Y 1993 Sulfhydryl drugs reduce neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the mouse. J Neural Transm Park Dis Dement Sect 6:45–52 [DOI] [PubMed] [Google Scholar]
- Przedborski S, Kostic V, Jackson-Lewis V, Naini AB, Simonetti S, Fahn S, Carlson E, Epstein CJ, Cadet JL 1992 Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J Neurosci 12:1658–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma Jr FJ, Sibley DR 1990 D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–1432 [DOI] [PubMed] [Google Scholar]
- Kindlundh AM, Lindblom J, Bergström L, Wikberg JE, Nyberg F 2001 The anabolic-androgenic steroid nandrolone decanoate affects the density of dopamine receptors in the male rat brain. Eur J Neurosci 13:291–296 [DOI] [PubMed] [Google Scholar]
- Estrada M, Varshney A, Ehrlich BE 2006 Elevated testosterone induces apoptosis in neuronal cells. J Biol Chem 281:25492–25501 [DOI] [PubMed] [Google Scholar]
- Gatson JW, Singh M 2007 Activation of a membrane-associated androgen receptor promotes cell death in primary cortical astrocytes. Endocrinology 148:2458–2464 [DOI] [PubMed] [Google Scholar]
- Hatzoglou A, Kampa M, Kogia C, Charalampopoulos I, TheodoropoulosPA, Anezinis P, Dambaki C, Papakonstanti EA, Stathopoulos EN, Stournaras C, Gravanis A, Castanas E 2005 Membrane androgen receptor activation induces apoptotic regression of human prostate cancer cells in vitro and in vivo. J Clin Endocrinol Metab 90:893–903 [DOI] [PubMed] [Google Scholar]
- Heidenreich KA 2003 Molecular mechanisms of neuronal cell death. Ann NY Acad Sci 991:237–250 [DOI] [PubMed] [Google Scholar]
- Martin SJ, Green DR 1995 Protease activation during apoptosis: death by a thousand cuts? Cell 82:349–352 [DOI] [PubMed] [Google Scholar]
- Choi WS, Canzoniero LM, Sensi SL, O'Malley KL, Gwag BJ, Sohn S, Kim JE, Oh TH, Lee EB, Oh YJ 1999 Characterization of MPP(+)-induced cell death in a dopaminergic neuronal cell line: role of macromolecule synthesis, cytosolic calcium, caspase, and Bcl-2-related proteins. Exp Neurol 159:274–282 [DOI] [PubMed] [Google Scholar]
- Cohen GM 1997 Caspases: the executioners of apoptosis. Biochem J 326(Pt 1):1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivotovsky B 2004 Apoptosis, necrosis and between. Cell Cycle 3:64–66 [PubMed] [Google Scholar]
- Jiang X, Wang X 2004 Cytochrome C-mediated apoptosis. Annu Rev Biochem 73:87–106 [DOI] [PubMed] [Google Scholar]
- Schulz JB, Lindenau J, Seyfried J, Dichgans J 2000 Glutathione, oxidative stress and neurodegeneration. Eur J Biochem 267:4904–4911 [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V 2003 Role of proteolytic activation of protein kinase CΔ in oxidative stress-induced apoptosis. Antioxid Redox Signal 5:609–620 [DOI] [PubMed] [Google Scholar]
- Shi Y 2004 Caspase activation: revisiting the induced proximity model. Cell 117:855–858 [DOI] [PubMed] [Google Scholar]
- Brodie C, Blumberg PM 2003 Regulation of cell apoptosis by protein kinase CΔ. Apoptosis 8:19–27 [DOI] [PubMed] [Google Scholar]
- Kikkawa U, Matsuzaki H, Yamamoto T 2002 Protein kinase CΔ (PKCΔ): activation mechanisms and functions. J Biochem (Tokyo) 132:831–839 [DOI] [PubMed] [Google Scholar]
- Kaul S, Kanthasamy A, Kitazawa M, Anantharam V, Kanthasamy AG 2003 Caspase-3 dependent proteolytic activation of protein kinase CΔ mediates and regulates 1-methyl-4-phenylpyridinium (MPP+)-induced apoptotic cell death in dopaminergic cells: relevance to oxidative stress in dopaminergic degeneration. Eur J Neurosci 18:1387–1401 [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG 2003 Dieldrin induces apoptosis by promoting caspase-3-dependent proteolytic cleavage of protein kinase CΔ in dopaminergic cells: relevance to oxidative stress and dopaminergic degeneration. Neuroscience 119:945–964 [DOI] [PubMed] [Google Scholar]
- Anantharam V, Kitazawa M, Wagner J, Kaul S, Kanthasamy AG 2002 Caspase-3-dependent proteolytic cleavage of protein kinase CΔ is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. J Neurosci 22:1738–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Kaul S, Zhang D, Anantharam V, Kanthasamy AG 2004 Suppression of caspase-3-dependent proteolytic activation of protein kinase CΔ by small interfering RNA prevents MPP+-induced dopaminergic degeneration. Mol Cell Neurosci 25:406–421 [DOI] [PubMed] [Google Scholar]
- Kaul S, Anantharam V, Kanthasamy A, Kanthasamy AG 2005 Wild-type α-synuclein interacts with pro-apoptotic proteins PKCΔ and BAD to protect dopaminergic neuronal cells against MPP+-induced apoptotic cell death. Brain Res Mol Brain Res 139:137–152 [DOI] [PubMed] [Google Scholar]
- Gavrielides MV, Gonzalez-Guerrico AM, Riobo NA, Kazanietz MG 2006 Androgens regulate protein kinase CΔ transcription and modulate its apoptotic function in prostate cancer cells. Cancer Res 66:11792–11801 [DOI] [PubMed] [Google Scholar]
- Mo Q, Lu SF, Hu S, Simon NG 2004 DHEA and DHEA sulfate differentially regulate neural androgen receptor and its transcriptional activity. Brain Res Mol Brain Res 126:165–172 [DOI] [PubMed] [Google Scholar]
- Lu SF, Mo Q, Hu S, Garippa C, Simon NG 2003 Dehydroepiandrosterone upregulates neural androgen receptor level and transcriptional activity. J Neurobiol 57:163–171 [DOI] [PubMed] [Google Scholar]
- Shakil T, Hoque AN, Husain M, Belsham DD 2002 Differential regulation of gonadotropin-releasing hormone secretion and gene expression by androgen: membrane versus nuclear receptor activation. Mol Endocrinol 16:2592–2602 [DOI] [PubMed] [Google Scholar]
- Belsham DD, Evangelou A, Roy D, Duc VL, Brown TJ 1998 Regulation of gonadotropin-releasing hormone (GnRH) gene expression by 5α-dihydrotestosterone in GnRH-secreting GT1-7 hypothalamic neurons. Endocrinology 139:1108–1114 [DOI] [PubMed] [Google Scholar]
- Adams FS, La Rosa FG, Kumar S, Edwards-Prasad J, Kentroti S, Vernadakis A, Freed CR, Prasad KN 1996 Characterization and transplantation of two neuronal cell lines with dopaminergic properties. Neurochem Res 21:619–627 [DOI] [PubMed] [Google Scholar]
- Rao PA, Molinoff PB, Joyce JN 1991 Ontogeny of dopamine D1 and D2 receptor subtypes in rat basal ganglia: a quantitative autoradiographic study. Brain Res Dev Brain Res 60:161–177 [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Mansour A, Healy DJ, Kuehn R, Zhou QY, Bunzow JR, Akil H, Civelli O, Watson Jr SJ 1991 Comparison of the distributions of D1 and D2 dopamine receptor mRNAs in rat brain. Neuropsychopharmacology 5:231–242 [PubMed] [Google Scholar]
- Sun F, Anantharam V, Zhang D, Latchoumycandane C, Kanthasamy A, Kanthasamy AG 2006 Proteasome inhibitor MG-132 induces dopaminergic degeneration in cell culture and animal models. Neurotoxicology 27:807–815 [DOI] [PubMed] [Google Scholar]
- Kaul S, Anantharam V, Yang Y, Choi CJ, Kanthasamy A, Kanthasamy AG 2005 Tyrosine phosphorylation regulates the proteolytic activation of protein kinase CΔ in dopaminergic neuronal cells. J Biol Chem 280:28721–28730 [DOI] [PubMed] [Google Scholar]
- Freshney RI 2005 Serum-free media. In: Culture of animal cells: a manual of basic technique. 5th ed. Chap 10. New York: Wiley-Liss [Google Scholar]
- Organization WH, Nieschlag E, Wang C, Handelsman D, Swerdloff R, Wu F, Einer-Jensen N, Waites G 1992 Guidelines for the use of androgens. Heidelberg: Springer [Google Scholar]
- Smith KW, Feldman HA, McKinlay JB 2000 Construction and field validation of a self-administered screener for testosterone deficiency (hypogonadism) in ageing men. Clin Endocrinol (Oxf) 53:703–711 [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Brune M, Nieschlag E 2002 Vascular reactivity in hypogonadal men is reduced by androgen substitution. J Clin Endocrinol Metab 87:5030–5037 [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Ostrowski NL, Wilson MA 1999 Gender differences in brain and behavior: hormonal and neural bases. Pharmacol Biochem Behav 64:655–664 [DOI] [PubMed] [Google Scholar]
- Mooradian AD, Morley JE, Korenman SG 1987 Biological actions of androgens. Endocr Rev 8:1–28 [DOI] [PubMed] [Google Scholar]
- Need EF, O'Loughlin PD, Armstrong DT, Haren MT, Martin SA, Tilley WD, Wittert GA, Buchanan G 3 March 2009 Serum testosterone bioassay evaluation in a large male cohort. Clin Endocrinol (Oxf) 10.1111/j.1365-2265.2009.03595.x [DOI] [PubMed] [Google Scholar]
- Kaufman JM, Vermeulen A 2005 The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev 26:833–876 [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Kaufman JM, Giagulli VA 1996 Influence of some biological indexes on sex hormone-binding globulin and androgen levels in aging or obese males. J Clin Endocrinol Metab 81:1821–1826 [DOI] [PubMed] [Google Scholar]
- Gray A, Feldman HA, McKinlay JB, Longcope C 1991 Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab 73:1016–1025 [DOI] [PubMed] [Google Scholar]
- Papadopoulou N, Charalampopoulos I, Anagnostopoulou V, Konstantinidis G, Föller M, Gravanis A, Alevizopoulos K, Lang F, Stournaras C 2008 Membrane androgen receptor activation triggers down-regulation of PI-3K/Akt/NF-κB activity and induces apoptotic responses via Bad, FasL and caspase-3 in DU145 prostate cancer cells. Mol Cancer 7:88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexaki VI, Dermitzaki E, Charalampopoulos I, Kampa M, Nifli AP, Gravanis A, Margioris AN, Castanas E 2006 Neuronal differentiation of PC12 cells abolishes the expression of membrane androgen receptors. Exp Cell Res 312:2745–2756 [DOI] [PubMed] [Google Scholar]
- Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, Verna JM 2001 Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol 65:135–172 [DOI] [PubMed] [Google Scholar]
- Jha N, Jurma O, Lalli G, Liu Y, Pettus EH, Greenamyre JT, Liu RM, Forman HJ, Andersen JK 2000 Glutathione depletion in PC12 results in selective inhibition of mitochondrial complex I activity. Implications for Parkinson’s disease. J Biol Chem 275:26096–26101 [DOI] [PubMed] [Google Scholar]
- Merad-Boudia M, Nicole A, Santiard-Baron D, Saillé C, Ceballos-Picot I 1998 Mitochondrial impairment as an early event in the process of apoptosis induced by glutathione depletion in neuronal cells: relevance to Parkinson’s disease. Biochem Pharmacol 56:645–655 [DOI] [PubMed] [Google Scholar]
- Ren J, Datta R, Shioya H, Li Y, Oki E, Biedermann V, Bharti A, Kufe D 2002 p73β is regulated by protein kinase CΔ catalytic fragment generated in the apoptotic response to DNA damage. J Biol Chem 277:33758–33765 [DOI] [PubMed] [Google Scholar]
- Jenner P 2003 Oxidative stress in Parkinson’s disease. Ann Neurol 53(Suppl 3):S26–S36; discussion S36–S38 [DOI] [PubMed] [Google Scholar]
- Gille G, Hung ST, Reichmann H, Rausch WD 2004 Oxidative stress to dopaminergic neurons as models of Parkinson’s disease. Ann NY Acad Sci 1018:533–540 [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Kaul S, Yang Y, Lahiri DK, Anantharam V, Kanthasamy A 2003 Proteolytic activation of proapoptotic kinase PKCΔ is regulated by overexpression of Bcl-2: implications for oxidative stress and environmental factors in Parkinson’s disease. Ann NY Acad Sci 1010:683–686 [DOI] [PubMed] [Google Scholar]
- Gamaley IA, Klyubin IV 1999 Roles of reactive oxygen species: signaling and regulation of cellular functions. Int Rev Cytol 188:203–255 [DOI] [PubMed] [Google Scholar]
- Nakamura H, Nakamura K, Yodoi J 1997 Redox regulation of cellular activation. Annu Rev Immunol 15:351–369 [DOI] [PubMed] [Google Scholar]
- Andersen JK 2004 Oxidative stress in neurodegeneration: cause or consequence? Nat Med 10(Suppl):S18–S25 [DOI] [PubMed] [Google Scholar]
- Ramsden M, Shin TM, Pike CJ 2003 Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience 122:573–578 [DOI] [PubMed] [Google Scholar]
- Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A 2001 Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem 77:1319–1326 [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Yao M, Pike CJ 2005 Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J Neurochem 94:1639–1651 [DOI] [PubMed] [Google Scholar]
- Orlando R, Caruso A, Molinaro G, Motolese M, Matrisciano F, Togna G, Melchiorri D, Nicoletti F, Bruno V 2007 Nanomolar concentrations of anabolic-androgenic steroids amplify excitotoxic neuronal death in mixed mouse cortical cultures. Brain Res 1165:21–29 [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA 2005 Testosterone’s anti-anxiety and analgesic effects may be due in part to actions of its 5α-reduced metabolites in the hippocampus. Psychoneuroendocrinology 30:418–430 [DOI] [PubMed] [Google Scholar]
- Luttge W 1979 Endocrine control of mammalian sexual behavior: an analysis of the potential role of testosterone metabolites. In: Beyer C, ed. Comprehensive endocrinology endocrine control of sexual behavior. New York: Raven Press; 341–364 [Google Scholar]
- Roselli CE, Resko JA 1993 Aromatase activity in the rat brain: hormonal regulation and sex differences. J Steroid Biochem Mol Biol 44:499–508 [DOI] [PubMed] [Google Scholar]
- Bains M, Cousins JC, Roberts JL 2007 Neuroprotection by estrogen against MPP+-induced dopamine neuron death is mediated by ERα in primary cultures of mouse mesencephalon. Exp Neurol 204:767–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Astous M, Morissette M, Di Paolo T 2004 Effect of estrogen receptor agonists treatment in MPTP mice: evidence of neuroprotection by an ERα agonist. Neuropharmacology 47:1180–1188 [DOI] [PubMed] [Google Scholar]
- Morissette M, Jourdain S, Al Sweidi S, Menniti FS, Ramirez AD, Di Paolo T 2007 Role of estrogen receptors in neuroprotection by estradiol against MPTP toxicity. Neuropharmacology 52:1509–1520 [DOI] [PubMed] [Google Scholar]
- Hill RA, Pompolo S, Jones ME, Simpson ER, Boon WC 2004 Estrogen deficiency leads to apoptosis in dopaminergic neurons in the medial preoptic area and arcuate nucleus of male mice. Mol Cell Neurosci 27:466–476 [DOI] [PubMed] [Google Scholar]
- Leibersperger H, Gschwendt M, Gernold M, Marks F 1991 Immunological demonstration of a calcium-unresponsive protein kinase C of the delta-type in different species and murine tissues. Predominance in epidermis. J Biol Chem 266:14778–14784 [PubMed] [Google Scholar]
- Goldberg M, Steinberg SF 1996 Tissue-specific developmental regulation of protein kinase C isoforms. Biochem Pharmacol 51:1089–1093 [DOI] [PubMed] [Google Scholar]
- Lotharius J, Dugan LL, O'Malley KL 1999 Distinct mechanisms underlie neurotoxin-mediated cell death in cultured dopaminergic neurons. J Neurosci 19:1284–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohda Y, Gemba M 2005 Cephaloridine induces translocation of protein kinase CΔ into mitochondria and enhances mitochondrial generation of free radicals in the kidney cortex of rats causing renal dysfunction. J Pharmacol Sci 98:49–57 [DOI] [PubMed] [Google Scholar]
- Yamakawa H, Ito Y, Naganawa T, Banno Y, Nakashima S, Yoshimura S, Sawada M, Nishimura Y, Nozawa Y, Sakai N 2000 Activation of caspase-9 and -3 during H2O2-induced apoptosis of PC12 cells independent of ceramide formation. Neurol Res 22:556–564 [DOI] [PubMed] [Google Scholar]
- Cadet JL, Brannock C 1998 Free radicals and the pathobiology of brain dopamine systems. Neurochem Int 32:117–131 [DOI] [PubMed] [Google Scholar]
- Choi HJ, Kim SW, Lee SY, Hwang O 2003 Dopamine-dependent cytotoxicity of tetrahydrobiopterin: a possible mechanism for selective neurodegeneration in Parkinson’s disease. J Neurochem 86:143–152 [DOI] [PubMed] [Google Scholar]
- Guillot TS, Shepherd KR, Richardson JR, Wang MZ, Li Y, Emson PC, Miller GW 2008 Reduced vesicular storage of dopamine exacerbates methamphetamine-induced neurodegeneration and astrogliosis. J Neurochem 106:2205–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF 1997 Selective colocalization of immunoreactivity for intracellular gonadal hormone receptors and tyrosine hydroxylase in the ventral tegmental area, substantia nigra, and retrorubral fields in the rat. J Comp Neurol 379:247–260 [DOI] [PubMed] [Google Scholar]
- Kumar RC, Thakur MK 2004 Androgen receptor mRNA is inversely regulated by testosterone and estradiol in adult mouse brain. Neurobiol Aging 25:925–933 [DOI] [PubMed] [Google Scholar]
- Kerr JE, Allore RJ, Beck SG, Handa RJ 1995 Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology 136:3213–3221 [DOI] [PubMed] [Google Scholar]
- Wu D, Lin G, Gore AC 2009 Age-related changes in hypothalamic androgen receptor and estrogen receptor α in male rats. J Comp Neurol 512:688–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur MK, Asaithambi A, Mukherjee S 2000 Synthesis and phosphorylation of androgen receptor of the mouse brain cortex and their regulation by sex steroids during aging. Mol Cell Biochem 203:95–101 [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR 2001 Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 86:724–731 [DOI] [PubMed] [Google Scholar]
- Downs JL, Urbanski HF 2006 Aging-related sex-dependent loss of the circulating leptin 24-h rhythm in the rhesus monkey. J Endocrinol 190:117–127 [DOI] [PubMed] [Google Scholar]
- Roselli CE, Kaler LW, Resko JA 1986 Hypothalamic aromatase activity in young and old male rats. Neurobiol Aging 7:121–125 [DOI] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ 2007 Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol 19:743–751 [DOI] [PubMed] [Google Scholar]
- Okun MS, McDonald WM, DeLong MR 2002 Refractory nonmotor symptoms in male patients with Parkinson disease due to testosterone deficiency: a common unrecognized comorbidity. Arch Neurol 59:807–811 [DOI] [PubMed] [Google Scholar]
- Ready RE, Friedman J, Grace J, Fernandez H 2004 Testosterone deficiency and apathy in Parkinson’s disease: a pilot study. J Neurol Neurosurg Psychiatry 75:1323–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova N, Lindau ST 2009 Salivary sex hormone measurement in a national, population-based study of older adults. J Gerontol B Psychol Sci Soc Sci 10.1093/geromb/gbn028 [DOI] [PMC free article] [PubMed] [Google Scholar]