Abstract
The GnRH decapeptide controls reproductive function through its release from neuroendocrine terminals in the median eminence, a site where there is a convergence of numerous nerve terminals and glial cells. Previous work showed dynamic changes in the GnRH-glial-capillary network in the median eminence under different physiological conditions. Because aging in rats is associated with a diminution of GnRH release and responsiveness to estradiol feedback, we examined effects of age and estradiol treatment on these anatomical interactions. Rats were ovariectomized at young (4 months), middle-aged (11 months), or old (22–23 months) ages, allowed 4 wk to recover, and then treated with vehicle or estradiol for 72 h followed by perfusion. Immunofluorescence of GnRH was measured, and immunogold electron microscopic analyses were performed to study the ultrastructural properties of GnRH neuroterminals and their microenvironment. Although the GnRH immunofluorescent signal showed no significant changes with age and estradiol treatment, we found that the median eminence underwent both qualitative and quantitative structural changes with age, including a disorganization of cytoarchitecture with aging and a decrease in the apposition of GnRH neuroterminals to glia with age and estradiol treatment. Thus, although GnRH neurons can continue to synthesize and transport peptide, changes in the GnRH neuroterminal-glial-capillary machinery occur during reproductive senescence in a manner consistent with a disconnection of these elements and a potential dysregulation of GnRH neurosecretion.
GnRH neuroterminals undergo substantial changes in their ultrastructural properties, and in their neural-glial relationships, during reproductive aging.
The GnRH decapeptide secreted at the base of the hypothalamus in the median eminence is critically involved in the control of reproductive function. The biological rhythms of GnRH release are responsible for pubertal development and the maintenance of adult reproductive function, but the role of GnRH in reproductive senescence is less clear (reviewed in Refs. 1 and 2). This process of reproductive aging in many female mammals, including humans and rats, is characterized by a gradual transition from regular reproductive cycles to irregular cycles to eventual acyclicity, and concomitant loss of fertility (3,4). A role of the hypothalamic GnRH neurons in this process is difficult to discern because all three levels of the hypothalamic-pituitary-gonadal axis change during aging, and the causes and consequences are not easily distinguishable. Nevertheless, there is evidence that GnRH neurons undergo age-related changes in biosynthesis, processing, and release of the GnRH decapeptide before reproductive failure, suggesting a contributory role of GnRH cells to reproductive failure, at least in rodents (reviewed in Ref. 2).
Neurosecretory terminals of hypothalamic releasing hormone neurons, including GnRH, are concentrated in the pericapillary region of the median eminence. This region is abundant in nerve terminals and glia, yet it contains very few synaptic contacts (5,6,7). Therefore, the mechanism for communication among neuroterminals in this region, including the coordination of pulses of GnRH release, is thought to involve volume (or nonsynaptic) transmission within the median eminence (5,8), a process that would enable communication among nerve terminals and glia through their release of neuroactive substances into the extracellular space. With regard to the neuroterminal-glial relationship, it was reported that glia of the median eminence may retract to expose the neuroterminal to both extracellular regulatory factors as well as to the portal vessels (9). This close relationship changes under different hormonal conditions (10,11), but little is known about how it may change during aging.
Previously, we reconstructed GnRH terminals from young (∼5 months) and old (∼24 months) ovariectomized rats in three dimensions, thereby revealing novel features of these cells and their microenvironment (12). Here, we extended that previous study by using both descriptive and quantitative measures in three age groups (young, middle-aged, and old), and two hormone treatments (estradiol or vehicle) at the light and electron microscopic level. In all cases, we related our microscopic analyses to the age and hormonal status of the animal. Together, these studies provide a novel morphological approach to understanding the hypothalamic changes that occur during reproductive aging.
Materials and Methods
Experimental animals
Female Sprague Dawley rats were purchased from a colony derived from the Harlan Sprague Dawley line (Houston, TX) and born and bred at the Animal Resources Center at the University of Texas at Austin. Rats were housed two per cage in a room with controlled temperature (∼21 C) and light cycles (12-h light, 12-h dark cycle, lights on at 0700 h). Food and water were available ad libitum. All animal experiments were performed using protocols approved by the Institutional Animal Care and Use Committee at the University of Texas at Austin following guidelines from The Guide for the Care and Use of Experimental Animals. Rats were assigned to use at one of three ages: young (∼4 months, n = 16), middle-aged (∼11 months, n = 16), and old (∼22 months, n = 16).
Surgical procedures
Rats at one of three ages, young (∼4 months), middle-aged (∼11 month), or old (∼22–23 months), were bilaterally ovariectomized under isoflurane anesthesia and allowed to recover for 4 wk (13,14,15). Capsules filled with 17β-estradiol (5% in cholesterol) or 100% cholesterol (vehicle) were soaked in saline for 24 h and then sc implanted into isoflurane-anesthetized rats. Seventy-two hours later, rats were anesthetized (0900–1100 h). Before perfusion, we collected 3 ml blood from the left ventricle. Serum was separated by centrifugation at 4000 rpm for 8 min and aliquoted for LH and estradiol assays. Rats were transcardially perfused with 0.1 m phosphate buffer (pH 7.4, 50 ml), followed by 4% paraformaldehyde and 0.125% glutaraldehyde in phosphate buffer (500 ml) (16,17). After perfusion, the pituitary was removed, postmortem pituitary weight was measured, and uterine diameter was recorded.
Tissue preparation
The brain was removed and postfixed in 4% paraformaldehyde overnight at 4 C and sectioned on a vibrating blade microtome (Leica VT1000S, Bannockburn, IL). We collected alternating sections of 40 μm thickness for light microscopy and 100 μm for electron microscopy through the entire median eminence region. Further procedures were identical to those described previously (12) and are presented here in brief. Sections for electron microscopy were cryoprotected by immersion in glycerol. Differential interference contrast images were taken for morphological orientation and comparison. A fragment of about 1 mm2 in area was excised from the lateral median eminence under a dissecting microscope (Leica S6D stereomicroscope, Heerbrugg, Switzerland), and rapidly plunged into liquid propane cooled to −180 C in a cryofixation system (Leica CPC, Vienna, Austria). Cryofixed tissue was then transferred to an automatic freeze-substitution system unit (Leica AFS, Vienna), infiltrated with Lowicryl HM20 resin (Electron Microscopy Sciences, Fort Washington, PA), and polymerized.
Fluorescence immunohistochemistry, microscopy, and analysis
One 40-μm section was chosen at the rostral, middle, and caudal levels of the median eminence of each rat for a total of three sections per rat for fluorescence immunohistochemistry. Sections were washed in PBS (0.012 m PO4; 0.154 m NaCl, pH 7.3), incubated in 10% normal goat serum for 1 h, and then incubated with mouse anti-GnRH primary antibody (HU11b at 1:500, kindly provided by Dr. H. F. Urbanski) for 3 d and then Texas red goat antimouse IgG secondary antibody (1:400, Vector Laboratories, Burlingame, CA) for 2 h. The primary antibody was extensively characterized by Dr. Urbanski who used RIA to show the specificity of HU11b to the bioactive GnRH molecule but not to the GnRH free acid, the precursor molecule, or other neuropeptides (7,18). The primary antibody has been tested in preadsorption control experiments in Urbanski’s and our laboratory, with no labeling found in those conditions (7,12,18). To analyze the localization and intensity of GnRH processes and neuroterminals, images were captured using a fluorescence microscope (Leica DM IRBE, Heidelberg, Germany) with a digital camera (Leica DFC 300), using the same settings. Quantification was performed using MetaMorph imaging software (MetaMorph 6.1). The fluorescent labeling of GnRH processes and neuroterminals from each image was thresholded at the same level, and the integrated number for each image was used for statistical analysis. The GnRH fluorescent signals were lower in the rostral sections compared with the caudal sections; to quantify fluorescent signals in a linear range, we used the caudal median eminence sections to set up the threshold settings and to eliminate saturation. Statistical analyses were performed using SPSS version 13.0 for Windows. The GnRH average intensity and distribution were compared for effects of age and hormone using two-way ANOVA. An effect was considered significant at P < 0.05.
Transmission electron microscopy tissue preparation and immunocytochemistry
Due to the intensive labor for electron microscopy preparation, we randomly chose embedded tissue from five rats from each of the six groups (total 30 rats) for ultrathin sectioning. Based on immunofluorescence results showing the greatest intensity of GnRH labeling in the caudal median eminence, this region was selected for all further electron microscopy procedures. Tissue blocks were trimmed on an ultramicrotome (Leica EM UC6, Vienna). Semithin sections (400 nm) stained with 2% toluidine blue were examined under a transmitted light microscope (Leica DMLB compound microscope, Germany) with Leica DFC digital color camera for morphological orientation. Three sets of serial ultrathin sections (70–80 nm thick) cut with a diamond knife (Diatome Ultra 45) were collected on formvar-coated gold slot grids (Electron Microscopy Sciences). For post-embedding immunogold labeling, sections were treated in 2% human serum albumin in Tris-buffered saline (0.005 m Tris and 0.3% NaCl) for 10 min. We incubated sections with mouse anti-GnRH (HU11b at 1:100) for 4 h and followed this with incubation using gold-tagged (10 nm) F(ab′)2 of goat antimouse IgG (1:20; Electron Microscopy Sciences) in Tris-buffered saline with 2% human serum albumin and polyethylene glycol (molecular weight 20,000; 5 mg/ml) for 1 h. Ultrastructural analyses were performed on a transmission electron microscope (Philips EM208, Eindhoven, The Netherlands) with an AMT HR 1Mb digital camera. Preadsorption control experiments were performed as previously described (7).
Quantitative analysis for transmission electron microscopy
Three ultrathin sections (4–5 μm apart) from the caudal median eminence of each rat were used for election microscopy data analysis. The neuroterminal region (10 by 800 μm) along the portal capillaries was identified in the lateral median eminence as described previously (12). This region was carefully defined and matched for all of the rats, with the investigator blind to treatment. For the organizational study, 10 images along the basal lamina of the lateral median eminence were taken at ×5600 electron microscope magnification. Each image covered an approximately 10 by 10 μm area near the portal capillary system. Glial elements were identified by higher electron density, large number of glycogen granules, and a distinctive outline (19), and pseudocolored in Photoshop 6.0. The basal lamina interface of the portal capillaries was outlined, and the length was measured in each image using NIH Image J software (Wayne Rasband, National Institute of Mental Health, Bethesda, MD; http://rsb.info.nih.gov/ij/). The extracellular space of the pericapillary region from each image was thresholded and quantified using MetaMorph software (MetaMorph 6.1). The neuroterminal density in each image was quantified, and the neuroterminals in apposition to the basal lamina were counted. Along the basal lamina in an area of about 10 by 1000 μm, each GnRH-immunopositive terminal was further individually photographed at ×28,000 electron microscope magnification. The distance between each GnRH neuroterminal to the nearest basal lamina point was measured, and only neuroterminals within 10 μm from the basal lamina and with a minimum size of 0.75 μm2 were included for data analysis. Using NIH Image J software, the area and perimeter of each GnRH neuroterminal fraction was measured. Within each GnRH neuroterminal, the threshold parameter was set to delineate and count large dense-core vesicle numbers and 10-nm immunogold particles in the neuroterminals. The mitochondrial area fraction in each terminal was also determined. Finally, the surface contact of GnRH neuroterminals and glial elements within a distance of 50 nm was outlined, and the percentage of membrane surface contact was calculated. To eliminate bias, analysis of morphology was performed blinded before statistical analysis. For publication purposes, the contrast and brightness of all electron microscopy images were slightly and similarly modified using software Photoshop 7.0. Statistical analyses were performed using SPSS version 13.0 for Windows. Effects of age and hormone treatment were analyzed using two-way ANOVA. Although multiple data points were collected per animal, because three sections were used with multiple sampling zones, the final unit of analysis is the animal, and each n represents the number of animals per group. Differences were considered significant at P < 0.05. When a significant main effect was found, post hoc analysis was performed using Bonferroni post hoc test.
RIA for LH and estradiol
LH concentrations in serum samples were determined by double-antibody RIA by Dr. Michael J. Woller, University of Wisconsin-Whitewater (13,20), using the rat LH RP-3 standard from the National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases (Rockville, MD) with reagents provided by Dr. A. Parlow (21). The antibody-antigen complex was precipitated using goat antirabbit γ-globulin (Biogenesis, Inc., Sandown, NH). Intraassay variability was 4.39%, and assay sensitivity was 0.4 ng/ml. Estradiol concentrations in serum were determined by RIA of duplicate samples using the Diagnostic Systems Laboratories (Webster, TX) ultrasensitive estradiol RIA kit (DSL-4800) according to the manufacturer’s instructions. This assay sensitivity was 2.2 pg/ml, and intraassay variability was 2.59%. Effects of age and hormone treatment, and their interactions, were compared using two-way ANOVA followed by post hoc analysis if indicated by a significant main effect. An effect was considered significant at P < 0.05.
Results
Epifluorescence microscopy
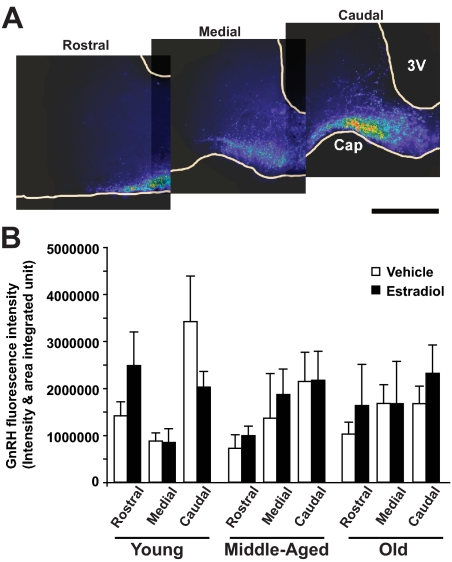
Immunofluorescence intensity of GnRH immunoreactive puncta was measured in three sections per rat, one each at the rostral, medial, and caudal median eminence levels, as shown in a representative series (Fig. 1A). Quantifications of fluorescence intensity showed that there was no statistically significant change in GnRH immunofluorescence with age or hormone treatment (Fig. 1B). In general, immunofluorescence intensity was higher in the caudal median eminence, and therefore this region was used for subsequent electron microscopy analyses.
Figure 1.
GnRH immunofluorescence intensity and distribution. A, Representative fluorescence micrographs through the median eminence are shown from rostral to caudal in a representative rat (young, vehicle treated). Similar results were seen for other ages and hormone treatments. GnRH fluorescence intensity is shown in pseudocolor (red, most intense; purple, least intense). The greatest GnRH immunofluorescence is found in the caudal median eminence. Scale bar, 500 μm. B, Quantitative analysis of GnRH immunofluorescence intensity through the median eminence was performed using MetaMorph software. Three sections were used per rat at the rostral, medial, and caudal levels of the median eminence (n = 8 rats per group). There was no significant effect of either age or hormone on GnRH fluorescence intensity. Cap, Portal capillary region; 3V, third ventricle.
Transmission electron microscopy
Organization and ultrastructure of the median eminence
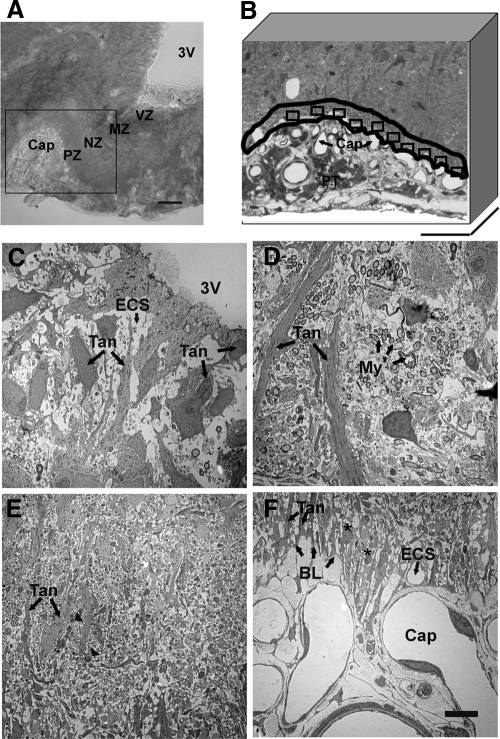
In the caudal median eminence, four zones were identified in the lateral portion of the median eminence (Fig. 2A): the ventricular zone, the myelinated axon zone, the neural profile zone, and the pericapillary zone. These zones were seen with both light microscopy (Fig. 2A) and electron microscopy (Fig. 2, C–F). In the ventricular zone, tanycyte cell bodies were seen to loosely line the walls of the third ventricle, and extended long basal processes oriented radially toward the portal capillary region (Fig. 2C). Basal to the ventricular zone, numerous neuronal axons covered with myelin sheaths were perpendicular to tanycyte processes (Fig. 2D). In the neural profile zone, neuronal axon swellings (puncta) containing large secretory vesicles were in close proximity to the tanycyte processes (Fig. 2E). Neuroterminals containing large secretory vesicles were separated from the basal lamina of the portal capillaries at the pericapillary zone (Fig. 2F). Although most of the portal capillary basal lamina was covered by tanycytic endfeet, some neuroterminals directly contacted the basal lamina (Fig. 3). Those neuroterminals in touch with the basal lamina had few large secretory vesicles in the cytoplasm. Therefore, without immunolabeled large vesicles, we could not identify whether these were GnRH neuroterminals. Finally, the median eminence was replete with extracellular space surrounding the neural and glial elements (Fig. 3).
Figure 2.
Light and electron microscopic images of the lateral median eminence. A, Transmitted light differential interference contrast image shows the lateral median eminence from a representative young vehicle-treated rat. The lateral median eminence can be visually delineated into zones beginning dorsomedially with the third ventricle (3V), followed by the myelinated axon zone (MZ), the neuronal profile zone (NZ), the pericapillary zone (PZ), and finally the portal capillary vasculature (Cap). The area outlined in the box was chosen for subsequent electron microscopy analyses. Scale bar, 100 μm. B, The pericapillary zone in this semithin section (500 μm in thickness) was traced along the portal capillary basal lamina, and a 10-μm boundary was drawn (outlined in black). Ten subregions (rectangles) along the basal lamina were chosen for further electron microscopic analyses. The pars tuberalis (PT) can also be seen. Scale bar, 100 μm. C–F, Electron microscopic images show the characteristic features of the four zones of the lateral median eminence of a representative vehicle-treated rat. C, In the ventricular zone (VZ; A), tanycyte cell bodies (Tan; arrow) are seen, some of which loosely line the walls of the third ventricle. D, In the myelinated axon zone (MZ; A), groups of axons covered with myelin sheaths (My; arrows) are perpendicular to tanycyte processes (Tan; arrow). E, In the neural profile zone (NZ; A), axon swellings (arrowheads; note that these are more apparent at higher magnification) are in close proximity to the tanycyte processes (Tan; arrows). F, In the pericapillary zone (PZ; A), neuroterminals (asterisks) are separated by tanycytic endfeet (Tan; arrows) from the basal lamina (BL; arrows) of the portal capillaries (Cap). The extracellular space (ECS; arrow) surrounding neuronal and glial elements is clearly visible in the ventricular and pericapillary zone. Scale bar (C–F), 5 μm.
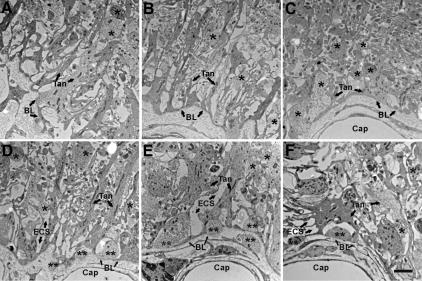
Figure 3.
Electron microscopic images of the pericapillary zone of the lateral median eminence are shown. A–C are from one representative vehicle-treated young rat with micrographs taken from most lateral (A) to medial (C) along the pericapillary zone. A, At the most lateral end of the median eminence, a few GnRH-immunopositive terminals (not identifiable at this magnification) are found. B, Moving more medially, the tanycyte processes gradually become organized and linear. C, In the most medial portion of the median eminence, a large number of neuroterminals are present, although relatively few GnRH-immunopositive terminals are found. Portal capillaries are covered by tanycytic endfeet, but few elongated tanycyte processes are found. D–F are representative data from three representative vehicle-treated rats: young (D), middle-aged (E), and old (F). Images were taken at a similar level of the median eminence pericapillary zone as that shown in B. In D–F, neuroterminals (single black asterisk), extracellular space (ECS; arrows), and the portal capillary (Cap) basal lamina (BL; arrows) can be seen. Similar profiles were seen for comparable estradiol-treated rats at each age (not shown). The neuroterminals apposed to the basal lamina (double black asterisks) have few large dense-core vesicles. The extracellular space (ECS) with low electron density surrounds neuronal and glial elements. Scale bar, 2 μm.
Several quantitative measures could be made in our electron microscopy study. The percentage of basal lamina area in contact with neuroterminals was quantified in 30 low-magnification electron microscopic images per rat (n = 5 rats per group), and no statistical difference was found with age (P = 0.537) and estradiol treatment (P = 0.907; Table 1). The percentage of extracellular space in the pericapillary zone was also measured and quantified in these same sections. No statistical difference was found for this parameter with age (P = 0.46) or hormone treatment (P = 0.68; Table 1).
Table 1.
Quantitative electron microscopy analysis of GnRH terminals and their surrounding environment in the pericapillary zone of the median eminence
| Group | % Basal lamina in contact with neuroendocrine terminals | % Extracellular space in the pericapillary zone | LDCV density per GnRH terminal area | GnRH immunogold particles per LDCV | Mitochondria area fraction |
|---|---|---|---|---|---|
| Young | |||||
| Vehicle | 8.51 ± 1.86 | 27.50 ± 2.50 | 7.49 ± 0.63 | 2.79 ± 0.19 | 3.01 ± 0.32 |
| E2 | 9.42 ± 1.29 | 24.92 ± 1.95 | 8.90 ± 0.85 | 3.10 ± 0.55 | 3.86 ± 0.38 |
| Middle-aged | |||||
| Vehicle | 8.30 ± 1.77 | 26.99 ± 1.45 | 8.74 ± 0.15 | 2.47 ± 0.21 | 4.55 ± 0.57 |
| E2 | 9.98 ± 4.16 | 29.63 ± 2.67 | 10.87 ± 0.72 | 3.25 ± 0.22 | 3.25 ± 0.50 |
| Old | |||||
| Vehicle | 12.27 ± 1.85 | 25.13 ± 0.82 | 12.36 ± 1.42 | 3.53 ± 0.43 | 3.29 ± 0.48 |
| E2 | 10.36 ± 1.76 | 27.10 ± 1.78 | 9.50 ± 0.74 | 2.58 ± 0.39 | 3.67 ± 0.55 |
The density of large dense-core vesicles (LDCV) in GnRH terminal increased with age (F = 5.36; P < 0.05; n = 5 rats in each group) with a significant increase in old compared with young rats (P < 0.01). Other quantitative measures showed no significant effects of age or hormone. E2, Estradiol.
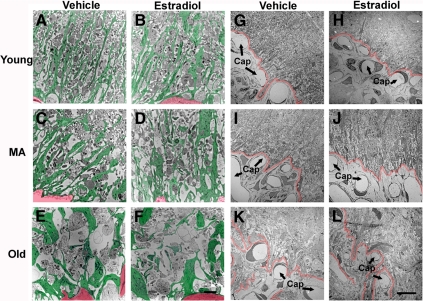
The organization of the pericapillary zone of the median eminence was further observed qualitatively using lower magnification electron microscopic images. In young rats, extended tanycyte processes were linear and oriented toward the portal capillary system. With age, the glial elements appeared larger, and their pattern of organization was less structured (Fig. 4, A–F). The boundary of the neuronal-glial elements and the portal capillary system became increasingly convoluted and less clearly delineated with progressive age (Fig. 4, G–L).
Figure 4.
Electron microscopic images (low magnification) showing the organization of the pericapillary zone of the median eminence. A–F, The pattern of glial processes in the pericapillary zone of the median eminence from one representative rat per group is shown. Glial processes are pseudocolored in green, and a corner of the portal capillary plexus (presented for orientation) is pseudocolored in red. Glial processes of young rats, irrespective of hormone treatment, tended to be narrow and linearly oriented toward the portal capillary system. These glial processes became larger and wider and this radial orientation diminished with age. Scale bar (A–F), 2 μm. G–L, The boundary between the pericapillary zone and the portal capillary region (Cap; arrows) is indicated in red for representative rats of each group. This boundary appeared to become increasingly convoluted and less clearly delineated with age. Scale bar (G–L), 10 μm. MA, Middle-aged.
GnRH neuroterminals and their properties
Because the focus of our study was GnRH neuroterminals, further electron microscopic studies were limited to the pericapillary zone of the lateral median eminence. GnRH-immunopositive neuroterminals were identified by the presence of 10-nm immunogold particles. The specificity of the GnRH antibody was tested in preadsorption control experiments carried out in adjacent sections; in these preadsorption experiments, no specific labeling was found. The relationship between GnRH-immunopositive terminals, glial processes, and the distance to capillary basal lamina as shown in Fig. 5 was quantified in randomly sampled regions of the pericapillary zone (cf. Fig. 2B). Only GnRH-immunopositive terminals that met the following criteria were included: a minimum 0.75-μm2 size, at least four labeled large dense-core vesicles, and within 10 μm to the portal capillary basal lamina. The largest GnRH-immunopositive terminal section imaged was 5.6 μm2. Between 106 and 150 terminals met criteria in each of the six groups, with a total of 718 GnRH-immunopositive terminals imaged and analyzed (Fig. 5, B–F, and Table 1). Statistical analysis showed a significant decrease in neuroterminal density of all terminals in the pericapillary zone, both GnRH immunopositive and immunonegative, with age (F = 6.30; P < 0.01; Fig. 5G). Post hoc analysis showed that terminal density was significantly lower in middle-aged and old rats when compared with young rats (P < 0.05 and P < 0.01, respectively). The density of GnRH-immunopositive terminals showed no statistical difference with age and estradiol treatment (data not shown).
Figure 5.
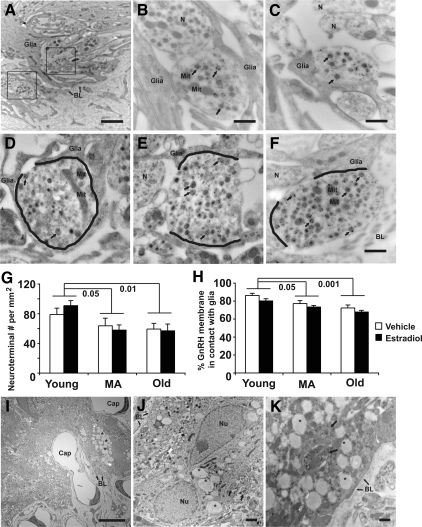
Electron microscopic images showing GnRH-immunopositive neuroterminals and surrounding microenvironmental changes with age. A–C, Electron microscopic images that include a mix of GnRH-immunopositive and -immunonegative terminals in the pericapillary zone are shown from a representative old vehicle-treated female rat. A, Two GnRH-immunopositive terminals (framed) are in close proximity to the basal lamina (BL with arrow) of the portal capillaries separated by glial elements (glia). Scale bar (A), 2 μm. The framed GnRH terminals in A are shown in higher magnification in B and C. B, A GnRH terminal containing large secretory vesicles (arrows), small vesicles, and mitochondria (Mit) was identified by immunogold particles. A GnRH-immunonegative neuroterminal (N) is in close proximity to the GnRH immunopositive terminal, and both are embedded in glial processes (glia). C, A GnRH-immunopositive terminal with labeled large secretory vesicles (arrows) is partially ensheathed by glia processes and is close to two GnRH-immunonegative terminals containing small vesicles (N). Scale bar (B and C), 500 nm. D–F, Electron microscopic images from representative young (D), middle-aged (E), and old (F) vehicle-treated rats show the interactions between GnRH-immunopositive neuroterminals and glial elements. The membrane contact between GnRH-immunopositive terminal and glial elements are marked in black. Immunogold labels for GnRH are seen in large secretory vesicle (arrows). Scale bar (D–F), 500 nm. G, The density of all neuroterminals within 10 μm of the basal lamina, including GnRH-immunopositive and -immunonegative terminals, decreased with age (P = 0.006), with young rats having significantly higher terminal density then middle-aged (P = 0.02) and old (P = 0.01) rats. H, The membrane contact between the GnRH-immunopositive terminals and glial elements was significantly decreased with age (main effect, P < 0.001; young vs. middle-aged, P < 0.05; young vs. old, P < 0.001) and estradiol treatment (P = 0.031). I–K, Electron microscopic images showing accumulated autophagic vacuoles (asterisk) in glial cells. I, Glial cells with autophagic vacuoles are found in the pericapillary zone of a representative vehicle-treated old rat. Scale bar (I), 10 μm. J, A glial cell with autophagic vacuoles is seen in a representative estradiol-treated old rat. Neuroterminals (arrows) have a close relationship to this glial cell. K, Some neuroterminals containing secretory vesicles are enclosed in the vacuoles (arrow). Scale bar (J and K), 2 μm. MA, Middle-aged; Nu, nucleus.
The cytoplasmic contents of GnRH-immunopositive terminals were also quantified. Within GnRH-immunopositive terminals, the density of large dense-core vesicles increased with age (F = 5.36; P < 0.05; Table 1 and Fig. 5, D–F) with a significant increase in old compared with young rats (P < 0.05). Although estradiol had no main effect on this endpoint, there was a significant interaction between age and estradiol (F = 5.16; P < 0.05). The number of immunogold particles per large dense-core vesicle was not affected by age or hormone (Table 1). The area fraction of mitochondria showed no difference with age and estradiol treatment (Table 1).
Relationship between GnRH terminals and glia
The surface contact between GnRH- immunopositive neuroterminals and glial profiles decreased with age (F = 13.5; P < 0.001; Fig. 5H) with a significant decrease in middle-aged (P < 0.05) and old (P < 0.001) compared with young rats. This interaction also decreased by estradiol capsule implantation compared with the vehicle (F = 5.26; P < 0.05; Fig. 5, D–F and H). In addition, there was a dramatic qualitative increase in the amount and size of autophagic vacuoles in middle-aged and old rats (Fig. 5, I–K). These vacuoles (0.5–2 μm in diameter) were accumulated in glial cytoplasm and exhibited low electron density. Membrane and neuroterminal structures were seen in some of the vacuoles (Fig. 5K). Few autophagic vacuoles were seen in the young rats.
Hormones and physiological characteristics of the experimental animals
LH serum concentrations decreased with age (F = 7.05; P < 0.01) and were significantly lower in old compared with young ovariectomized rats (P < 0.01). Negative feedback from estradiol treatment decreased LH concentrations only in the young group. The efficacy of estradiol replacement was confirmed by the estradiol RIA results, with significantly higher estradiol levels in the estrogen- compared with the vehicle-treated group at all three ages (P < 0.001). Estradiol treatment also increased pituitary weight in all age groups (F = 7.67; P < 0.05), and pituitary weight increased significantly with age (F = 7.11; P < 0.01). Importantly, rats with pituitary tumors were excluded from the study. The diameter of the uterus significantly increased with estradiol treatment at all three ages (F = 29.34; P < 0.001), demonstrating that estradiol had a uterotrophic effect at all ages.
Discussion
The contribution of the hypothalamic GnRH system to the process of reproductive senescence is becoming better understood through measures of GnRH gene expression, the GnRH release pattern, and GnRH neuronal morphology and by investigations of inputs to GnRH circuits (reviewed in Ref. 2). In aging rats, both pulsatile release of GnRH (3,22,23) and the GnRH/LH surge (24,25,26) are diminished, indicating functional changes to this circuit. Here, we demonstrate age- and estradiol-related ultrastructural changes of the GnRH neuroterminal-glia-capillary network of the median eminence. Specifically, we show organizational differences in the cytoarchitecture of this region, decreased density of neuroterminals in the pericapillary zone, diminished contacts between GnRH and glial membranes, and increased numbers of large dense-core vesicles within GnRH terminals. We speculate that these alterations may contribute to the senescence of the hypothalamic-pituitary-gonadal axis.
GnRH profiles in the median eminence show no change with age at the light microscopic level
During the process of GnRH synthesis, secretory vesicles from the perikarya are transported down the axon caudally through the hypothalamus to the median eminence where the decapeptide is stored and released from GnRH neuroterminals after high-frequency stimulation into the portal capillary system (27). Within GnRH-containing axons, clusters of secretory vesicles form axonal swellings (Herring bodies) often referred to as puncta or varicosities that can be identified by immunohistochemistry at the light microscopic level (7,28). A study in male rats showed a reduction in GnRH fiber immunostaining in the median eminence with age (29). In an intact female rat study comparing young (3–4 months, regular cycling) and middle-aged (10–15 months, persistent estrus) rats, no significant differences in GnRH fiber immunoreactivity and distribution were found (30). Another study compared the peptide content of GnRH in extracts from the median eminence and medial basal hypothalamus of young (2 months, diestrus) and old (22 months, irregularly cycling with long diestrous periods) female rats and showed no difference (31). These data are consistent with our current results in the ovariectomized aging model showing that the average GnRH fluorescence intensity representing GnRH axon and terminals through the median eminence showed no significant change with age and estradiol treatment. Therefore, GnRH neurons are capable of continuing to synthesize and transport GnRH well into advanced age.
It is surprising that there is relatively little change in GnRH immunoreactivity in the median eminence at a time when there are robust age-related declines in pituitary gonadotropin function and levels (22,23). This disparity could be interpreted to mean that there is a disconnection between GnRH release and the LH response, possibly due to alterations in properties of the pituitary GnRH receptor. However, this does not appear to be the only explanation, because a failure to release adequate amounts of GnRH in reproductive aging has been proposed for decades (30,32,33). Therefore, it is likely that the light microscopic level of analysis is inadequate to ascertain functional properties of GnRH terminals, including their capacity to store and release the peptide, and for the peptide to gain access to the portal capillary vasculature. Our electron microscopy preparation, post-embedding immunohistochemistry, and computer analyses allowed us to seek and quantify such ultrastructural evidence for age- and hormone-related changes in GnRH neuroterminals.
The structure and cytoplasmic contents of GnRH-immunopositive terminals undergo age-related changes
Using cryo-embedding immunogold electron microscopy, we performed both qualitative and quantitative analyses of GnRH terminals and their environment. A number of significant changes with age were found. First, although the density of terminals in the pericapillary zone of the median eminence decreased in middle-aged and old rats when compared with young rats (P < 0.05 and P < 0.01, respectively; Fig. 5G), the relative proportion of those terminals that were GnRH immunopositive showed no change with age and hormone treatment. Second, within GnRH terminals, the density of large dense-core vesicles increased with age (young vs. middle-aged, P < 0.05; young vs. old, P < 0.001; Table 1), suggesting that the synthesis of secretory vesicles increased or the release of secretory vesicles decreased with age, two possibilities that are not mutually exclusive. We have performed three-dimensional reconstructions of GnRH terminals in young and old ovariectomized rats, and we noticed that the large dense-core vesicles were highly concentrated in terminals of the old compared with the young rats (12). The current results add quantitative support to that previous qualitative observation. In addition to storing neuropeptide, it has been suggested that large dense-core vesicles may regulate receptor trafficking (7,34), so the change in secretory vesicle density may also affect this function. Other properties of GnRH terminals measured in our quantitative analyses did not change with age, including the area fraction of mitochondria, consistent with other electron microscopy reports showing that the volume density of mitochondria remains constant throughout the lifespan (35,36) and indicating that the GnRH neuroterminal energy demands do not change substantially during reproductive aging. We also noted that in young rats, most of the neuroterminals apposed to the capillary basal lamina had few large vesicles left in neuroterminals, whereas in old rats, many GnRH-immunopositive terminals with a high density of secretory vesicles contacted the basal lamina. Together these data suggest that the pattern of GnRH release may change with aging.
GnRH neuroterminal-glia-portal capillary interactions: effects of age
Glial cells have been shown to interact with GnRH neurons in the rodent (32,37,38), nonhuman primate (39,40), and human hypothalamus (41). In the median eminence, glial cells including tanycytes and astrocytes are in close apposition to GnRH axons and neuroterminals (6,9,32,41,42) and regulate GnRH secretion (43,44,45,46). Earlier light and electron microscopic studies comparing young (1 month) and middle-aged (14 months) male and female rats showed evidence that tanycytes undergo age-related changes with a marked increase in neutral lipid droplets present in the cytoplasm (47). In the present study, we found that in young rats, tanycytic processes enwrapping neuronal axons tended to be linear and oriented from the ventricle toward the portal capillary system. With age, these tanycytic processes became larger and appeared disorganized. In addition, the boundary of the neuronal-glial elements and the portal capillary system as defined by the basal lamina became increasingly convoluted and less clearly delineated with age. These age-related changes in the capillary system might alter GnRH peptide transportation to the portal capillaries after it is released from neuroterminals. Glial regulation of microvasculature has been shown to play an important role to maintain neural function (48). In our study, whether the observed structural changes resulted in changes of GnRH release needs to be further investigated, but it suggests that the underlying anatomical framework and neuronal-glial communication is substantially different. Furthermore, we observed a dramatically increased amount of autophagic vacuoles in glia in aged rats similar to the results seen in Brawer and Walsh (47). These vacuoles were accumulated in glial cytoplasm with low electron density especially in the pericapillary zones of the central portion of the median eminence and the infundibulum.
At higher magnification, we quantified the surface contacts between the GnRH neuroterminals and glial processes. Similar to the dynamic interactions of glia and GnRH neuroterminals reported throughout the estrous cycle (42), we observed an overall decrease in membrane contacts in estradiol-treated rats in all three ages. A small but significant age-related decrease in these membrane contacts was also detected, a result that confirmed and extended our previous report on young and old ovariectomized rats (12). The plasticity of the neuroterminal-glial surface interactions is only beginning to be understood, but it likely involves several cell-surface molecules in the median eminence (49). Polysialic acid-neural cell adhesion molecule (PSA-NCAM) (50) is suggested to be involved in the control of GnRH neuroterminal-glial plasticity (51,52). Contactin, a neural surface protein highly expressed on GnRH neuroterminals, was shown to mediate adhesive communication with astrocytes (53). SynCAM1, an adhesion molecule required for synaptic assembly, is expressed in both GnRH cell lines (GT1-7 cells) and astrocytes (49). Whether this neuronal-glial interaction is altered by steroid hormones needs additional evidence, but considering that estrogen receptors have been found in astrocyte, tanycyte, and endothelial cells in the median eminence (54), it is possible that estradiol may alter the network of glial cells and therefore indirectly regulate GnRH secretion.
Although we saw no morphological changes of extracellular space in the pericapillary region with age and estradiol treatment, extracellular factors such as neurotrophic factors, neurotransmitters, and neuromodulators involved in volume transmission might change with age and hormone status. The roles of gliotransmission have been extensively studied in synapses (55), but few or no synaptic contacts are found in the median eminence (6). Whether gliotransmission molecules such as glutamate and ATP are involved in neuropeptide release and how gliotransmission may change with age are interesting ideas that warrant further study. The arrangement of neuroterminals, glia, and capillary morphology and their regulation by age and hormone provide a system to fine-tune a response to the demands of neuronal activity. Our data provide further support to the idea that glial cells are involved in remodeling the median eminence.
In summary, our data showing ultrastructural changes in the GnRH neuroterminal-glia-capillary relationship support age- and hormone-related hypothalamic control at the level of GnRH neuroterminals. Changes to glia and capillaries in this brain region are also likely to have an impact on non-GnRH terminals that converge in the median eminence, and our quantitative measure of total neuroterminal density in the pericapillary zone showed an age-related decrease that was not limited to the GnRH system. Further studies of changes to GnRH neuroterminals and their surrounding microenvironment are central to understanding senescence of reproductive and other hypothalamic neural systems that are involved in neuroendocrine functions.
Acknowledgments
We thank Kristen Reynolds, Dr. Jacqueline Maffucci, and Sonya Hughes for help with tissue preparation, Deena Walker for help with hormone assays, and Monique M. Monita for assistance with data analysis. We are grateful to John Mendenhall and Dr. Angela Bardo for valuable microscopy advice, Dr. A. F. Parlow (National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases, Rockville, MD) for provision of LH RIA reagents, and Dr. Michael Woller (University of Wisconsin-Whitewater, Whitewater, WI) for generously performing the LH RIA in his laboratory. Special thanks to Dr. H. F. Urbanski who kindly provided us with the GnRH antibody.
Footnotes
This work was supported by funding from the National Institutes of Health (AG16765 and AG028051 to A.C.G.) and a University Continuing Fellowship from The University of Texas at Austin (to W.Y.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 9, 2009
References
- Gore AC 2002 GnRH: the master molecule of reproduction. Boston: Kluwer Academic Publishers [Google Scholar]
- Yin W, Gore AC 2006 Neuroendocrine control of reproduction aging: roles of GnRH neurons. Reproduction 131:403–414 [DOI] [PubMed] [Google Scholar]
- Scarbrough K, Wise PM 1990 Age-related changes in pulsatile luteinizing hormone release precede the transition to estrous acyclicity and depend upon estrous cycle history. Endocrinology 126:884–890 [DOI] [PubMed] [Google Scholar]
- Huang HH, Marshall S, Meites J 1976 Capacity of old versus young female rats to secrete LH, FSH and prolactin. Biol Reprod 14:538–543 [DOI] [PubMed] [Google Scholar]
- Kawakami S, Ichikawa M, Murahashi K, Hirunagi K, Tsukamura H, Maeda K 1998 Excitatory amino acids act on the median eminence nerve terminals to induce gonadotropin-releasing hormone release in female rats. Gen Comp Endocrinol 112:372–382 [DOI] [PubMed] [Google Scholar]
- Durrant AR, Plant TM 1999 A study of the gonadotropin releasing hormone neuronal network in the median eminence of the rhesus monkey (Macaca mulatta) using a post-embedding immunolabelling procedure. J Neuroendocrinol 11:813–821 [DOI] [PubMed] [Google Scholar]
- Yin W, Mendenhall JM, Bratton SB, Oung T, Janssen WG, Morrison JH, Gore AC 2007 Novel localization of NMDA receptors within neuroendocrine gonadotropin-releasing hormone terminals. Exp Biol Med (Maywood) 232:662–673 [PubMed] [Google Scholar]
- Agnati LF, Bjelke B, Fuxe K 1995 Volume versus wiring transmission in the brain: a new theoretical frame for neuropsychopharmacology. Med Res Rev 15:33–45 [DOI] [PubMed] [Google Scholar]
- Theodosis DT, MacVicar B 1996 Neurone-glia interactions in the hypothalamus and pituitary. Trends Neurosci 19:363–367 [DOI] [PubMed] [Google Scholar]
- Prevot V, Dutoit S, Croix D, Tramu G, Beauvillain J 1998 Semi-quantitative ultrastructural analysis of the localization and neuropeptide content of gonadotropin releasing hormone nerve terminals in the median eminence throughout the estrous cycle of the rat. Neuroscience 84:177–191 [DOI] [PubMed] [Google Scholar]
- King JC, Rubin BS 1994 Dynamic changes in LHRH neurovascular terminals with various endocrine conditions in adults. Horm Behav 28:349–356 [DOI] [PubMed] [Google Scholar]
- Yin W, Mendenhall JM, Monita M, Gore AC 2009 Three-dimensional properties of GnRH neuroterminals in the median eminence of young and old rats. J Comp Neurol 517:284–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Oung T, Woller MJ 2002 Age-related changes in hypothalamic gonadotropin-releasing hormone and N-methyl-d-aspartate receptor gene expression, and their regulation by oestrogen, in the female rat. J Neuroendocrinol 14:300–309 [DOI] [PubMed] [Google Scholar]
- Funabashi T, Kleopoulos SP, Brooks PJ, Kimura F, Pfaff DW, Shinohara K, Mobbs CV 2000 Changes in estrogenic regulation of estrogen receptor a mRNA and progesterone receptor mRNA in the female rat hypothalamus during aging: an in situ hybridization study. Neurosci Res 38:85–92 [DOI] [PubMed] [Google Scholar]
- Gibbs RB 2000 Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging 21:107–116 [DOI] [PubMed] [Google Scholar]
- Gore AC, Yeung G, Morrison JH, Oung T 2000 Neuroendocrine aging in the female rat: the changing relationship of hypothalamic gonadotropin-releasing hormone neurons and N-methyl-d-aspartate receptors. Endocrinology 141:4757–4767 [DOI] [PubMed] [Google Scholar]
- Miller BH, Gore AC 2002 NMDA receptor subunit expression in gonadotropin-releasing hormone neurons changes during reproductive senescence in the female rat. Endocrinology 143:3568–3574 [DOI] [PubMed] [Google Scholar]
- Urbanski HF 1991 Monoclonal antibodies to luteinizing hormone-releasing hormone: production, characterization, and immunocytochemical application. Biol Reprod 44:681–686 [DOI] [PubMed] [Google Scholar]
- Gruetter R 2003 Glycogen: the forgotten cerebral energy store. J Neurosci Res 74:179–183 [DOI] [PubMed] [Google Scholar]
- Gore AC, Oung T, Yung S, Flagg RA, Woller MJ 2000 Neuroendocrine mechanisms for reproductive senescence in the female rat: gonadotropin-releasing hormone neurons. Endocrine 13:315–323 [DOI] [PubMed] [Google Scholar]
- Vella S, Gussick J, Woller M, Waechter-Brulla D 2001 Modification of cell perifusion for extended study of hormone release in the rat pituitary. Methods Cell Sci 23:197–204 [DOI] [PubMed] [Google Scholar]
- Wise PM, Cohen IR, Weiland NG, London ED 1988 Aging alters the circadian rhythm of glucose utilization in the suprachiasmatic nucleus. Proc Natl Acad Sci USA 85:5305–5309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano A, Kimura F 2000 Electrical activity of the pulse generator of gonadotropin-releasing hormone in 26-month-old female rats. Neuroendocrinology 72:199–207 [DOI] [PubMed] [Google Scholar]
- Rubin BS, Lee CE, King JC 1994 A reduced proportion of luteinizing hormone (LH)-releasing hormone neurons express Fos protein during the preovulatory or steroid-induced LH surge in middle-aged rats. Biol Reprod 51:1264–1272 [DOI] [PubMed] [Google Scholar]
- Lloyd JM, Hoffman GE, Wise PM 1994 Decline in immediate early gene expression in gonadotropin-releasing hormone neurons during proestrus in regularly cycling, middle-aged rats. Endocrinology 134:1800–1805 [DOI] [PubMed] [Google Scholar]
- Wise PM 1982 Alterations in the proestrous pattern of median eminence LHRH, serum LH, FSH, estradiol and progesterone concentrations in middle-aged rats. Life Sci 12:165–173 [DOI] [PubMed] [Google Scholar]
- King JA, Millar RP 1982 Structure of chicken hypothalamic luteinizing hormone-releasing hormone. I. Structural determination on partially purified material. J Biol Chem 257:10722–10728 [PubMed] [Google Scholar]
- Goldsmith P, Ganong W 1975 Ultrastructural localization of luteinizing hormone-releasing hormone in the median eminence of the rat. Brain Res 97:183–193 [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Sladek Jr JR 1980 Age-related changes in dopamine, LHRH and somatostatin in the rat hypothalamus. Neurobiol Aging 1:27–37 [DOI] [PubMed] [Google Scholar]
- Rubin BS, King JC, Bridges RS 1984 Immunoreactive forms of luteinizing hormone-releasing hormone in the brains of aging rats exhibiting persistent vaginal estrus. Biol Reprod 31:343–351 [DOI] [PubMed] [Google Scholar]
- Bestetti GE, Reymond MJ, Blanc F, Boujon CE, Furrer B, Rossi GL 1991 Functional and morphological changes in the hypothalamopituitary-gonadal axis of aged female rats. Biol Reprod 45:221–228 [DOI] [PubMed] [Google Scholar]
- Kozlowski GP, Coates PW 1985 Ependymoneuronal specializations between LHRH fibers and cells of the cerebroventricular system. Cell Tissue Res 242:301–311 [DOI] [PubMed] [Google Scholar]
- Terasawa E 1998 Cellular mechanism of pulsatile LHRH release. Gen Comp Endocrinol 112:283–295 [DOI] [PubMed] [Google Scholar]
- Shuster SJ, Riedl M, Li X, Vulchanova L, Elde R 1999 Stimulus-dependent translocation of κ-opioid receptors to the plasma membrane. J Neurosci 19:2658–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoni-Freddari C, Fattoretti P, Casoli T, Di Stefano G, Giorgetti B, Balietti M 2008 Brain aging: the zinc connection. Exp Gerontol 43:389–393 [DOI] [PubMed] [Google Scholar]
- Monteiro RA, Henrique RM, Rocha E, Marini-Abreu MM, Oliveira MH, Silva MW 1998 Age-related changes in the volume of somata and organelles of cerebellar granule cells. Neurobiol Aging 19:325–332 [DOI] [PubMed] [Google Scholar]
- King JC, Letourneau RJ 1994 Luteinizing hormone-releasing hormone terminals in the median eminence of rats undergo dramatic changes after gonadectomy, as revealed by electron microscopic image analysis. Endocrinology 134:1340–1351 [DOI] [PubMed] [Google Scholar]
- Romero MT, Silverman AJ, Wise PM, Witkin JW 1994 Ultrastructural changes in gonadotropin-releasing hormone neurons as a function of age and ovariectomy in rats. Neuroscience 58:217–225 [DOI] [PubMed] [Google Scholar]
- Witkin JW, Ferin M, Popilskis SJ, Silverman AJ 1991 Effects of gonadal steroids on the ultrastructure of GnRH neurons in the rhesus monkey: synaptic input and glial apposition. Endocrinology 129:1083–1092 [DOI] [PubMed] [Google Scholar]
- Perera AD, Plant TM 1997 Ultrastructural studies of neuronal correlates of the pubertal reaugmentation of hypothalamic gonadotropin-releasing hormone (GnRH) release in the rhesus monkey (Macaca mulatta). J Comp Neurol 385:71–82 [DOI] [PubMed] [Google Scholar]
- Baroncini M, Allet C, Leroy D, Beauvillain JC, Francke JP, Prevot V 2007 Morphological evidence for direct interaction between gonadotrophin-releasing hormone neurones and astroglial cells in the human hypothalamus. J Neuroendocrinol 19:691–702 [DOI] [PubMed] [Google Scholar]
- Prevot V, Croix D, Rialas CM, Poulain P, Fricchione GL, Stefano GB, Beauvillain JC 1999 Estradiol coupling to endothelial nitric oxide stimulates gonadotropin-releasing hormone release from rat median eminence via a membrane receptor. Endocrinology 140:652–659 [DOI] [PubMed] [Google Scholar]
- Dhandapani K, Mahesh V, Brann D 2003 Astrocytes and brain function: implications for reproduction. Exp Biol Med (Maywood) 228:253–260 [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Lorenz B, DonCarlos LL 2008 The role of glia in the hypothalamus: implications for gonadal steroid feedback and reproductive neuroendocrine output. Reproduction 135:419–429 [DOI] [PubMed] [Google Scholar]
- Dziedzic B, Prevot V, Lomniczi A, Jung H, Cornea A, Ojeda SR 2003 Neuron-to-glia signaling mediated by excitatory amino acid receptors regulates ErbB receptor function in astroglial cells of the neuroendocrine brain. J Neurosci 23:915–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Seranno S, Estrella C, Loyens A, Cornea A, Ojeda SR, Beauvillain JC, Prevot V 2004 Vascular endothelial cells promote acute plasticity in ependymoglial cells of the neuroendocrine brain. J Neurosci 24:10353–10363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawer JR, Walsh RJ 1982 Response of tanycytes to aging in the median eminence of the rat. Am J Anat 163:247–256 [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M 2007 Glial regulation of the cerebral microvasculature. Nat Neurosci 10:1369–1376 [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Lomzicai A, Sandau US 2008 Glial-gonadotrophin hormone (GnRH) neurone interactions in the median eminence and the control of GnRH secretion. J Neuroendocrinol 20:732–742 [DOI] [PubMed] [Google Scholar]
- Gascon E, Vutskits L, Kiss JZ 2007 Polysialic acid-neural cell adhesion molecule in brain plasticity: from synapses to integration of new neurons. Brain Res Rev 56:101–118 [DOI] [PubMed] [Google Scholar]
- Perera AD, Verbalis JG, Mikuma N, Majumdar SS, Plant TM 1993 Cholecystokinin stimulates gonadotropin-releasing hormone release in the monkey (Macaca mulatta). Endocrinology 132:1723–1728 [DOI] [PubMed] [Google Scholar]
- Parkash J, Kaur G 2005 Neuronal-glial plasticity in gonadotropin-releasing hormone release in adult female rats: role of the polysialylated form of the neural cell adhesion molecule. J Endocrinol 186:379–409 [DOI] [PubMed] [Google Scholar]
- Parent AS, Mungenast AE, Lomniczi A, Sandau US, Peles E, Bosch MA, Rønnekleiv OK, Ojeda SR 2007 A contactin-receptor-like protein tyrosine phosphatase β complex mediates adhesive communication between astroglial cells and gonadotrophin-releasing hormone neurones. J Neuroendocrinol 19:847–859 [DOI] [PubMed] [Google Scholar]
- Langub Jr MC, Watson Jr RE 1992 Estrogen receptor-immunoreactive glia, endothelia, and ependyma in guinea pig preoptic area and median eminence: electron microscopy. Endocrinology 130:364–372 [DOI] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG 2007 The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med 13:54–63 [DOI] [PubMed] [Google Scholar]