Abstract
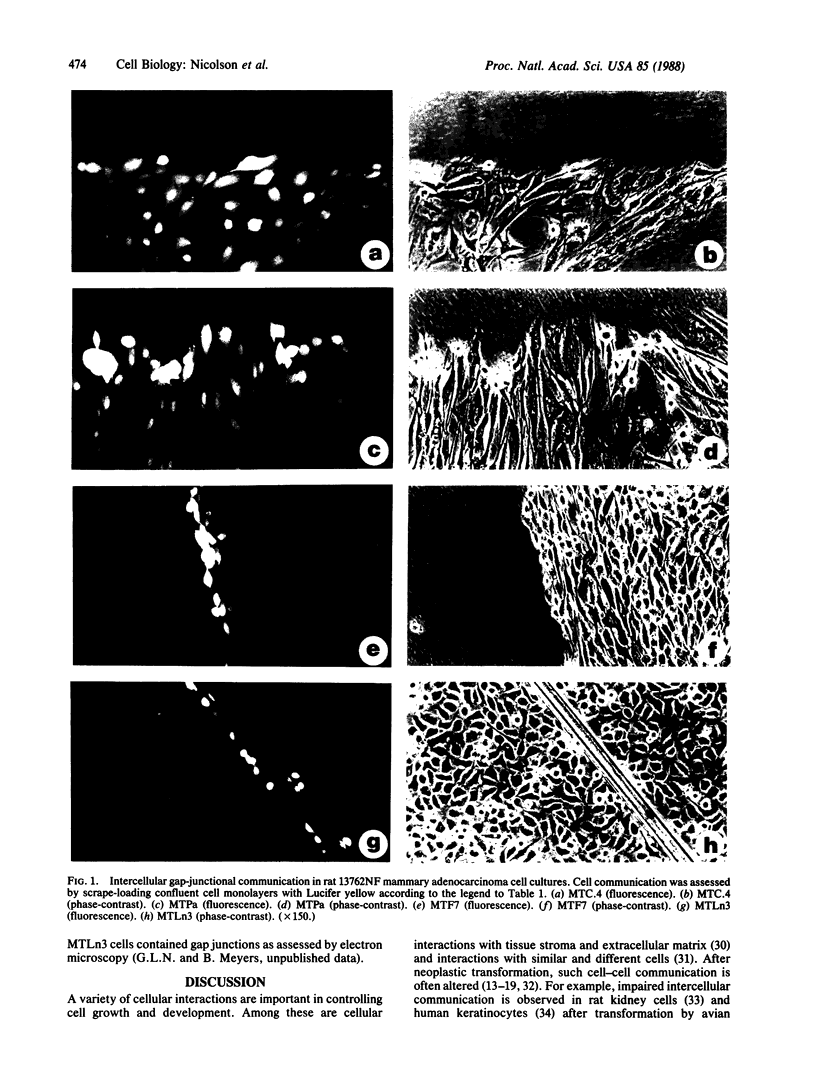
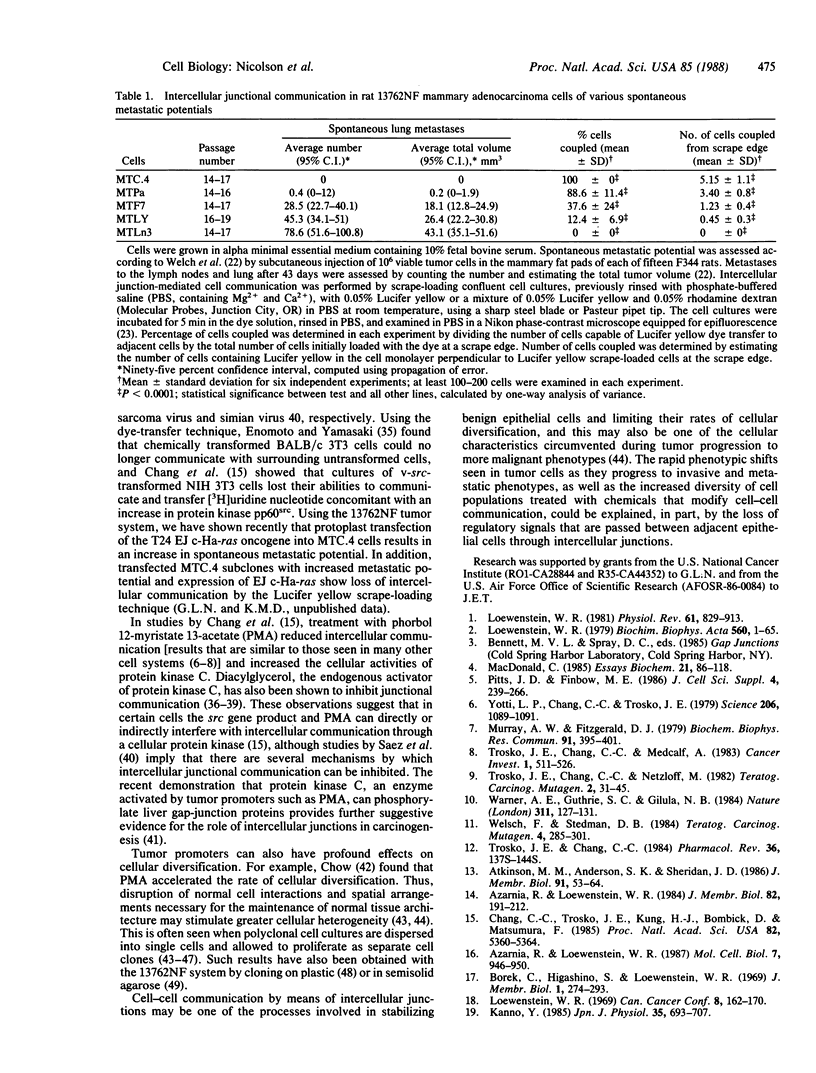
A series of rat 13762NF mammary adenocarcinoma cell sublines and clones of various spontaneous pulmonary metastatic potentials from the mammary fat pads of syngeneic rats were examined for their intercellular junctional communication. Using the scrape-loading dye-transfer technique to introduce Lucifer yellow (Mr 457) into cells, we measured the abilities of 13762NF cells to transfer dye to adjacent cells. There was an excellent correlation between loss of Lucifer yellow dye transfer and spontaneous metastatic potential (average total volume of lung metastases inversely correlated to % cells coupled, r = 0.93; average total number of lung metastases inversely correlated to % cells coupled, r = 0.91). The data suggest that high metastatic potentials are closely correlated with loss of intercellular junctional communication in these malignant mammary tumor cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. M., Anderson S. K., Sheridan J. D. Modification of gap junctions in cells transformed by a temperature-sensitive mutant of Rous sarcoma virus. J Membr Biol. 1986;91(1):53–64. doi: 10.1007/BF01870214. [DOI] [PubMed] [Google Scholar]
- Atkinson M. M., Menko A. S., Johnson R. G., Sheppard J. R., Sheridan J. D. Rapid and reversible reduction of junctional permeability in cells infected with a temperature-sensitive mutant of avian sarcoma virus. J Cell Biol. 1981 Nov;91(2 Pt 1):573–578. doi: 10.1083/jcb.91.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarnia R., Loewenstein W. R. Intercellular communication and the control of growth: XI. Alteration of junctional permeability by the src gene in a revertant cell with normal cytoskeleton. J Membr Biol. 1984;82(3):207–212. doi: 10.1007/BF01871630. [DOI] [PubMed] [Google Scholar]
- Azarnia R., Loewenstein W. R. Polyomavirus middle T antigen downregulates junctional cell-to-cell communication. Mol Cell Biol. 1987 Feb;7(2):946–950. doi: 10.1128/mcb.7.2.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M. J., Hall H. G., Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982 Nov 7;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Chang C. C., Trosko J. E., Kung H. J., Bombick D., Matsumura F. Potential role of the src gene product in inhibition of gap-junctional communication in NIH/3T3 cells. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5360–5364. doi: 10.1073/pnas.82.16.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow D. A. Variant generation and selection: an in vitro model of tumor progression. Int J Cancer. 1984 Apr 15;33(4):541–545. doi: 10.1002/ijc.2910330419. [DOI] [PubMed] [Google Scholar]
- Corsaro C. M., Migeon B. R. Comparison of contact-mediated communication in normal and transformed human cells in culture. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4476–4480. doi: 10.1073/pnas.74.10.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J. S., Baumgarten I. M., Harley E. H. Studies on the mechanism of phorbol ester-induced inhibition of intercellular junctional communication. Carcinogenesis. 1985 Sep;6(9):1353–1358. doi: 10.1093/carcin/6.9.1353. [DOI] [PubMed] [Google Scholar]
- Enomoto T., Yamasaki H. Lack of intercellular communication between chemically transformed and surrounding nontransformed BALB/c 3T3 cells. Cancer Res. 1984 Nov;44(11):5200–5203. [PubMed] [Google Scholar]
- Enomoto T., Yamasaki H. Rapid inhibition of intercellular communication between BALB/c 3T3 cells by diacylglycerol, a possible endogenous functional analogue of phorbol esters. Cancer Res. 1985 Aug;45(8):3706–3710. [PubMed] [Google Scholar]
- Flagg-Newton J., Simpson I., Loewenstein W. R. Permeability of the cell-to-cell membrane channels in mammalian cell juncton. Science. 1979 Jul 27;205(4404):404–407. doi: 10.1126/science.377490. [DOI] [PubMed] [Google Scholar]
- Gainer H. S., Murray A. W. Diacylglycerol inhibits gap junctional communication in cultured epidermal cells: evidence for a role of protein kinase C. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1109–1113. doi: 10.1016/0006-291x(85)90300-6. [DOI] [PubMed] [Google Scholar]
- Iijima N., Yamamoto T., Inoue K., Soo S., Matsuzawa A. Experimental studies on dynamic behavior of cells in subcutaneous solid tumor (MH 134-C3H-He). Jpn J Exp Med. 1969 Jun;39(3):205–221. [PubMed] [Google Scholar]
- Kanno Y. Modulation of cell communication and carcinogenesis. Jpn J Physiol. 1985;35(5):693–707. doi: 10.2170/jjphysiol.35.693. [DOI] [PubMed] [Google Scholar]
- Lin Z. X., Kavanagh T., Trosko J. E., Chang C. C. Inhibition of gap junctional intercellular communication in human teratocarcinoma cells by organochlorine pesticides. Toxicol Appl Pharmacol. 1986 Mar 30;83(1):10–19. doi: 10.1016/0041-008x(86)90318-2. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication and the control of growth. Biochim Biophys Acta. 1979 Feb 4;560(1):1–65. doi: 10.1016/0304-419x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981 Oct;61(4):829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Transfer of information through cell junctions and growth control. Proc Can Cancer Conf. 1969;8:162–170. [PubMed] [Google Scholar]
- MacDonald C. Gap junctions and cell-cell communication. Essays Biochem. 1985;21:86–118. [PubMed] [Google Scholar]
- McNeil P. L., Murphy R. F., Lanni F., Taylor D. L. A method for incorporating macromolecules into adherent cells. J Cell Biol. 1984 Apr;98(4):1556–1564. doi: 10.1083/jcb.98.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner K. M., Kawaguchi T., Uba G. W., Nicolson G. L. Clonal drift of cell surface, melanogenic, and experimental metastatic properties of in vivo-selected, brain meninges-colonizing murine B16 melanoma. Cancer Res. 1982 Nov;42(11):4631–4638. [PubMed] [Google Scholar]
- Muir J. G., Murray A. W. Mimicry of phorbol ester responses by diacylglycerols. Differential effects on phosphatidylcholine biosynthesis, cell-cell communication and epidermal growth factor binding. Biochim Biophys Acta. 1986 Feb 21;885(2):176–184. doi: 10.1016/0167-4889(86)90086-8. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Fitzgerald D. J. Tumor promoters inhibit metabolic cooperation in cocultures of epidermal and 3T3 cells. Biochem Biophys Res Commun. 1979 Nov 28;91(2):395–401. doi: 10.1016/0006-291x(79)91535-3. [DOI] [PubMed] [Google Scholar]
- Neri A., Welch D., Kawaguchi T., Nicolson G. L. Development and biologic properties of malignant cell sublines and clones of a spontaneously metastasizing rat mammary adenocarcinoma. J Natl Cancer Inst. 1982 Mar;68(3):507–517. [PubMed] [Google Scholar]
- Nicolson G. L. Tumor cell instability, diversification, and progression to the metastatic phenotype: from oncogene to oncofetal expression. Cancer Res. 1987 Mar 15;47(6):1473–1487. [PubMed] [Google Scholar]
- Pitts J. D., Finbow M. E. The gap junction. J Cell Sci Suppl. 1986;4:239–266. doi: 10.1242/jcs.1986.supplement_4.15. [DOI] [PubMed] [Google Scholar]
- Pitts J. D., Kam E. Communication compartments in mixed cell cultures. Exp Cell Res. 1985 Feb;156(2):439–449. doi: 10.1016/0014-4827(85)90550-6. [DOI] [PubMed] [Google Scholar]
- Poste G., Doll J., Fidler I. J. Interactions among clonal subpopulations affect stability of the metastatic phenotype in polyclonal populations of B16 melanoma cells. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6226–6230. doi: 10.1073/pnas.78.10.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. Cancer as a dynamic developmental disorder. Cancer Res. 1985 Jul;45(7):2935–2942. [PubMed] [Google Scholar]
- Rubin H. Early origin and pervasiveness of cellular heterogeneity in some malignant transformations. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5121–5125. doi: 10.1073/pnas.81.16.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. M. Roles of cell-to-cell communication in development. Biol Reprod. 1985 Feb;32(1):27–42. doi: 10.1095/biolreprod32.1.27. [DOI] [PubMed] [Google Scholar]
- Steinberg M., Defendi V. Patterns of cell communication and differentiation in SV40 transformed human keratinocytes. J Cell Physiol. 1981 Oct;109(1):153–159. doi: 10.1002/jcp.1041090117. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Sáez J. C., Bennett M. V., Spray D. C. Carbon tetrachloride at hepatotoxic levels blocks reversibly gap junctions between rat hepatocytes. Science. 1987 May 22;236(4804):967–969. doi: 10.1126/science.3576214. [DOI] [PubMed] [Google Scholar]
- Takeda A., Hashimoto E., Yamamura H., Shimazu T. Phosphorylation of liver gap junction protein by protein kinase C. FEBS Lett. 1987 Jan 5;210(2):169–172. doi: 10.1016/0014-5793(87)81330-3. [DOI] [PubMed] [Google Scholar]
- Trosko J. E., Chang C. C. Adaptive and nonadaptive consequences of chemical inhibition of intercellular communication. Pharmacol Rev. 1984 Jun;36(2 Suppl):137S–144S. [PubMed] [Google Scholar]
- Trosko J. E., Chang C. C., Medcalf A. Mechanisms of tumor promotion: potential role of intercellular communication. Cancer Invest. 1983;1(6):511–526. doi: 10.3109/07357908309020276. [DOI] [PubMed] [Google Scholar]
- Trosko J. E., Chang C. C., Netzloff M. The role of inhibited cell-cell communication in teratogenesis. Teratog Carcinog Mutagen. 1982;2(1):31–45. doi: 10.1002/1520-6866(1990)2:1<31::aid-tcm1770020105>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Warner A. E., Guthrie S. C., Gilula N. B. Antibodies to gap-junctional protein selectively disrupt junctional communication in the early amphibian embryo. Nature. 1984 Sep 13;311(5982):127–131. doi: 10.1038/311127a0. [DOI] [PubMed] [Google Scholar]
- Welch D. R., Krizman D. B., Nicolson G. L. Multiple phenotypic divergence of mammary adenocarcinoma cell clones. I. In vitro and in vivo properties. Clin Exp Metastasis. 1984 Oct-Dec;2(4):333–355. doi: 10.1007/BF00135172. [DOI] [PubMed] [Google Scholar]
- Welch D. R., Neri A., Nicolson G. L. Comparison of 'spontaneous' and 'experimental' metastasis using rat 13762 mammary adenocarcinoma metastatic cell clones. Invasion Metastasis. 1983;3(2):65–80. [PubMed] [Google Scholar]
- Welsch F., Stedman D. B. Inhibition of metabolic cooperation between Chinese hamster V79 cells by structurally diverse teratogens. Teratog Carcinog Mutagen. 1984;4(3):285–301. doi: 10.1002/tcm.1770040304. [DOI] [PubMed] [Google Scholar]
- Yancey S. B., Edens J. E., Trosko J. E., Chang C. C., Revel J. P. Decreased incidence of gap junctions between Chinese hamster V-79 cells upon exposure to the tumor promoter 12-O-tetradecanoyl phorbol-13-acetate. Exp Cell Res. 1982 Jun;139(2):329–340. doi: 10.1016/0014-4827(82)90257-9. [DOI] [PubMed] [Google Scholar]
- Yotti L. P., Chang C. C., Trosko J. E. Elimination of metabolic cooperation in Chinese hamster cells by a tumor promoter. Science. 1979 Nov 30;206(4422):1089–1091. doi: 10.1126/science.493994. [DOI] [PubMed] [Google Scholar]
- el-Fouly M. H., Trosko J. E., Chang C. C. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res. 1987 Feb;168(2):422–430. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]