Abstract
GH-releasing peptide-2 (GHRP-2) is a synthetic peptide that increases circulating GH and IGF-I levels. It also binds to CD36, a scavenger receptor for oxidized low-density lipoprotein (OxLDL), and may prevent cellular uptake of this proatherogenic complex. To determine its potential antiatherogenic effects, GHRP-2 (20 μg twice daily) was administered sc to ApoE−/− mice for 12 wk. GHRP-2 increased circulating IGF-I 1.2- to 1.6-fold and decreased circulating interferon-γ by 66%. Although GHRP-2 did not alter atherosclerotic plaque area, it decreased aortic production of superoxide as assessed by dihydroethidium staining. GHRP-2 decreased aortic gene expression of 12/15-lipoxygenase by 92% and reduced the aortic expression of interferon-γ and macrophage migration inhibitory factor. In cultured aortic smooth muscle cells, GHRP-2 prevented the OxLDL-induced generation of peroxides, down-regulation of IGF-I receptor, and apoptosis. In macrophages, GHRP-2 reduced lipid accumulation with OxLDL exposure. In summary, GHRP-2 exerts antioxidant effects in vivo and in vitro but does not reduce plaque burden. The lack of an antiatherogenic effect may be due to GH-dependent effects in vivo, thereby blunting the effect of increased IGF-I.
GHRP-2 has beneficial effects on vascular cells in vitro and reduces oxidative stress in vivo but does not reduce atherosclerosis in a mouse model.
Cardiovascular disease is the leading cause of death in the developed world, accounting for more than one third of all deaths in the United States (1). The underlying etiology responsible for most cardiovascular disease is atherosclerosis. Atherosclerosis has a complicated pathogenesis involving inflammation, vascular cell dysfunction, accumulation of lipid and minerals, and abnormalities in cell division, migration, metabolism, oxidative stress, and apoptosis (2,3). IGF-I is produced primarily in the liver and mediates many of the effects of GH. We have recently reported that sc infusion of IGF-I to ApoE−/− mice decreased the size of atherosclerotic lesions as well as the number of macrophages and TNF-α expression in the lesions (4). IGF-I administration also led to a decrease in oxidative stress and inflammatory cytokine production as well as increases in vascular endothelial nitric oxide synthase (eNOS) expression and circulating endothelial progenitor cells.
GH-releasing peptides (GHRP) are small synthetic peptides that stimulate GH production and secretion via their stimulation of the ghrelin receptor (5), resulting in increased circulating IGF-I. They act independently of and synergistically with GHRH to promote GH release from the anterior pituitary gland and are an attractive method to pharmacologically stimulate the GH/IGF-I axis. In addition to their central effects, peripheral effects of GHRP include actions in the cardiovascular system via the G protein-coupled receptor, GH secretagogue receptor (GHSR)-1α (6) or via interaction with CD36, a scavenger receptor for the proatherogenic molecule, oxidized low-density lipoprotein (OxLDL) (7). Importantly, GHRP and OxLDL share a common CD36 binding site (7). GHRP can bind to several tissues including myocardium, arteries, and veins (8). Ghrelin, the endogenous GH secretagogue, has been shown to be a potent vasodilator (9,10). Additionally, in a rat model of myocardial infarction, ghrelin inhibited the sympathetic nervous system and improved cardiac function and decreased myocardial remodeling (11).
In the ApoE−/− model of atherosclerosis, the GHRP hexarelin (12) and GHRP analog EP80317 (13) are reported to prevent progression of atherosclerosis in a CD36-dependent manner while activating PPARγ and transporters involved in cholesterol transport and metabolism. GHRP-2 (dAla-dβNal-Ala-Trp-dPhe-Lys-NH2) is a hexapeptide member of the subclass of GHRP that also binds to CD36 (7). We administered GHRP-2 to ApoE−/− mice to determine whether GHRP-2 can inhibit the progression of atherosclerosis. Additionally, we sought to determine whether GHRP-2 can inhibit generation of reactive oxygen species, lipid accumulation, apoptosis of vascular cells, and inflammation.
Materials and Methods
Animal studies and quantification of atherosclerosis
Animal protocols were approved by the Institutional Animal Care and Use Committee at Tulane University. Eight-week-old male apolipoprotein E (ApoE)-deficient mice (The Jackson Laboratory, Bar Harbor, ME) were fed a Western-style diet (42% total calories from fat, 0.15% cholesterol; Harlan Teklad Diet TD.88137) ad libitum. Mice were treated for 12 wk beginning at 9 wk of age with twice-daily sc injections of either 20 μg GHRP-2 (a dose that significantly increases GH and IGF-I levels) (14) or PBS. Mice were kept in a temperature-controlled room on a 12-h light, 12-h dark cycle, and body weight and food intake were recorded periodically. Pulse and blood pressure were measured weekly by tail cuff method (BP-2000; Visitech Systems, Apex, NC). Nonfasting blood samples were obtained from the submandibular vein and serum samples stored at −80 C. Mice were euthanized after an overnight fast, and serum was collected and stored at −80 C. Mice were perfused through the left ventricle at physiological pressure with saline for 5 min followed by PBS containing 4% paraformaldehyde and 5% sucrose (fixation solution) for 15 min. The heart and aorta were dissected to approximately 1 mm past the iliac bifurcation, removed together, and further fixed in fixation solution at 4 C for 24 h. For some mice, tissue was collected after saline perfusion without fixation. The base of the heart containing the aortic root was embedded in Optimal Cutting Temperature (OCT) medium (Sakura Finetek, Torrance, CA), frozen on dry ice, and later stored at −80 C for cryosections. Aortas from these mice were cleaned of periadventitial fat with the aid of a dissecting microscope and saved either at −80 C or in RNA later solution (Ambion, Austin, TX) at −20 C. Aortas used for en face analysis of atherosclerosis were stained with Oil Red O (ICN Biomedical, Inc., Aurora, OH), and atherosclerosis was quantified using Image-Pro Plus version 6.0 software (Media Cybernetics Inc., Silver Spring, MD) as previously described (4). Atherosclerotic plaque burden was also measured in cross-sections at the aortic valve using Oil Red O or hematoxylin and eosin (H&E) staining as previously described (4). Paraffin sections of the aortic valve area adjacent to those used for H&E staining were used for immunostaining of macrophages with anti-Mac-3 (BD PharMingen, San Diego, CA) as previously described (4). Normal rat IgG (Santa Cruz Biotechnology, Santa Cruz, CA) served as a negative control.
Biochemical assays
IGF-I and IGF-binding protein (IGFBP)-3 levels were measured in duplicate using a mouse/rat-specific IGF-1 ELISA (Diagnostic Systems Laboratories, Webster, TX) and mouse/rat IGFBP-3 ELISA (Mediagnost, Tübingen, Germany), respectively. Serum levels of other circulating factors (listed in Table 1) were measured in quadruplicate by multiplex ELISA using a custom cytokine array (Quantibody; RayBiotech, Norcross, GA). Urine was collected for 24 h in the presence of the antioxidant butylated hydroxytoluene (1 mmol/liter; Cayman Chemical, Ann Arbor, MI). Urine concentrations of 8-isoprostane and creatinine were measured as previously described (4) using commercially available assays (Cayman Chemical). Serum GH levels were measured with rat/mouse GH ELISA (Millipore, Bedford, MA). For 12/15-lipoxygenase immunochemistry, we used rabbit anti-15-lipoxygenase antibody (Santa Cruz; 1:50) followed by staining with rabbit VECTASTAIN ABC peroxidase kit (Vector Laboratories, Burlingame, CA) and developing with diaminobenzidine peroxidase substrate (Vector). Mac-3- or 12/15-lipoxygenase-immunopositive plaque area was quantified using Image Pro Plus version 6.0 (Media Cybernetics).
Table 1.
GHRP-2 effect on serum cytokine levels
| PBS | GHRP-2 | P value | |
|---|---|---|---|
| GH (ng/ml)a | 2.0 ± 0.6 | 1.8 ± 0.5 | 0.93d |
| IGF-I (ng/ml)a | 180 ± 11 | 240 ± 20 | 0.02 |
| IGFBP-2 (ng/ml) | 46 ± 7 | 27 ± 6 | 0.049 |
| IGFBP-3 (ng/ml)a | 291 ± 8 | 333 ± 15 | 0.022 |
| IGFBP-5 (ng/ml)b | 18 ± 1 | 15 ± 2 | 0.22 |
| IFN-γ (ng/ml)c | 5.3 ± 1.2 | 1.8 ± 0.6 | 0.027 |
| IL-6 (pg/ml)c | 125 ± 46 | 357 ± 143 | 0.37d |
| IL-10 (pg/ml) | 2600 ± 700 | 2900 ± 1500 | 0.24d |
| Leptin (ng/ml) | 2.2 ± 0.9 | 3.0 ± 1.0 | 0.67d |
| MCP-1 (pg/ml) | 53 ± 7 | 63 ± 6 | 0.49d |
| RANTES (pg/ml) | 610 ± 64 | 600 ± 87 | 0.91 |
| SDF-1α (pg/ml)b | 790 ± 670e | 640 ± 430e | 0.96d |
| TNF-α (pg/ml)c | 0 ± 0 | 35 ± 35f | 0.30 |
Fasting levels of GH, IGF-I, IGFBP-2, IGFBP-3, IGFBP-5, IFN-γ, IL-6, IL-10, leptin, MCP-1, Regulated upon Activation Normal T-cell Expressed and Secreted (RANTES), stromal cell-derived factor-1α (SDF-1α), and TNF-α.
a–c Except as follows, n = 9 for all groups:
n = 15 per group;
n = 8 in the PBS group;
n = 8 in the GHRP-2 group.
P value obtained by Mann Whitney U test.
Only two mice per group had detectable levels of SDF-1α.
Only one mouse had detectable levels of TNF-α.
Detection of vascular superoxide and apoptosis
For visualization of superoxide, 20-μm cryosections of the aortic root (just distal to the aortic valve) were stained with dihydroethidium (DHE; Invitrogen, Carlsbad, CA) for 1 h at 37 C as previously described (4). Adjacent sections were stained in parallel with DHE plus 500 U/ml polyethylene glycol-superoxide dismutase (PEG-SOD; Sigma Chemical Co., St. Louis, MO), a scavenger of superoxide. Paraffin sections (6 μm, adjacent to those stained with H&E) of the aortic valve area were used for staining apoptotic cells via the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) method using the in situ cell death detection kit (Roche, Indianapolis, IN) on slides treated with proteinase K (50 μg/ml in Tris-HCl, pH 7.4) for 30 min at 37 C. For each slide, one section was treated with deoxyribonuclease I (Pierce, Rockford, IL; 500 U/ml) for 20 min at 37 C to serve as a positive control in detecting fragmented DNA. Slides were coverslipped with Vectashield mounting medium containing propidium iodide (Vector) to counterstain DNA.
Quantitative real-time RT-PCR
Aortas were removed from RNA later solution and homogenized in Tripure isolation reagent (Roche). Total RNA was then isolated after the Tripure protocol using Phase Lock Gels (Eppendorf, Hamburg, Germany) to assist in phase separation. RNA was purified using an RNeasy mini kit (QIAGEN, Valencia, CA). cDNA synthesis was performed using the RT2 first strand kit (SuperArray, Frederick, MD). Gene expression profiling was performed in a 96-well plate using a custom RT2 Profiler PCR array (SuperArray) to examine the aortic expression of 86 genes of interest. Real-time PCR was performed using a 40-cycle two-step PCR protocol in the iCycler IQ real-time detection system (Bio-Rad, Hercules, CA).
Aortic smooth muscle cell (SMC) peroxide production, apoptosis assays, and immunoblotting
Human aortic SMC (hASMC) were obtained from Lonza (Allendale, NJ) and maintained in SMC basal medium with included serum and supplements (Lonza). Cells at passages 6–12 were used for experiments after growing to approximately 80–90% confluence. Unless otherwise specified, growth medium was removed, and cells were treated in serum-free media (SFM) (DMEM/F12; Life Technologies, Inc., Rockville, MD). Native LDL (nLDL) was isolated from human plasma by sodium bromide stepwise density gradient centrifugation and oxidized with CuSO4 to prepare OxLDL as previously described (15). The value for thiobarbituric acid-reactive substances in OxLDL was 37.2 ± 1.2 nmol malondialdehyde per milligram protein. Thiobarbituric acid-reactive substances were not detectable in nLDL. Apoptosis was measured using cytoplasmic lysates in a cell death detection ELISA (Roche) according to the manufacturer’s protocol. hASM cells were grown in 24-well plates and treated for 24 h in SFM with nLDL or OxLDL (60 μg/ml) with or without 50 μm GHRP-2. Treatment with the nonspecific protein kinase inhibitor staurosporine (0.5 μm) served as a positive control. Cell viability assay was performed using a calcein acetoxymethyl ester (calcein-AM) probe (Invitrogen), which is converted to a fluorescent form, calcein, after digestion in viable cells with intracellular esterase. To assay cell viability, hASM cells were loaded with calcein-AM (1 μmol) for 30 min, briefly washed with medium, and imaged in a fluorescent microscope, or the fluorescent signal was quantified with a microplate reader (Synergy; Biotek Instruments, Winooski, VT). Immunoblotting was performed in parallel experiments after overnight treatment with LDLs. To assess IGF-I-dependent Akt phosphorylation, cells were additionally exposed to 3 nm IGF-I for 15 min. hASM cells were washed with PBS and lysed in RIPA buffer containing complete Mini EDTA-free protease inhibitor cocktail (Roche). Lysates were run on 10% SDS-PAGE followed by immunoblot using a rabbit antibody against the β-chain of human IGF-I- receptor (IGF-IR) (Santa Cruz), phosphor-Akt (Ser473), and Akt (Cell Signaling Technology, Beverly, MA) with mouse anti-β-actin (Sigma) serving as a loading control. Immunoblotting was repeated six times. Peroxide production was assayed using the peroxide-sensitive dye 6-carboxy-2′,7′-dichlorodihydro-fluorescein diacetate (CDC-H2F; Molecular Probes, Eugene, OR). hASM cells were grown to approximately 90% confluence in a 96-well plate and incubated with 10 μg/ml CDC-H2F for 1 h at 37 C. Cells were treated with 50 μm GHRP-2, anti-CD36, or normal IgG (nIgG) for 30 min. At this point, nLDL or OxLDL (60 μg/ml) was added, and plates were incubated for 3 h. Fluorescence was measured with an excitation wavelength set at 485 ± 20 and an emission wavelength set at 535 ± 20 with a Synergy HT multidetection microplate reader (Bio-Tek Instruments).
Foam cell assay
Foam cell formation was assayed using THP-1 cells (American Type Culture Collection, Rockville, MD) differentiated in RPMI 1640 (American Type Culture Collection), 10% fetal bovine serum, 0.05 mm 2-mercaptoethanol, and 50 ng/ml phorbol myristate acetate for 4 d. Cells were serum starved for 24 h in SFM (with 2-mercaptoethanol and phorbol myristate acetate) and then treated overnight in SFM with or without GHRP-2 (50 μm) or ghrelin (50 μm). OxLDL or nLDL (80 μg/ml) was then added, and cells were incubated for 48 h. After paraformaldehyde fixation, cells were stained with Oil Red O (5 mg/ml in 60% isopropanol) for 30 min. Image-Pro Plus software was used to determine relative lipid accumulation, as indicated by Oil Red O-positive area. Alternatively, peripheral blood mononuclear cells (PBMC) were isolated from whole blood using Ficoll and cultured in RPMI 1640 medium plus 10% heat-inactivated fetal bovine serum. PBMC were then differentiated into macrophages via 5-d treatment in medium containing macrophage colony-stimulating factor (50 ng/ml; Sigma). For experiments with PBMC-derived macrophages, OxLDL-induced lipid accumulation was quantified by extracting the Oil Red O with 100% isopropanol, reading absorbance at 571 nm and normalizing that signal to cell protein (milligrams).
Statistical analysis
All numerical data are expressed as mean ± sem. Statistical analysis was performed using GraphPad Prism 4 software. Unless otherwise specified, two-tailed unpaired Student t tests were performed to determine statistical significance. For data that were not normally distributed, Mann Whitney U test was performed as indicated for nonparametric analysis. Where appropriate, ANOVA was used to compare multiple groups followed by post hoc analysis with Dunnett’s multiple comparison test. Differences were considered significant at P < 0.05.
Results
GHRP-2 increases weight gain and circulating IGF-I in ApoE−/− mice
Mice that received GHRP-2 gained more weight than PBS-treated mice over the 12-wk experiment (9.0 ± 0.3 vs. 7.9 ± 0.3 g, P = 0.02); however, the GHRP-2 group did not differ significantly from the PBS group in total body weight at the end of the experimental period (31.0 ± 0.5 vs. 29.8 ± 0.5 g, P = 0.13) (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). To determine whether increased appetite could explain any increase in weight gain, 24 h food consumption was measured periodically (supplemental Table 1). On experimental d 3, GHRP-2-treated mice ate 16.5% more food than control (PBS-treated) mice (3.2 ± 0.07 vs. 2.8 ± 0.07 g/d, P < 0.001); however, by d 4, the increase in food consumption with GHRP-2 treatment decreased to that seen in control mice, with the exception of the wk-4 time point. This suggests that the orexigenic effect of GHRP-2 was transient. Mice treated with GHRP-2 had significantly higher IGF-I levels than PBS-treated mice (supplemental Fig. 2). This increase was robust during the first 2 months of the experiment: a 56% increase at wk 4, 51% at wk 7, and 17% at wk 11. Two-way ANOVA revealed that both age (P < 0.01) and GHRP-2 treatment (P < 0.0001) had a significant effect on IGF-I levels. At the time of killing, GHRP-2-treated mice had 29% higher fasting IGF-I levels than PBS-treated controls. Fasting GH levels were not increased by GHRP-2 at the time of killing (Table 1); however, the acute GH increase after GHRP-2 treatment was verified in a separate experiment (supplemental Fig. 4) and revealed a 3-fold increase in GH levels 4 h after injection of GHRP-2. GHRP-2 did not alter blood pressure or heart rate. Mice treated with GHRP-2 had an average heart rate of 672 ± 7 beats/min and a systolic blood pressure of 116 ± 2 mm Hg compared with controls with a heart rate of 678 ± 11 (P > 0.6) and a systolic blood pressure of 116 ± 2 mm Hg (P > 0.9). Additionally, total fasting cholesterol/cholesteryl esters were not changed by GHRP-2 treatment (710 ± 37 vs. 620 ± 51 mg/dl in the PBS group, P = 0.17; n = 7 per group). Fast protein liquid chromatography revealed no difference in the overall serum lipoprotein profile between the two groups in terms of protein content (supplemental Fig. 3).
GHRP-2 reduces vascular oxidative stress but not atherosclerosis burden
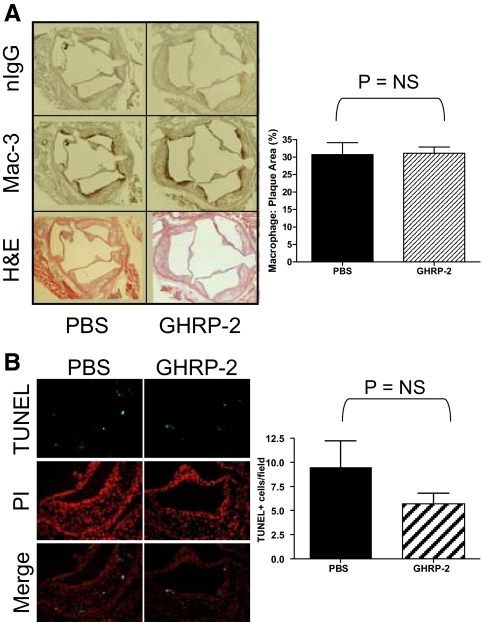
Atherosclerotic plaque burden was quantified by both longitudinal analyses of the entire aorta and cross-sectional analysis at the aortic valve. En face analysis showed that GHRP-2 treatment did not alter the amount of plaque present throughout the entire aorta (Fig. 1A). GHRP-2 treatment did not alter the amount of plaque present in aortic valve cross-sections (Fig. 1B). To determine whether GHRP-2 exerts an antioxidant effect, we measured superoxide generation by staining frozen sections of the aorta taken just distal to the aortic valve with DHE (Fig. 1C). GHRP-2 reduced vascular production of superoxide by approximately 55% (P < 0.01; n = 7 PBS, n = 6 GHRP-2). The antioxidant effect of GHRP-2 was not systemic. 8-Isoprostane (8-iso prostaglandin F2α) was measured in the urine as an index of the overall redox state. The 8-isoprostane level in GHRP-2-treated mice was not different from that of PBS mice at 4 wk (GHRP-2, 50.4 ± 4.5 ng/mg creatinine; PBS, 63.2 ± 18.4 ng/mg creatinine) and 12 wk (GHRP-2, 74.5 ± 14.3 ng/mg creatinine; PBS. 58.1 ± 8.4 ng/mg creatinine). The relative abundance of macrophages in the plaques was assayed by immunohistochemistry for the macrophage marker Mac-3 (Fig. 2A). GHRP-2 treatment had no effect on the ratio of Mac-3-immunoreactive area to total plaque area. TUNEL staining was used to see whether treatment with GHRP-2 reduced apoptosis in atherosclerotic plaques in aortic valve cross-sections of ApoE−/− mice (Fig. 2B). GHRP-2 did not change the number of TUNEL-positive cells (GHRP-2 vs. PBS, P = 0.22).
Figure 1.
GHRP-2 effect on atherosclerosis and vascular superoxides in ApoE−/− mice. A, En face analysis of atherosclerosis. Representative images of aortas obtained from mice treated twice per day with either PBS or GHRP-2 and stained with Oil Red O (ORO). Atherosclerotic burden is expressed as a percentage of plaque area to total area. n = 15 for each group. B, Atherosclerosis in cross-sections of the aortic valve. Paraffin-embedded cross-sections were stained with H&E and cryosections with Oil Red O. Scale bar, 200 μm. n = 25 (PBS) or n = 28 (GHRP-2). C, Superoxide levels in ascending aorta. Cross-sections of the ascending aorta were stained with DHE to visualize superoxide. For each aorta, a serial section was also treated with PEG-SOD to establish a background level of fluorescence. Images were taken at ×200 magnification. Quantitative data are expressed relative to PBS-treated mice. n = 7 (PBS) or n = 6 (GHRP-2). *, P < 0.01 vs. PBS. D, 12/15-Lipoxygenase immunohistochemistry. Sections were stained with an anti-12/15-lipoxygenase antibody or normal rabbit IgG as a negative control, and immunoreactive area is expressed as a ratio of positive (brown) area to total plaque area determined by the adjacent H&E-stained slide. n = 5 per group. *, P < 0.05 vs. PBS. NS, Not significant.
Figure 2.
GHRP-2 effect on macrophage levels and cell apoptosis in atherosclerotic plaque. A, Macrophage immunohistochemistry. Sections were stained with an anti-Mac-3 antibody or with nIgG as a negative control. Macrophage levels are expressed as a ratio of Mac-3-positive (brown) area to the total plaque area determined by the adjacent H&E-stained slide. n = 9 per group. B, TUNEL staining of apoptotic cells. Apoptotic nuclei were detected with a TUNEL-fluorescein kit and counterstained with propidium iodide (PI). Images were taken at ×200 magnification. n = 11 mice per group. NS, Not significant.
GHRP-2 treatment reduces interferon-γ (IFN-γ) and aortic expression of 12/15-lipoxygenase
GHRP-2 treatment significantly decreased serum IFN-γ by 70%, IGFBP-2 by 40%, and increased circulating IGFBP-3 levels by 14% (Table 1). GHRP-2 did not change circulating levels of IGFBP-5, IL-6, IL-10, leptin, monocyte chemoattractant protein-1 (MCP-1), Regulated upon Activation Normal T-cell Expressed and Secreted (RANTES) (CCL5), stromal cell-derived factor-1α (SDF-1α), or TNF-α. To determine the effect of GHRP-2 on aortic gene expression, RT-PCR was performed to profile 86 genes of interest including those involved in the GH/IGF axis, inflammation, oxidative stress, and lipid transport or metabolism. A complete list of genes and results from the RT2 Profiler PCR array is in Supplemental Table 2. GHRP-2 treatment dramatically reduced the aortic expression of 12/15-lipoxygenase, an enzyme that mediates generation of reactive oxygen species, eicosanoids, and OxLDL. Immunohistochemical analysis of aortic valve cross-sections revealed a 40% decrease in 12/15-lipoxygenase immunoreactivity with GHRP-2 treatment (Fig. 1D). GHRP-2 also reduced the vascular expression of the cytokines interferon-γ and macrophage migration inhibitory factor (MIF) (Table 2). There was no significant change in IGF-IR mRNA levels with GHRP-2 treatment (supplemental Table 2).
Table 2.
Aortic gene expression profiling by RT-PCR
| mRNA expression | PBS | GHRP-2 | P value |
|---|---|---|---|
| 15-LOX | 1.00 ± 0.09 | 0.08 ± 0.01 | 0.015 |
| IFN-γ | 1.00 ± 0.05 | 0.21 ± 0.01 | 0.051 |
| MIF | 1.00 ± 0.10 | 0.46 ± 0.03 | 0.048 |
| SOD1 | 1.00 ± 0.12 | 0.40 ± 0.03 | 0.052 |
| SOD3 | 1.00 ± 0.06 | 1.68 ± 0.06 | 0.017 |
| vWF | 1.00 ± 0.02 | 1.70 ± 0.06 | 0.041 |
Gene expression in GHRP-2-treated mouse aortas relative to the PBS-treated group. n = 6 per group.
GHRP-2 protects vascular SMC from the cytotoxic effects of OxLDL
To determine whether GHRP-2 treatment could protect vascular SMC from OxLDL-induced apoptosis, we incubated hASM cells with SFM, nLDL, or OxLDL for 24 h in the presence or absence of GHRP-2 (Fig. 3A). OxLDL caused a 4.5-fold increase in apoptosis compared with SFM (P < 0.01), and this effect was reduced by cotreatment with GHRP-2 (51% reduction, OxLDL vs. OxLDL plus GHRP-2, P < 0.01). GHRP-2 treatment reduced apoptosis in OxLDL-treated cells to levels that were not statistically different from serum-free conditions (P > 0.05). The GHRP-2-induced protective effect against OxLDL cytotoxicity was confirmed with a cell viability assay using calcein-AM. OxLDL reduced hASM cell viability by 83 ± 8%, and pretreatment with ghrelin or nIgG had no effect; however, preincubation with GHRP-2 (50 μm) or an antibody against CD36 (20 μg/ml) markedly prevented the reduction in cell viability (GHRP-2/OxLDL, 16 ± 5% decrease in cell viability, P < 0.05 vs. OxLDL; CD36 antibody/OxLDL, 27 ± 9% decrease in cell viability, P < 0.05 vs. OxLDL).
Figure 3.
GHRP-2 effect on OxLDL-induced cell apoptosis, IGF-IR down-regulation and generation of peroxides in hASMC. A, GHRP-2 rescues hASMC from OxLDL-induced apoptosis. hASMC were treated with SFM, nLDL, or OxLDL (60 μg/ml) for 24 h in the absence or presence of 50 μm GHRP-2, and 0.5 μm staurosporine served as a positive control. Average absorbance is expressed relative to OxLDL-treated cells. All samples were assayed in duplicate. Data represent the average of four independent experiments. *, P < 0.01 vs. OxLDL. B, GHRP-2 prevents IGF-IR down-regulation in hASMC exposed to OxLDL. hASMC were treated with nLDL or OxLDL (60 μg/ml) overnight in the absence or presence of 50 μm GHRP-2, and immunoblotting was performed. Data represent the average of six independent experiments. C, GHRP-2 suppresses OxLDL-induced peroxide generation in hASMC. Peroxide levels were measured by quantification of CDC-H2F fluorescence of hASMC exposed to LDL (60 μg/ml) and treated with GHRP-2, anti-CD36 antibody, or nIgG. D, GHRP-2 prevents the decrease in IGF-I-dependent-Akt phosphorylation in hASMC exposed to OxLDL. hASMC were treated with nLDL or OxLDL (60 μg/ml) overnight in the absence or presence of 50 μm GHRP-2, followed by exposure to IGF-I (3 nm) for 15 min, and immunoblotting was performed. A representative immunoblot from two separate experiments is shown. NS, Not significant.
We have previously shown that the apoptotic effect of OxLDL is in part mediated by OxLDL-induced down-regulation of IGF-IR. To determine whether GHRP-2 could inhibit IGF-IR down-regulation, we exposed hASM to nLDL or OxLDL for 16 h with or without GHRP-2. Immunoblotting for IGF-IR demonstrated that OxLDL decreased the IGF-IR level in hASM cells (P < 0.05 vs. nLDL), and GHRP-2 blocked this OxLDL-induced down-regulation (Fig. 3B). We also examined IGF-I-dependent signaling activity. Consistent with the down-regulation of IGF-IR, OxLDL significantly reduced IGF-I-dependent phosphorylation of Akt, and GHRP-2 prevented the OxLDL-induced decrease in Akt phosphorylation (Fig. 3D). Because OxLDL-induced oxidative stress mediates the down-regualtion of IGF-IR and apoptosis (15), we measured peroxide levels in OxLDL-treated hASM cells with and without preincubation with GHRP-2 or an antibody against CD36 (Fig. 3C). OxLDL treatment enhanced peroxide generation in hASM (Fig. 3C), and this increase was markedly inhibited by both GHRP-2 and anti-CD36 antibody.
GHRP-2 reduces lipid content of macrophages
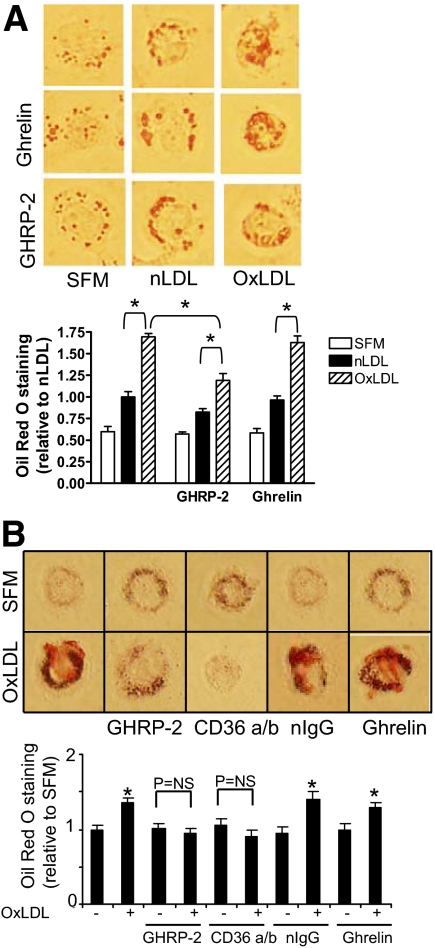
To determine whether GHRP-2 inhibits lipid internalization, we exposed THP-1-derived macrophages to nLDL or OxLDL (80 μg/ml) with or without pretreatment with either GHRP-2 (50 μm) or ghrelin (50 μm) and used Oil Red O staining to visualize the cellular lipid content (Fig. 4A). GHRP-2, but not ghrelin, reduced the lipid content of OxLDL-treated macrophages. Two-way ANOVA used to examine both the type of LDL exposure and the presence or absence of GHRP-2 revealed that both factors significantly impacted the average Oil Red O-positive area (P < 0.001 for both variables), and there is no interaction between the variables (P > 0.2). Treatment with GHRP-2 did not significantly change Oil Red O positivity for nLDL-treated macrophages (P > 0.05, nLDL plus GHRP-2 vs. nLDL).
Figure 4.
GHRP-2 effect on OxLDL-induced lipid accumulation in cultured macrophages. A, GHRP-2 reduces lipid accumulation in THP-1 macrophages. THP-1-derived macrophages were treated in SFM, nLDL, or OxLDL and stained with Oil Red O with or without pretreatment with GHRP-2 or ghrelin (50 μm); representative images were taken at ×400 magnification. Quantification of Oil Red O-positive cell area is expressed relative to nLDL-treated cells; data represent mean ± sem from 10 fields per treatment condition. *, P < 0.05. Data are representative of five independent experiments. B, GHRP-2 reduces lipid accumulation in PBMC-derived macrophages. Macrophages were treated in SFM or OxLDL in the presence of GHRP-2, anti-CD36 antibody, nIgG, or ghrelin. Images of representative cells are shown at ×400 magnification. Data shown as Oil Red O staining are normalized for total milligrams protein and expressed relative to untreated cells (SFM). Each bar is the mean ± sem of three wells. Data are representative of three independent experiments. *, P < 0.01 vs. SFM-treated cells.
Similarly to THP-1 cells, GHRP-2 decreased OxLDL-induced lipid uptake in macrophages derived from human peripheral blood monocytes (Fig. 4B). Additionally, a CD36 blocking antibody was able to prevent OxLDL-induced lipid accumulation, confirming the important role of CD36 as a scavenger receptor for OxLDL. Ghrelin, the endogenous GH secretagogue, did not prevent OxLDL-induced lipid uptake, suggesting that the effect of GHRP-2 is not mediated via the ghrelin receptor. Similarly, nIgG was unable to prevent the OxLDL-induced increase in Oil Red O staining.
Discussion
Our data indicate that GHRP-2 can rescue vascular SMC from many of the toxic effects of OxLDL in vitro, including reactive oxygen species generation, down-regulation of IGF-IR, decrease in cell viability, and apoptosis. Furthermore, GHRP-2 can prevent lipid internalization in macrophages in vitro. Nevertheless, GHRP-2 administration in vivo failed to reduce atherosclerotic plaque burden, despite evidence of bioactivity as indicated by its ability to increase circulating IGF-I.
This is the first report of GHRP-2 preventing apoptosis in cultured SMC. This effect is likely mediated via GHRP-2 binding to the CD36 receptor because it is mimicked by anti-CD36 receptor antibody, and ghrelin (which does not bind to CD36) is without effect. The ability of GHRP-2 to prevent SMC apoptosis is likely related at least in part to its ability to block OxLDL-induced reactive oxygen species formation and prevent IGF-IR down-regulation. Indeed, we have previously shown that in cultured vascular SMC, IGF-I signaling through the phosphatidylinositol 3-kinase/Akt pathway can prevent OxLDL-induced apoptosis (16). We show here that GHRP-2 treatment preserved Akt signaling (a key survival pathway) in OxLDL-exposed cells (Fig. 3D). OxLDL binding to the scavenger receptor CD36 triggers lipoxygenase-generated reactive oxygen species formation that leads to IGF-IR down-regulation and apoptosis (15). Thus, our present finding that GHRP-2 can prevent the peroxide generation and subsequent decrease in IGF-IR protein levels triggered by OxLDL treatment could in part explain the pro-survival effect of GHRP-2 in SMC.
The ability of GHRP-2 to inhibit OxLDL-induced apoptosis may be important in atherogenesis because OxLDL colocalizes with apoptotic intimal SMC in vivo (17). Excess SMC apoptosis in ApoE−/− mice can lead to increased thinning of the fibrous cap, loss of extracellular matrix, intimal inflammation, and accumulation of cell debris (18), all similar to advanced unstable human lesions. Furthermore, chronic SMC apoptosis increases atherosclerotic plaque area and calcification (19). Despite the antiapoptotic effect of GHRP-2 in vitro, TUNEL staining failed to detect a significant reduction in apoptosis in plaques in vivo. However, apoptosis was assessed only at 12 wk, and it is possible that a transient antiapoptotic effect of GHRP-2 in vivo was no longer present in advanced atheromas after 12 wk treatment with the Western diet. Indeed, in a rat model of pressure overload-induced heart failure, GHRP-2 treatment reduced the number of apoptotic cardiomyocytes at 3 wk (20). Alternatively, it is possible that pleiotropic effects of GHRP-2 in vivo negated its ability to block OxLDL-induced SMC apoptosis in vivo.
We report here an antioxidant effect of GHRP-2 both in vitro and in vivo. GHRP-2 markedly reduced peroxide generation in cultured aortic SMC exposed to OxLDL and superoxide levels in cross sections of aortas stained with DHE. This antioxidant effect of GHRP-2 in the vasculature was not systemic because urinary 8-isoprostane levels were not altered with GHRP-2 treatment. Mechanisms for the antioxidant effects of GHRP-2 in vivo are likely multiple, potentially including both its ability to bind to the CD36 scavenger receptor and to increase circulating IGF-I levels. Indeed, we have recently shown that continuous infusion of IGF-I in ApoE−/− mice on a Western diet also decreased aortic superoxide production (4). Additionally, aortic gene expression profiling revealed that GHRP-2 reduced 12/15-lipoxygenase mRNA levels by greater than 12-fold and also decreased 12/15-lipoxygenase staining in plaques as detected by immunohistochemistry. 12/15- Lipoxygenase is present in atherosclerotic lesions colocalized with OxLDL, and in vitro preparations of mammalian 12/15-lipoxygenase can oxidize human LDL (21). Beyond the oxidation of LDL, products of lipoxygenase can have additional inflammatory effects. For example, one product of 12/15-lipoxygenase is 12(S)-HETE, which has been shown to increase the expression of MCP-1 in mouse macrophages (22). Thus, the ability of GHRP-2 to markedly decrease aortic 12/15-lipoxygenase expression could play a critical role in its vascular antioxidant effects.
SOD, including intracellular isoforms (SOD1 and SOD2) and extracellular (cell surface-bound) SOD3 scavenge superoxides, and they also produce hydrogen peroxide that could reach toxic concentrations in some cellular compartments. We have shown previously that cotreatment of hASMC with OxLDL and PEG-SOD (an enzyme with preferential intracellular distribution) potentiated oxidant-induced toxicity; however, a cell-nonpermeable SOD form was protective (34). Here we report that GHRP-2 decreases vascular intracellular SOD1 expression and significantly increases aortic levels of extracellular SOD3 (see Table 2). These data taken together with our previous findings suggest that GHRP-2-induced SOD1 inhibition could decrease the toxicity of intracellular H2O2 levels. The increase in surface-bound SOD3 and the dramatic down-regulation of 12/15-lipoxygenase expression potentially mediate the local vascular antioxidant effect of GHRP-2 consistent with our DHE-based vascular superoxide data (see Fig. 1C).
GHRP-2 decreased lipid uptake in two different cell lines of cultured macrophages exposed to OxLDL. This result was likely attributable to the interaction of GHRP-2 with CD36 and not GHSR because ghrelin, the natural ligand for GHSR, did not alter OxLDL uptake. Additionally, EP80317, a GHRP analog that interacts with CD36 but does not stimulate GH secretion, has been shown to inhibit OxLDL uptake in peritoneal macrophages (13). Hexarelin, another GHRP that interacts with CD36, also inhibits OxLDL uptake in THP-1 macrophages (12). Although GHRP-2 reduced OxLDL uptake in cultured macrophages, there was no reduction in macrophage staining in atherosclerotic plaques of ApoE−/− mice treated with GHRP-2 and no decrease in Oil Red O staining in cross-sections of the aortic valve area. This finding is inconsistent with the decrease in macrophage infiltration seen in aortic plaques of ApoE−/− mice infused sc with IGF-I for 12 wk (4). Although the increase in circulating IGF-I achieved with GHRP-2 administration (∼1.2- to ∼1.6-fold increase) was lower than that achieved with IGF-I infusion (∼2-fold increase), it appears unlikely that this difference would account for the marked variance between the effects of GHRP-2 and IGF-I infusions on plaque macrophage infiltration and, indeed, on atherosclerotic plaque burden. The role of IGF-I in atherosclerosis has been the subject of much debate (reviewed in Ref. 23). Indeed, some studies implicate IGF-I in neointimal formation in models of arterial injury of either rat aortas or mouse carotid arteries (24,25). It is likely that the proliferative and migratory effects of IGF-I on SMC promote neointima formation in these injury models; however, these models differ significantly from the atherogenic ApoE−/− mouse model, where a proatherogenic diet and dyslipidemic phenotype promote atherosclerosis rather than direct physical injury to the endothelium. In ApoE−/− mice, it is possible that the antiapoptotic and antioxidant effects dominate over the migratory and mitotic effects of IGF-I. Clearly, both models are useful in evaluating the role of IGF-I in the natural history of atherosclerosis and in the type of injury that occurs during percutaneous coronary interventions.
Because GHRP-2 infusion increases both GH and IGF-I levels (5,14), and IGF-I administration reduces GH levels (26), it is quite likely that the potential beneficial effect of increasing IGF-I via GHRP-2 treatment on macrophage infiltration and plaque burden was negated by effects of increased GH. Consistent with this hypothesis, GH overexpression in ApoE−/− mice (which, similarly to GHRP, raised both GH and IGF-I) has been shown to enhance atherosclerosis, increase inflammatory markers, and raise blood pressure despite beneficial changes in circulating lipoproteins (27). GH has also been shown to stimulate secretion of inflammatory cytokines such as IFN-γ by macrophages in vitro (28).
Our finding that GHRP-2 does not reduce atherosclerosis in ApoE−/− mice contrasts with previous reports of antiatherogenic effects of the GHRP EP80317 (13) and hexarelin (12). Associated with its antiatherogenic effect, EP80317 reduced total cholesterol, whereas GHRP-2 did not. Additionally, in contrast to GHRP-2, EP80317 does not promote GH release and thus would not be expected to augment circulating IGF-I. Similarly, the study by Avallone et al. (12) demonstrating the antiatherogenic effect of hexarelin used a low dose of hexarelin that reportedly did not stimulate GH release. Taken together, it appears that there are key differences among GHRP in terms of their ability to both stimulate the GH/IGF-I axis and alter circulating lipoproteins, which may explain their differing effects in the ApoE−/− model of atherosclerosis.
Aortic gene expression profiling and measurement of circulating cytokines revealed that GHRP-2 had pleiotropic effects, some of which could be antiatherogenic. GHRP-2 reduced circulating IFN-γ levels and vascular IFN-γ expression. Intraperitoneal administration of IFN-γ to ApoE−/− mice has been shown to increase both atherosclerotic lesion size and the number of T lymphocytes present in the lesions (29). Mice deficient in both ApoE and the IFN-γ receptor have less atherosclerosis with relatively more extracellular matrix compared with ApoE−/− mice (30). Additionally, male mice deficient in both IFN-γ and ApoE have less plaque burden than ApoE−/− male mice (31). GHRP-2 also decreased expression of the proinflammatory cytokine macrophage MIF. MIF has been demonstrated to be atherogenic in multiple models of atherosclerosis. For example, ApoE−/− mice treated with an antibody to MIF had a decrease in plaque volume compared with mice treated with a control antibody (32). Additionally, mice deficient in both MIF and LDL receptor had decreased atherosclerosis accompanied with decreased SMC proliferation in vitro (33).
In conclusion, GHRP-2 protects SMC from OxLDL-induced IGF-IR down-regulation, peroxide generation, and apoptosis in vitro. GHRP-2 also prevents lipid uptake in cultured macrophages. GHRP-2 administration in vivo led to a complicated array of changes. Some of these changes, such as increasing IGF-I, reducing vascular oxidative stress, and reducing circulating IFN-γ, are potentially antiatherogenic. However, GHRP-2 failed to reduce plaque macrophage infiltration and overall plaque burden, indicating that at least in the ApoE−/− model of atherosclerosis, the net effect of GHRP-2 treatment is neutral in terms of plaque volume. In view of the antiatherosclerotic effects of increasing IGF-I (4), it is possible that the potential IGF-I-dependent antiatherosclerotic effects of GHRP-2 were negated by increased GH levels. Additional studies are needed to investigate the mechanisms mediating the ability of GHRP-2 to promote SMC survival, inhibit oxidative stress, and prevent OxLDL uptake in macrophages.
Supplementary Material
Acknowledgments
We are grateful for the help of John Wolpers, Mathew Irimpen, Jean-Luc Delafontaine, Phil Chou, and Ajay Reddy in analysis of aortic histopathology. Thanks also to GeorgeAnn Reynolds for her assistance with experimental design.
Footnotes
This work was supported by National Institutes of Health R01HL070241 (to P.D.) and R01HL080682 (to P.D.) and Tulane Research Enhancement Fund (to P.D.).
Disclosure Summary: J.S.T., S.S., Y.H., C.V., C.B., and P.D. have no disclosures.
First Published Online October 9, 2009
Abbreviations: ApoE, Apolipoprotein E; calcein-AM, calcein acetoxymethyl ester; CDC-H2F, 6-carboxy-2′,7′-dichlorodihydro-fluorescein diacetate; DHE, dihydroethidium; GHRP, GH-releasing peptide; GHSR, GH secretagogue receptor; hASMC, human aortic SMC; H&E, hematoxylin and eosin; IFN-γ, interferon-γ; IGF-IR, IGF-I receptor; IGFBP, IGF-binding protein; MCP-1, monocyte chemoattractant protein-1; MIF, macrophage migration inhibitory factor; nIgG, normal IgG; nLDL, native low-density lipoprotein; OxLDL, oxidized low-density lipoprotein; PBMC, peripheral blood mononuclear cells; PEG-SOD, polyethylene glycol-superoxide dismutase; SFM, serum-free media; SMC, smooth muscle cells; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling.
References
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y; American Heart Association Statistics Committee and Stroke Statistics S 2008 Heart disease and stroke statistics: 2008 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117:e25–e146 [DOI] [PubMed] [Google Scholar]
- Lusis AJ 2000 Atherosclerosis. Nature 407:233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Witztum JL 2001 Atherosclerosis. The road ahead. Cell 104:503–516 [DOI] [PubMed] [Google Scholar]
- Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, Song YH, Titterington J, Delafontaine P 2007 IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 27:2684–2690 [DOI] [PubMed] [Google Scholar]
- Bowers CY 2000 Growth hormone releasing peptides: physiology and clinical applications. Curr Opin Endocrinol Diabetes 7:168–174 [Google Scholar]
- McKee KK, Tan CP, Palyha OC, Liu J, Feighner SD, Hreniuk DL, Smith RG, Howard AD, Van der Ploeg LH 1997 Cloning and characterization of two human G protein-coupled receptor genes (GPR38 and GPR39) related to the growth hormone secretagogue and neurotensin receptors. Genomics 46:426–434 [DOI] [PubMed] [Google Scholar]
- Demers A, McNicoll N, Febbraio M, Servant M, Marleau S, Silverstein R, Ong H 2004 Identification of the growth hormone-releasing peptide binding site in CD36: a photoaffinity cross-linking study. Biochem J 382:417–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papotti M, Ghè C, Cassoni P, Catapano F, Deghenghi R, Ghigo E, Muccioli G 2000 Growth hormone secretagogue binding sites in peripheral human tissues. J Clin Endocrinol Metab 85:3803–3807 [DOI] [PubMed] [Google Scholar]
- Okumura H, Nagaya N, Enomoto M, Nakagawa E, Oya H, Kangawa K 2002 Vasodilatory effect of ghrelin, an endogenous peptide from the stomach. J Cardiovasc Pharmacol 39:779–783 [DOI] [PubMed] [Google Scholar]
- Kleinz MJ, Maguire JJ, Skepper JN, Davenport AP 2006 Functional and immunocytochemical evidence for a role of ghrelin and des-octanoyl ghrelin in the regulation of vascular tone in man. Cardiovasc Res 69:227–235 [DOI] [PubMed] [Google Scholar]
- Soeki T, Kishimoto I, Schwenke DO, Tokudome T, Horio T, Yoshida M, Hosoda H, Kangawa K 2008 Ghrelin suppresses cardiac sympathetic activity and prevents early left ventricular remodeling in rats with myocardial infarction. Am J Physiol Heart Circ Physiol 294:H426–H432 [DOI] [PubMed] [Google Scholar]
- Avallone R, Demers A, Rodrigue-Way A, Bujold K, Harb D, Anghel S, Wahli W, Marleau S, Ong H, Tremblay A 2006 A growth hormone-releasing peptide that binds scavenger receptor CD36 and ghrelin receptor up-regulates sterol transporters and cholesterol efflux in macrophages through a peroxisome proliferator-activated receptor γ-dependent pathway. Mol Endocrinol 20:3165–3178 [DOI] [PubMed] [Google Scholar]
- Marleau S, Harb D, Bujold K, Avallone R, Iken K, Wang Y, Demers A, Sirois MG, Febbraio M, Silverstein RL, Tremblay A, Ong H 2005 EP 80317, a ligand of the CD36 scavenger receptor, protects apolipoprotein E-deficient mice from developing atherosclerotic lesions. FASEB J 19:1869–1871 [DOI] [PubMed] [Google Scholar]
- Alba M, Fintini D, Bowers CY, Parlow AF, Salvatori R 2005 Effects of long-term treatment with growth hormone-releasing peptide-2 in the GHRH knockout mouse. Am J Physiol Endocrinol Metab 289:E762–E767 [DOI] [PubMed] [Google Scholar]
- Higashi Y, Peng T, Du J, Sukhanov S, Li Y, Itabe H, Parthasarathy S, Delafontaine P 2005 A redox-sensitive pathway mediates oxidized LDL-induced downregulation of insulin-like growth factor-1 receptor. J Lipid Res 46:1266–1277 [DOI] [PubMed] [Google Scholar]
- Li Y, Higashi Y, Itabe H, Song YH, Du J, Delafontaine P 2003 Insulin-like growth factor-1 receptor activation inhibits oxidized LDL-induced cytochrome C release and apoptosis via the phosphatidylinositol 3 kinase/Akt signaling pathway. Arterioscler Thromb Vasc Biol 23:2178–2184 [DOI] [PubMed] [Google Scholar]
- Okura Y, Brink M, Itabe H, Scheidegger KJ, Kalangos A, Delafontaine P 2000 Oxidized low-density lipoprotein is associated with apoptosis of vascular smooth muscle cells in human atherosclerotic plaques. Circulation 102:2680–2686 [DOI] [PubMed] [Google Scholar]
- Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, Bennett MR 2006 Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med 12:1075–1080 [DOI] [PubMed] [Google Scholar]
- Clarke MC, Littlewood TD, Figg N, Maguire JJ, Davenport AP, Goddard M, Bennett MR 2008 Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ Res 102:1529–1538 [DOI] [PubMed] [Google Scholar]
- Xu XB, Pang JJ, Cao JM, Ni C, Xu RK, Peng XZ, Yu XX, Guo S, Chen MC, Chen C 2005 GH-releasing peptides improve cardiac dysfunction and cachexia and suppress stress-related hormones and cardiomyocyte apoptosis in rats with heart failure. Am J Physiol Heart Circ Physiol 289:H1643–H1651 [DOI] [PubMed] [Google Scholar]
- Belkner J, Wiesner R, Rathman J, Barnett J, Sigal E, Kühn H 1993 Oxygenation of lipoproteins by mammalian lipoxygenases. Eur J Biochem 213:251–261 [DOI] [PubMed] [Google Scholar]
- Wen Y, Gu J, Vandenhoff GE, Liu X, Nadler JL 2008 Role of 12/15-lipoxygenase in the expression of MCP-1 in mouse macrophages. Am J Physiol Heart Circ Physiol 294:H1933–H1938 [DOI] [PubMed] [Google Scholar]
- Conti E, Carrozza C, Capoluongo E, Volpe M, Crea F, Zuppi C, Andreotti F 2004 Insulin-like growth factor-1 as a vascular protective factor. Circulation 110:2260–2265 [DOI] [PubMed] [Google Scholar]
- Cercek B, Fishbein MC, Forrester JS, Helfant RH, Fagin JA 1990 Induction of insulin-like growth factor I messenger RNA in rat aorta after balloon denudation. Circ Res 66:1755–1760 [DOI] [PubMed] [Google Scholar]
- Zhu B, Zhao G, Witte DP, Hui DY, Fagin JA 2001 Targeted overexpression of IGF-I in smooth muscle cells of transgenic mice enhances neointimal formation through increased proliferation and cell migration after intraarterial injury. Endocrinology 142:3598–3606 [DOI] [PubMed] [Google Scholar]
- Giustina A, Veldhuis JD 1998 Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev 19:717–797 [DOI] [PubMed] [Google Scholar]
- Andersson IJ, Ljungberg A, Svensson L, Gan LM, Oscarsson J, Bergström G 2006 Increased atherosclerotic lesion area in apoE deficient mice overexpressing bovine growth hormone. Atherosclerosis 188:331–340 [DOI] [PubMed] [Google Scholar]
- Sodhi A, Tripathi A 2008 Prolactin and growth hormone induce differential cytokine and chemokine profile in murine peritoneal macrophages in vitro: involvement of p-38 MAP kinase, STAT3 and NF-κB. Cytokine 41:162–173 [DOI] [PubMed] [Google Scholar]
- Whitman SC, Ravisankar P, Elam H, Daugherty A 2000 Exogenous interferon-γ enhances atherosclerosis in apolipoprotein E−/− mice. Am J Pathol 157:1819–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C 1997 IFN-γ potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest 99:2752–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman SC, Ravisankar P, Daugherty A 2002 IFN-γ deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E−/− mice. J Interferon Cytokine Res 22:661–670 [DOI] [PubMed] [Google Scholar]
- Burger-Kentischer A, Göbel H, Kleemann R, Zernecke A, Bucala R, Leng L, Finkelmeier D, Geiger G, Schaefer HE, Schober A, Weber C, Brunner H, Rütten H, Ihling C, Bernhagen J 2006 Reduction of the aortic inflammatory response in spontaneous atherosclerosis by blockade of macrophage migration inhibitory factor (MIF). Atherosclerosis 184:28–38 [DOI] [PubMed] [Google Scholar]
- Pan JH, Sukhova GK, Yang JT, Wang B, Xie T, Fu H, Zhang Y, Satoskar AR, David JR, Metz CN, Bucala R, Fang K, Simon DI, Chapman HA, Libby P, Shi GP 2004 Macrophage migration inhibitory factor deficiency impairs atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 109:3149–3153 [DOI] [PubMed] [Google Scholar]
- Sukhanov S, Higashi Y, Shai SY, Itabe H, Ono K, Parthasarathy S, Delafontaine P 2006 Novel effect of oxidized low-density lipoprotein; cellular ATP depletion via down regulation of glyceraldehyde-3-phosphate dehydrogenase. Circ Res 99:191–200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.