Abstract
Facilitative glucose transporters (GLUTs) including GLUT9, accelerate the facilitative diffusion of glucose across the plasma membrane. Studies in GLUT2-deficient mice suggested the existence of another GLUT in the mammalian β-cell responsible for glucose sensing. The objective of this study was to determine the expression and function of GLUT9 in murine and human β-cells. mRNA and protein expression levels were determined for both isoforms of GLUT9 in murine and human isolated islets as well as insulinoma cell lines (MIN6). Immunohistochemistry and subcellular localization were performed to localize the protein within the cell. Small interfering RNA knockdown of GLUT9 was used to determine the effect of this transporter, in the presence of GLUT2, on cell metabolism and insulin secretion in MIN6 and INS cells. In this report we demonstrate that GLUT9a and GLUT9b are expressed in pancreatic islets and that this expression localizes to insulin-containing β-cells. Subcellular localization studies indicate that mGLUT9b is found associated with the plasma membrane as well as in the high-density microsome fraction and low-density microsome fraction, whereas mGLUT9a appears to be located only in the high-density microsome and low-density microsome under basal conditions. Functionally GLUT9 appears to participate in the regulation of glucose-stimulated insulin secretion in addition to GLUT2. small interfering RNA knockdown of GLUT9 results in reduced cellular ATP levels that correlate with reductions in glucose-stimulated insulin secretion in MIN6 and INS cells. These studies confirm the expression of GLUT9a and GLUT9b in murine and human β-cells and suggest that GLUT9 may participate in glucose-sensing in β-cells.
The expression of glucose transporters GLUT9a and GLUT9b in murine and human β-cells is confirmed, and the results suggest that GLUT9 may participate in glucose sensing in β-cells.
The movement of glucose into cells is regulated by membrane-associated glucose transporters (GLUTs) that allow for the facilitative transport of hexose sugars across the plasma membrane. Two families of hexose transporters have been described. One group mediates the active cotransport of sodium and glucose (SLC5A family-sodium-dependent glucose transporters). The second family of transporters (SLC2A family-GLUTs) accelerates the transport of glucose along the gradient by facilitative diffusion across the cell membrane (1,2). Fourteen mammalian GLUTs have been identified and are expressed in a tissue-specific manner. GLUTs have intrinsic or inducible glucose transport activity and display variable affinities to specific hexose sugars (2,3). These transporters have 12 putative transmembrane-spanning helices and share conserved domains as signature patterns of glucose transporters (4,5). GLUT9 is a facilitative glucose transporter that is expressed as two splice variants differing only in their amino terminus (6,7). The GLUT9 gene codes for two alternative RNAs, suggesting that two different promoters may transcriptionally regulate GLUT9a and GLUT9b. Human GLUT9, which is very similar to mouse GLUT9, is a high-affinity GLUT with a Michaelis constant (Km) of 0.61 ± 0.16 mm (8). GLUT9 also transports fructose with a Km of 0.42 ± 0.09 mm, lower than the Km for the primary fructose transporter, GLUT5, of 15 ± 4 mm (9). Prior studies by our laboratory demonstrated that human and mouse GLUT9a are distributed in most tissues, whereas GLUT9b is predominately expressed in liver and kidney in mouse and kidney and placenta in humans (6,7,10). Also, we have shown that levels of mGLUT9a and mGLUT9b are elevated in the kidney and liver of diabetic mice (10).
Pancreatic β-cells are responsible for detecting changes in the concentration of blood glucose to signal insulin secretion and maintain glucose homeostasis. Uptake of glucose and subsequent phosphorylation by glucokinase initiates glucose-stimulated insulin secretion. The metabolism of glucose-6-phosphate generates ATP, which triggers closure of ATP-inhibited potassium channels, β-cell depolarization, calcium influx, and calcium-dependent exocytosis (4,11,12,13). To date, the primary GLUT found in β-cells is the low-affinity transporter, GLUT2 (Km ∼17 mm) (4,11,12,13,14,15,16,17,18). Mice lacking GLUT2 display a hyperglycemic, hypoinsulinemic phenotype (19), resulting from an impairment in the first phase of insulin secretion. β-Cells isolated from GLUT2-deficient mice lack the initial, rapid phase of insulin secretion in response to glucose stimulation but retain the second-phase insulin secretion. Maintenance of the second phase of insulin secretion in GLUT2-deficient mice is associated with the metabolism of glucose, suggesting that β-cells may express a second high-affinity GLUT system that participates in the regulation of insulin secretion (19,20). Furthermore, GLUT1, GLUT3, or GLUT8 do not compensate for the loss of GLUT2 (4,11,20).
In this study, we provide experimental evidence that GLUT9a and GLUT9b are expressed in pancreatic islets of Langerhans and that this expression is primarily restricted to insulin secreting β-cells. Functionally GLUT9 participates in the regulation of insulin secretion as small interfering RNA (siRNA) knockdown of GLUT9 results in reduced intracellular ATP levels and the attenuation of glucose-stimulated insulin secretion.
Materials and Methods
Cell culture and siRNA transfection
MIN6 cells (21) were maintained in DMEM containing 25 mm glucose, supplemented with 15% heat-inactivated fetal bovine serum, 50 μm β-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin, 100 μg/ml l-glutamine, 10 mm HEPES, and 1 mm sodium pyruvate, in 5% CO2-95% air at 37 C. MIN6 cells were used between passages 20 and 30.
INS cells were maintained in RPMI 1640 with 11.1 mmol d-glucose supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 mmol HEPES, 2 mmol/liter l-glutamine, 1 mmol/liter sodium pyruvate, and 50 μmol/liter β-mercaptoethanol, in 5% CO2-95% air at 37 C (22).
Mouse islet isolation and dispersion
Islets were isolated from C57BL female mice (Jackson Labs, Bar Harbor, ME) by collagenase digestion as previously described (23). Islets were cultured overnight in complete CMRL-1066 (Invitrogen, Carlsbad, CA) containing 2 mm l-glutamine (Cambrex, Walkersville, MD), 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT), 100 U/ml penicillin, 100 μg/ml streptomycin (Cambrex), and 5 mm glucose in 5% CO2-95% air at 37 C. Human islets were obtained from the Islet Cell Resource Center at Washington University School of Medicine (St. Louis, MO). Before experiments, the human islets were cultured for 48 h at 37 C in complete CMRL-1066 tissue culture medium in 5% CO2-95% air at 37 C. For immunostaining, islets were dispersed into individual cells by trypsin digestion as previously described (23).
RNA isolation and RT-PCR analysis
Total RNA was extracted from cultured cells and isolated islets using RNeasy RNA isolation kit (QIAGEN, Chatsworth, CA). cDNA was synthesized from total RNA using Superscript III (Invitrogen) following the manufacturer’s instructions. For each cDNA synthesis, the absence of reverse transcriptase was used as an internal control. Expression of mGLUT9a and mGLUT9b was determined using a nested RT-PCR analysis. Primers used to identify mGLUT9a were: forward, 5′-GGG TCA CCA GCA GAG GAG-3′ and reverse, 5′-TTC AAA GAG AAG GTA GCG TGG GCT-3′, followed by forward, 5′-TCA CCA GCA GAG GAG GAC AAA GAA-3′ and reverse, 5′-TGG ACC AAG GCA GGG ACA A-3′, which generated a band of 658 bp.
To identify mGLUT9b, the following primers were used: forward, 5′-TGA AAA GAA CTC CGC AGA AAC CAA-3′ and reverse, 5′-AGT TGG TAG CTG GCC ATG GTG ATA-3′, followed by forward, 5′-GAA ACC AAG GAA AGC CAG CGG AAA-3′ and reverse, 5′-CAG AAG CTC CAG CAC AGA CAC CAG-3′, which generated a band of 857 bp. Expression of GAPDH was used as an internal RNA control. Primers used were: forward, 5′-AGT GGA GAT TGT TGC CAT CAA CGA-3′ and reverse, 5′-GGG AGT CGC TGC TGT TGA AGT CGC AGG A-3′, which generated a band of 791bp.
Expression of hGLUT9a and hGLUT9b was studied by nested PCR analysis as follows: hGLUT9a forward, 5′-ACT GAG ACC CAT GGC AAG GAA A-3′ and hGLUT9b forward, 5′-ATG AAG CTC AGT AAA AAG GAC-3′ with common reverse primer: 5′-GAG TGT CTG GGT CTA TTG GA-3′. For the nested reaction, the following primers were used: hGLUT9a forward, 5′-TAG GAA TTC CAA GGA ACT GGG CCT-3′ and hGLUT9b forward, 5′-ACG TCC ATG CCT TCT TTC CCA TGA-3′ with common reverse primer, 5′-CGA GGA GAA GAT GAA GAA AGT GAT TCA GCG-3′, which generated bands of 280 and 189 bp, respectively. Expression of actin was used as a control. Primers used were: forward, 5′-TGC GTG ACA TTA AGG AGA AG-3′ and reverse, 5′-CTG CAT CCT GTC GGC AAT G-3′, which generated a band of 316 bp.
siRNA transfection
The polyamine transfection reagent, Trans IT-TKO (Mirus Corp., Madison, WI) was used to transfect the MIN-6 cells according to the manufacturer’s instructions. siRNA targeting mouse GLUT9 (sense, GGA AGU CCA CAU UGC UGG Utt; antisense, ACC AGC AAU GUG GAC UUC Ctc), GAPDH (positive control) (AM 4624) as well as negative control siRNA (no. 5) (AM 4642) were purchased from Ambion (Austin, TX). Experiments were performed 48–72 h after transfection.
siRNA was transfected into the INS cells by reverse transfection with the transfection reagent Lipofectamine RNAimax (Invitrogen) according to the manufacturer’s instructions. siRNA targeting rat GLUT9 (sense, UUG UCA AAU CUA UAC GUU GCA AUC UAU; antisense, AGA UUG CAA CGU AUA GAU UUG ACaa), and the negative control scrambled RNA was purchased from IDT (Coralville IA).
siRNA-mediated knockdown was assessed using quantitative PCR 72 h after transfection. Expression of rat GLUT9 was studied using primers forward, 5′-AGT CAA CTG GCT CTC CAA CTT CGT-3′ and reverse, 5′-CGA ATG CCT GGC TGA TTT CTG CAT-3′, which generate 176-bp bands. GAPDH was used as a control using primers forward, 5′-ACA AGA TGG TGA AGG TCG GTG TGA-3′ and reverse, 5′-AGC TTC CCA TTC TCA GCC TTG ACT-3′, which generated a 199-bp band.
Western blot analysis
Western blot analysis was performed as previously described (10) using rabbit antisera generated against peptides of mGLUT9a (MDSRELALASLMC) or mGLUT9b (MKLSEKNSAETKESC) at dilutions of 1:5000. Preimmune serum was used as a negative control. Human GLUT9 was also analyzed using hGLUT9a- and hGLUT9b-specific antisera generated in rabbits at a dilution of 1:1000 (7). Horseradish peroxidase-conjugated preabsorbed goat antirabbit and goat antimouse antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) were used at a dilution of 1:10,000.
Immunohistochemistry studies
Dispersed islet cells were centrifuged onto slides and MIN6 cells were grown on coverslips. Cells were washed twice with PBS, fixed for 20 min in 3% paraformaldehyde, and permeabilized with 0.1% Tween 20 for 10 min and then blocked with nonspecific antiserum containing 20% normal goat serum in 2% BSA/PBS for 30 min. Cells were incubated for 1 h with rabbit anti-GLUT9a (20 μg/ml), rabbit anti-GLUT9b (20 μg/ml), or guinea pig antihuman insulin diluted 1:250 (Linco, St. Louis, MO) in 2% BSA/PBS, washed with PBS, and probed with goat antirabbit Alexa 488 antibody or goat anti-guinea pig Alexa 546 antibody (1:200 in 2% BSA/PBS). Cells were washed with PBS and nuclei were stained with TOPRO-3 iodide for 10 min. Cells were then washed in PBS and mounted using Vectashield (Vector Laboratories, Burlingame, CA). Specimens were examined by confocal microscopy using a C1 confocal microscope (Nikon, Tokyo, Japan).
Protein isolation and subcellular fractionation
MIN6 cell protein lysates were prepared by treatment of 500,000 cells with 200 μl of lysis buffer [50 mm Tris-HCl, 150 mm NaCl, 0.5% Nonidet P-40, 10% glycerol, 1 mm EDTA, and protease inhibitor (PI) mix of 1.4 μg/ml aprotinin; 1 μg/ml each leupeptin, antipain, benzamidine, chymostatin, pepstatin A; 5 μg/ml trypsin inhibitor; 87 μg/ml phenylmethylsulfonyl fluoride; Sigma, St. Louis, MO)] for 30 min on ice. For gel electrophoresis, 10 μg protein was solubilized in 15 μl vol Laemmli buffer (24).
For the isolation of plasma membrane (PM), high-density microsome (HDM) and low-density microsome (LDM) fractions, MIN6 cells were collected in homogenization buffer [20 mm Tris, 1 mm EDTA, 255 mm sucrose (pH 7.4)] with PI mix, homogenized in a Dounce homogenizer, and centrifuged at 600 × g for 10 min. Supernatants were then centrifuged at 14,000 × g for 15 min, and the resulting pellet was resuspended in homogenization buffer with PI mix and overlaid on an equal volume of 38% sucrose cushion and centrifuged at 100,000 × g for 1 h using a swinging bucket rotor. The PM fraction, obtained at the interface of sample and the sucrose cushion, was then centrifuged at 50,000 × g for 30 min, and the pellet containing the PM fraction was resuspended in 10 mm Tris. The supernatant remaining after the 14,000 × g spin was removed and centrifuged a second time at 50,000 × g for 30 min to obtain the HDM fraction The resultant supernatant was centrifuged a third time at 200,000 × g for 75 min to isolate the LDM fraction. The HDM and LDM factions were suspended in 10 mm Tris. Protein was quantified using the BCA reagent (Bio-Rad, Hercules, CA), and the purity of the subcellular fractions was determined by Western blot analysis using antibodies to Na/K-ATPase protein for the PM fraction (a generous gift from Dr. Robert Mercer, Washington University School of Medicine, St. Louis, MO), GM130, a Golgi matrix protein, for the LDM fraction (PharMingen, San Diego, CA) and calnexin, an integral endoplasmic reticulum membrane protein, for the HDM fraction (Stressgen, Victoria, British Columbia, Canada).
Insulin secretion
Previously described methods were used to evaluate glucose-stimulated insulin secretion by MIN6 cells (25,26). In brief, MIN6 cells (4 × 105) were washed two times followed by 1 h preincubation in insulin secretion assay buffer (Krebs-Ringer solution containing HEPES, sodium bicarbonate, and 0.1% BSA. One hour later, the buffer was replaced with insulin secretion assay buffer containing 2 or 25 mm glucose, the cells were incubated for 1 h at 37 C, the assay media were then removed and centrifuged to remove detached cells, and insulin content was determined by the Metabolism Core of the Diabetes Research and Training Center at Washington University. Similar methods were used to measure insulin secretion from the primary murine β-cells and as described elsewhere (12). For INS cells, the cells were plated and reverse transfected with siRNA at a density of about 2 × 106 cells/well in a six-well plate. Forty-eight hours after transfection, media were changed from culture media containing 11.1 mmol/liter glucose to fresh media containing 5 mmol/liter glucose. Seventy-two hours after transfection, insulin secretion was assayed. Cells were washed twice with 1 ml of Hanks’ balanced salt solution with 3 mmol/liter glucose followed by a 2-h preincubation in 2 ml of the same buffer. Insulin secretion was then measured using static incubation for 1 h in 2 ml of Hanks’ balanced salt solution containing 2 or 25 mmol/liter glucose. Insulin was measured using Alpco Ultrasensitive rat insulin ELISA kit (ALPCO Diagnostics, Salem NH).
Intracellular ATP assay
MIN6 cells (4 × 105 cells/volume, six-well plate) were removed from tissue culture plates using 1 ml cell dissociation solution (Sigma) followed by repeated pipetting, transferred to a 1.5-ml pellet disposable tube (Fisher, Hampton, NH), and centrifuged for 1 min at 7000 rpm. Cell dissociation solution was discarded, the pellet washed twice with 1 ml PBS, and then extracted in 200 ml 0.1 n NaOH. The pellet was homogenized with the pestle and heated in 80 C water bath for 20 min. The homogenate (100 μl) was neutralized with a 50-ml mixture of 0.15 n HCl and 0.1 m Tris HCl (pH 6.6) to form 34 mm Tris HCl (pH 8.1). The extract was then analyzed for ATP analysis by enzymatic cycling reaction as previously described (27). ATP values were normalized to the amount of protein in the cell lysate. For INS cells, cells were plated, transfected, and had media changed as in insulin secretion assay. Seventy-two hours after transfection, cells removed and assay performed as with MIN6 cell.
Statistical analysis
Statistical analysis was performed using Student’s t test for comparing changes in ATP levels between control and siRNA-treated cells. Results are expressed as means ± se for at least three separate experiments. Significance was defined at P < 0.05.
Results
Expression of GLUT9 in β-cells
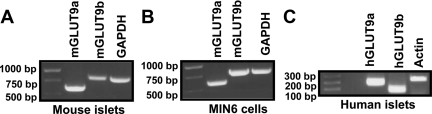
The expression of mGLUT9a and mGLUT9b was examined by nested RT-PCR using RNA isolated from B6SJLF1/J mouse islets, MIN6 insulinoma cells, and human islets. As shown in Fig. 1, A and C, mRNA for both mGLUT9a and mGLUT9b is present in isolated mouse and human islets. We were unable to detect the presence of mGLUT9a and mGLUT9b mRNA in mouse acinar (exocrine) tissue (data not shown), suggesting that pancreatic expression of GLUT9 may be selective for endocrine tissue. Mouse insulinoma MIN6 cells, which represent a homogenous cell population that retain many of the phenotypes of primary β-cells, have been widely used to study β-cell responses in vitro (21). Similar to islets, MIN6 cells express mGLUT9a and mGLUT9b as determined by RT-PCR (Fig. 1B), suggesting that both variants of GLUT9 are expressed by insulin-producing β-cells.
Figure 1.
GLUT9 mRNA detected in MIN6 cells, isolated mouse islets, and isolated human islets. A, Expression of GLUT9 variants in mouse islets. Mouse islets were isolated by collagenase digestion as described in Materials and Methods. RNA was isolated and RT-PCR analysis was performed using primers specific for mGLUT9a, mGLUT9b, and GAPDH (control). B, Expression of GLUT9 variants in MIN6 cells. RNA was isolated from MIN6 cells and RT-PCR analysis was performed using primers specific for mGLUT9a, mGLUT9b, and GAPDH (control). C, Expression of GLUT9 variants in human islets. RNA was isolated from isolated human islets and RT-PCR analysis was performed using primers specific for hGLUT9a, hGLUT9b, and actin (control). Results are representative of three independent experiments.
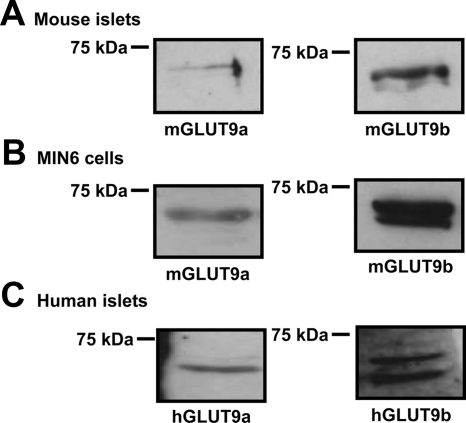
Using antibodies raised against N-terminal peptides of mGLUT9a and mGLUT9b, the expression of each variant of GLUT9 was examined in mouse islets (Fig. 2A) and MIN6 cells (Fig. 2B) by Western blot analysis seen as bands representing the glycosylated forms of the proteins, similar to that seen previously in kidney and liver studies (10). GLUT9b protein is consistently detected as a double band representing differentially glycosylated forms of the protein. Similar to mouse islets and MIN6 cells, both hGLUT9a and hGLUT9b are present in human islets as determined by Western analysis using antibodies generated against the N terminus of each of the human GLUT9 splice variants (Fig. 2C).
Figure 2.
GLUT9 protein expression in MIN6 cells, isolated mouse islets, and isolated human islets. A, GLUT9 protein expression in isolated mouse islets. Mouse islets were isolated by collagenase digestion as described in Materials and Methods. Cell lysates were analyzed by Western blot using antibodies to mGLUT9a and mGLUT9b. B, GLUT9 protein expression in MIN6 cells. MIN6 cell lysates were analyzed by Western blot using antibodies to mGLUT9a and mGLUT9b. C, GLUT9 protein expression in isolated human islets. Cell lysates were prepared from isolated human islets and analyzed by Western blot using antibodies to hGLUT9a and hGLUT9b. Preimmune serum was used as a negative control. Results are representative of three independent experiments.
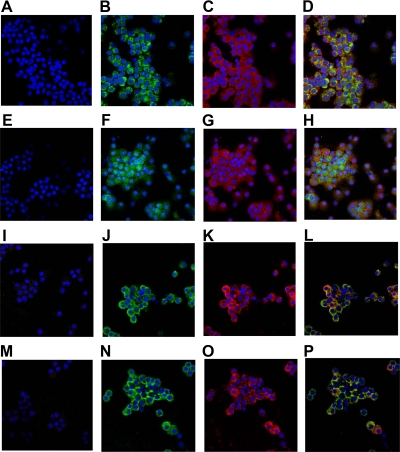
To further characterize the expression of mGLUT9 in mouse islets, immunohistochemistry was performed. Isolated mouse islets were dispersed into single cells by trypsin digestion and cytospun onto slides before staining. Rabbit IgG was used as a negative control (Fig. 3, A, E, I, and M). An antiinsulin antibody was used to identify insulin-producing β-cells within the dispersed islet population (Fig. 3, C and G). As seen in Fig. 3B (mGLUT9a) and Fig. 3F (mGLUT9b), both variants are detected in dispersed mouse islets by immunohistochemistry, and expression of mGLUT9a and mGLUT9b appears to be specific for β-cells as determined by colocalization with insulin (Fig. 3, D and H). Furthermore, when dispersed human islets were subjected to immunohistochemical analysis, hGLUT9a and hGLUT9b were also detected (Fig. 3, J and N) and colocalized with the insulin-positive cells (Fig. 3, K and O), suggesting that expression is β-cell specific (Fig. 3, L and P).
Figure 3.
Immunolocalization of GLUT9 in dispersed mouse and human islets. A–H, GLUT9 expression in dispersed mouse islets. Isolated mouse islets were dispersed into single cells by trypsin digestion and analyzed by immunohistochemistry using primary antibodies to mGLUT9a (B and D) or mGLUT9b (F and H) followed by incubation with Alexafluor-488 conjugated secondary antibody (green). β-Cells within the population were identified using a primary antibody to insulin followed by incubation with an Alexafluor-546 conjugated secondary antibody (red) (C and D, G and H). Nuclei were visualized by TO-PRO-3 iodide staining (blue). I–P, GLUT9 expression in dispersed human islets. Human islets were dispersed into single cells by trypsin digestion and analyzed by immunohistochemistry using primary antibodies to hGLUT9a (J and L) or hGLUT9b (N and P) followed by incubation with Alexafluor-488 conjugated secondary antibody (green). β-Cells within the population were identified using an antibody to insulin followed by incubation with Alexafluor-546 conjugated secondary antibody (red) (K and L, O and P). Nuclei were visualized by TO-PRO-3 iodide staining (blue). Rabbit IgG was used as a negative control (A, E, I, and M). Results are representative of three independent experiments.
Localization of GLUT9 in β-cells
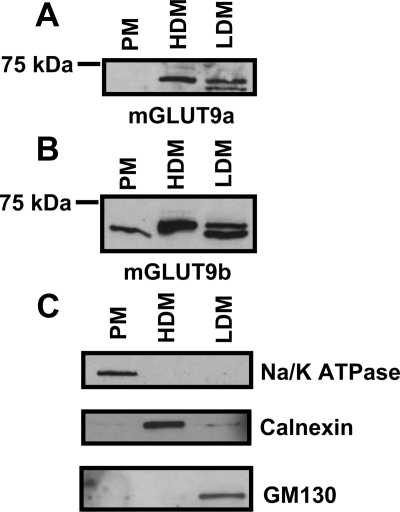
To further examine the subcellular localization of mGLUT9, MIN6 cells were homogenized in sucrose buffer and centrifuged to obtain PM, HDM, and LDM fractions. These fractions were then analyzed for the presence of mGLUT9a and mGLUT9b by Western blot (Fig. 4). Consistent with its cellular localization as determined by histology, mGLUT9b is present in all three cellular fractions, whereas mGLUT9a is present only in the HDM and LDM fractions, suggesting an intracellular localization under basal conditions. The purity of each fraction was determined by Western blot analysis of Na/K ATPase (PM), calnexin (HDM), and GM130 (LDM) (Fig. 4C). Further investigation will determine whether mGLUT9a and mGLUT9b localization changes in response to various stimuli.
Figure 4.
Subcellular localization of GLUT9 in MIN6 cells. A and B, GLUT9 protein expression in MIN6 subcellular fractions. MIN6 cells were homogenized and subcellular fractions were isolated: PM, HDM, and LDM. Subcellular fractions were analyzed by Western blot using antibodies to mGLUT9a (A) and mGLUT9b (B). Fractions were also analyzed by Western blot using antibodies to Na/K-ATPase (PM), calnexin (HDM), and GM130 (LDM) to determine the relative purity of the fractions (C). Results are representative of three independent experiments.
GLUT9 function in β-cells
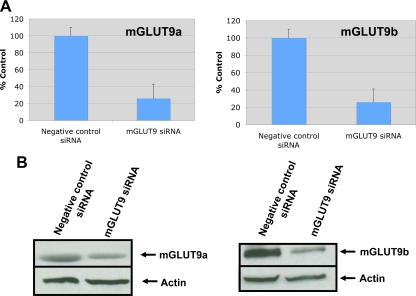
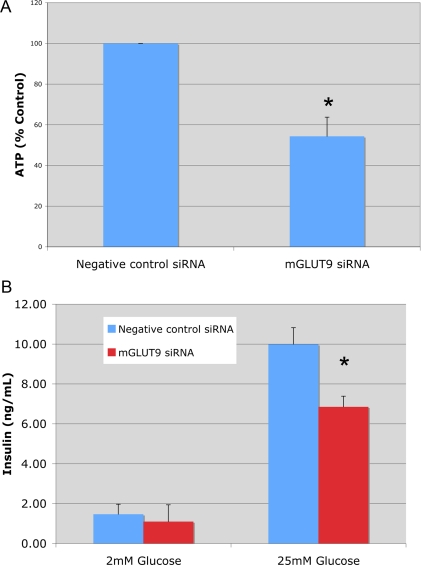
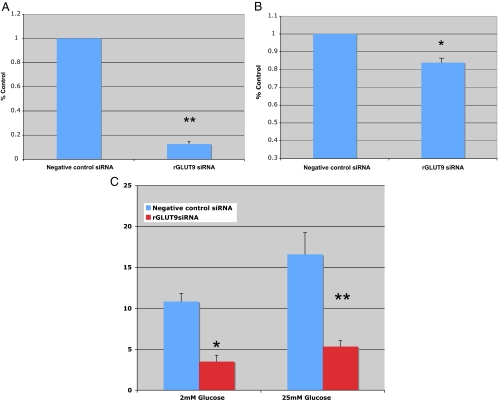
The cellular function of GLUT9 in β-cells was examined using a siRNA knockdown strategy in MIN6 cells. Using this methodology, the mRNA and protein expression levels of mGLUT9a and mGLUT9b in MIN6 cells transiently transfected with siRNA targeting GLUT9 are reduced by about 60–70% compared with the nonspecific siRNA control (Western blot analysis, Fig. 5). In MIN6 cells deficient in GLUT9 (a and b), basal intracellular levels of ATP are reduced by about 50% compared with control cells (Fig. 6A). Consistent with reductions in ATP levels, glucose-stimulated insulin secretion by MIN6 cells is attenuated by 30% compared with MIN6 cells transfected with negative control siRNA (Fig. 6B). The same experiments were repeated in a different cell line, rat insulinoma cell line INS, and the findings agreed with those in the MIN6. In INS cells transiently transfected with siRNA targeting rat GLUT9, mRNA levels were reduced by 87% (Fig. 7A). As with the MIN6 cells, ATP levels were significantly lower in the rGLUT9 siRNA-treated cells (Fig. 7B). Consistent with this decrease, glucose-stimulated insulin secretion by INS cells was significantly decreased by 68% compared with INS cell transfected with negative control siRNA (Fig. 7C). These findings suggest that GLUT9 participates, in part, in the regulation of glucose-stimulated insulin secretion by β-cells.
Figure 5.
siRNA knockdown of GLUT9 expression in MIN6 cells. A, Real-time RT-PCR analysis of mGLUT9a and mGLUT9b expression in MIN6 cells after transfection with mGLUT9 siRNA or scrambled siRNA for 48 h. B, Western blot analysis of MIN6 cell lysates after transfection with mGLUT9 siRNA or scrambled siRNA for 72 h. Results are typical of several experiments.
Figure 6.
Effect of mGLUT9 siRNA on MIN6 intracellular ATP levels and insulin secretion. A, MIN6 cells were transfected with siRNA targeting mouse GLUT9 or with negative control siRNA for 72 h before ATP analysis. Cells were then starved (1 h) and treated for 1 h with 2 or 25 mm glucose, washed with PBS and extracted with NaOH. ATP was measured by enzymatic cycling reactions. Results are the average of three independent experiments ± sem. *, P < 0.05; B, MIN6 cells were transfected with siRNA targeting GLUT9 or negative control siRNA for 72 h before insulin secretion. Cells were then starved (1 h) and treated for 1 h with 2 or 25 mm glucose. Insulin secretion was determined by RIA. Results are the average of three independent experiments ± sem. *, P < 0.05.
Figure 7.
Effect of rGLUT9 siRNA on rat INS cells. A, Effect on ATP: real-time RT-PCR analysis of rGLUT9 expression in INS cells after transfection with rGLUT9 siRNA or scrambled siRNA for 72 h. *, P < 0.001. Results are the average of three independent experiments ± sem. *, P < 0.05. B, Effect on INS intracellular ATP levels. INS cells were transfected with siRNA targeting rat GLUT9 or negative control siRNA for 72 h before ATP analysis. Cells were then starved (1 h) and treated for 1 h with 2 or 25 mm glucose, washed with PBS, and extracted with NaOH. ATP was measured by enzymatic cycling reactions. Results are the average of three independent experiments ± sem. *, P < 0.01. C, Effect on INS insulin secretion. INS cells were transfected with siRNA targeting GLUT9 or with negative control siRNA for 72 h before insulin secretion. Cells were then starved (1 h) and treated for 1 h with 2 or 25 mm glucose. Insulin secretion was determined by RIA. Results are the average of three independent experiments ± sem. *, P < 0.03; **, P < 0.05.
Discussion
Recently, we have shown that mGLUT9a and mGLUT9b are expressed in a tissue-selective fashion, and the levels of expression are elevated in kidney and liver of diabetic mice (10). These findings suggest that GLUT9 may participate in the regulation of glucose homeostasis in these tissues and that the expression of GLUT9 may be differentially regulated by the diabetic state (6,7,10). In the current study, we expand the tissue selective expression of GLUT9a and GLUT9b to insulin-producing β-cells found in islets of Langerhans. The expression of GLUT9a and -9b was shown in both rodent and human β-cells.
The ability of pancreatic β-cells to sense extracellular glucose concentrations and deliver the appropriate amount of insulin is based on the high Km of glucokinase for glucose and the low-affinity high-Km glucose transporter GLUT2 (4,11,12,14,15,16,17,18). Thorens and coworkers (19) have shown that mice deficient in GLUT2 display a hyperglycemic, hypoinsulinemic phenotype that is associated with attenuation of the first-phase insulin secretion. In contrast, the prolonged second phase of insulin secretion in response to glucose is retained in these GLUT-2-deficient mice. Thorens and colleagues (11,19,20) speculated that a yet-unidentified high-affinity GLUT may be responsible for this retention of the second phase of insulin secretion in the absence of GLUT2 expression but ruled out increased expression of GLUT1, GLUT3, and GLUT8 under these conditions. Additional studies by Yamada et al. (28) identified the presence of a high-affinity, low-Km GLUT (Km 1.6 mm) in addition to GLUT2 (Km 17 mm) in MIN6 cells. Whereas the low-Km transporter was assumed to be GLUT1, which is expressed at very low levels in β-cells, additional studies have shown that either GLUT1 or GLUT2 can restore glucose-stimulated insulin secretion to normal levels in GLUT2-deficient cells, suggesting that the Km of the transporter is not limiting because GLUT1 is a high-affinity, low-Km transporter.
We now propose that our results are consistent with GLUT9 functioning as the high-affinity GLUT found in β-cells. We show that GLUT9 is expressed in mouse and human β-cells and that reduction in GLUT9 expression (siRNA knockdown) results in reduced intracellular ATP levels in MIN6 cells. Consistent with reductions in cellular ATP levels, glucose-stimulated insulin secretion is attenuated in MIN6 cells and in INS cells in which GLUT9 levels have been reduced using siRNA. siRNA knockdown of GLUT9 does not fully inhibit glucose-induced insulin secretion, presumably due to the presence of GLUT2 in these cells. Despite the presence of GLUT2, however, knockdown of GLUT9 in MIN6 and INS cells results in a significant decrease in insulin secretion, suggesting that GLUT2 is not the only glucose-sensing transporter. Due to the lower Km of GLUT9 compared with GLUT2, it is presumed that GLUT9 is functional, even during normal blood glucose levels. This is surprising because glucose stimulation of insulin secretion occurs above 5 mm glucose. As seen by the immunohistochemistry and subcellular fractionation; however, it appears the majority of GLUT9 protein is not present on the PM in the basal state. It is possible that fluctuations in glucose levels may regulate GLUT9 translocation to the cell surface, thus affecting insulin secretion. Importantly, the relative contribution of each of these transporters to the first and second phase of glucose-stimulated insulin secretion from β-cells remains to be elucidated but is an area we are further exploring.
Acknowledgments
We thank David Sonderman for technical assistance and Colleen Bratcher (in laboratory of J.A.C.) and Maria Remedi and Dr. Colin Nichols (Washington University) for their expert assistance in islet isolation and thoughtful discussions concerning GLUT9. We also thank the Diabetes Research Training Center (Washington University) for their expertise in performing the RIA assay.
Footnotes
This work was supported by National Institutes of Health (NIH) Training Grant 5 T32 DK0007296-27 (to S.A.E), NIH Training Grant Supplement to T32 DK007130/T32 DK007120 (to M.D.), NIH Grant DK52194 (to J.A.C), and an American Diabetes Association research grant (to K.H.M.).
Disclosure Summary: The authors have nothing to declare.
First Published Online October 6, 2009
Abbreviations: GLUT, Facilitative glucose transporter; HDM, high-density microsome; Km, Michaelis constant; LDM, low-density microsome; PI, protease inhibitor; PM, plasma membrane; siRNA, small interfering RNA.
References
- Wood IS, Trayhurn P 2003 Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr 89:3–9 [DOI] [PubMed] [Google Scholar]
- Thorens B 1996 Glucose transporters in the regulation of intestinal, renal, and liver glucose fluxes. Am J Physiol 270:G541–G553 [DOI] [PubMed] [Google Scholar]
- Olson AL, Pessin JE 1996 Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu Rev Nutr 16:235–256 [DOI] [PubMed] [Google Scholar]
- Joost HG, Thorens B 2001 The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review). Mol Membr Biol 18:247–256 [DOI] [PubMed] [Google Scholar]
- Joost HG, Bell GI, Best JD, Birnbaum MJ, Charron MJ, Chen YT, Doege H, James DE, Lodish HF, Moley KH, Moley JF, Mueckler M, Rogers S, Schürmann A, Seino S, Thorens B 2002 Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol Endocrinol Metab 282:E974–E976 [DOI] [PubMed] [Google Scholar]
- Carayannopoulos MO, Schlein A, Wyman A, Chi M, Keembiyehetty C, Moley KH 2004 GLUT9 is differentially expressed and targeted in the preimplantation embryo. Endocrinology 145:1435–1443 [DOI] [PubMed] [Google Scholar]
- Augustin R, Carayannopoulos MO, Dowd LO, Phay JE, Moley JF, Moley KH 2004 Identification and characterization of human glucose transporter-like protein-9 (GLUT9): alternative splicing alters trafficking. J Biol Chem 279:16229–16236 [DOI] [PubMed] [Google Scholar]
- Manolescu AR, Augustin R, Moley K, Cheeseman C 2007 A highly conserved hydrophobic motif in the exofacial vestibule of fructose transporting SLC2A proteins acts as a critical determinant of their substrate selectivity. Mol Membr Biol 24:455–463 [DOI] [PubMed] [Google Scholar]
- Kane S, Seatter MJ, Gould GW 1997 Functional studies of human GLUT5: effect of pH on substrate selection and an analysis of substrate interactions. Biochem Biophys Res Commun 238:503–505 [DOI] [PubMed] [Google Scholar]
- Keembiyehetty C, Augustin R, Carayannopoulos MO, Steer S, Manolescu A, Cheeseman CI, Moley KH 2006 Mouse glucose transporter 9 splice variants are expressed in adult liver and kidney and are up-regulated in diabetes. Mol Endocrinol 20:686–697 [DOI] [PubMed] [Google Scholar]
- Thorens B 2001 GLUT2 in pancreatic and extra-pancreatic gluco-detection. Mol Membr Biol 18:265–273 (Review) [DOI] [PubMed] [Google Scholar]
- MacDonald PE, Joseph JW, Rorsman P 2005 Glucose-sensing mechanisms in pancreatic β-cells. Philos Trans R Soc Lond B Biol Sci 360:2211–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschinsky FM 1996 Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes 45:223–241 [DOI] [PubMed] [Google Scholar]
- Johnson JH, Newgard CB, Milburn JL, Lodish HF, Thorens B 1990 The high Km glucose transporter of islets of Langerhans is functionally similar to the low affinity transporter of liver and has an identical primary sequence. J Biol Chem 265:6548–6551 [PubMed] [Google Scholar]
- Thorens B, Sarkar HK, Kaback HR, Lodish HF 1988 Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and β-pancreatic islet cells. Cell 55:281–290 [DOI] [PubMed] [Google Scholar]
- Orci L, Thorens B, Ravazzola M, Lodish HF 1989 Localization of the pancreatic β cell glucose transporter to specific plasma membrane domains. Science 245:295–297 [DOI] [PubMed] [Google Scholar]
- Scheepers A, Joost HG, Schürmann A 2004 The glucose transporter families SGLT and GLUT: molecular basis of normal and aberrant function. JPEN J Parenter Enteral Nutr 28:364–371 [DOI] [PubMed] [Google Scholar]
- Tal M, Liang Y, Najafi H, Lodish HF, Matschinsky FM 1992 Expression and function of GLUT-1 and GLUT-2 glucose transporter isoforms in cells of cultured rat pancreatic islets. J Biol Chem 267:17241–17247 [PubMed] [Google Scholar]
- Guillam MT, Hümmler E, Schaerer E, Yeh JI, Birnbaum MJ, Beermann F, Schmidt A, Dériaz N, Thorens B 1997 Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat Genet 17:327–330 [DOI] [PubMed] [Google Scholar]
- Guillam MT, Dupraz P, Thorens B 2000 Glucose uptake, utilization, and signaling in GLUT2-null islets. Diabetes 49:1485–1491 [DOI] [PubMed] [Google Scholar]
- Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, Oka Y, Yamamura K 1990 Establishment of a pancreatic β cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 127:126–132 [DOI] [PubMed] [Google Scholar]
- Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB 2000 Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49:424–430 [DOI] [PubMed] [Google Scholar]
- Kelly CB, Blair LA, Corbett JA, Scarim AL 2003 Isolation of islets of Langerhans from rodent pancreas. Methods Mol Med 83:3–14 [DOI] [PubMed] [Google Scholar]
- Laemmli UK 1970 Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- Ramshur EB, Rull TR, Wice BM 2002 Novel insulin/GIP co-producing cell lines provide unexpected insights into Gut K-cell function in vivo. J Cell Physiol 192:339–350 [DOI] [PubMed] [Google Scholar]
- Eizirik SSaD 1994 Culture of human pancreatic islet cells. In: GE J, ed. Methods in molecular medicine: human cell culture protocols. Totowa, NJ: Humana; 391–407 [DOI] [PubMed] [Google Scholar]
- Chi MM, Hoehn A, Moley KH 2002 Metabolic changes in the glucose-induced apoptotic blastocyst suggest alterations in mitochondrial physiology. Am J Physiol Endocrinol Metab 283:E226–E232 [DOI] [PubMed] [Google Scholar]
- Yamada K, Nakata M, Horimoto N, Saito M, Matsuoka H, Inagaki N 2000 Measurement of glucose uptake and intracellular calcium concentration in single, living pancreatic β-cells. J Biol Chem 275:22278–22283 [DOI] [PubMed] [Google Scholar]