Abstract
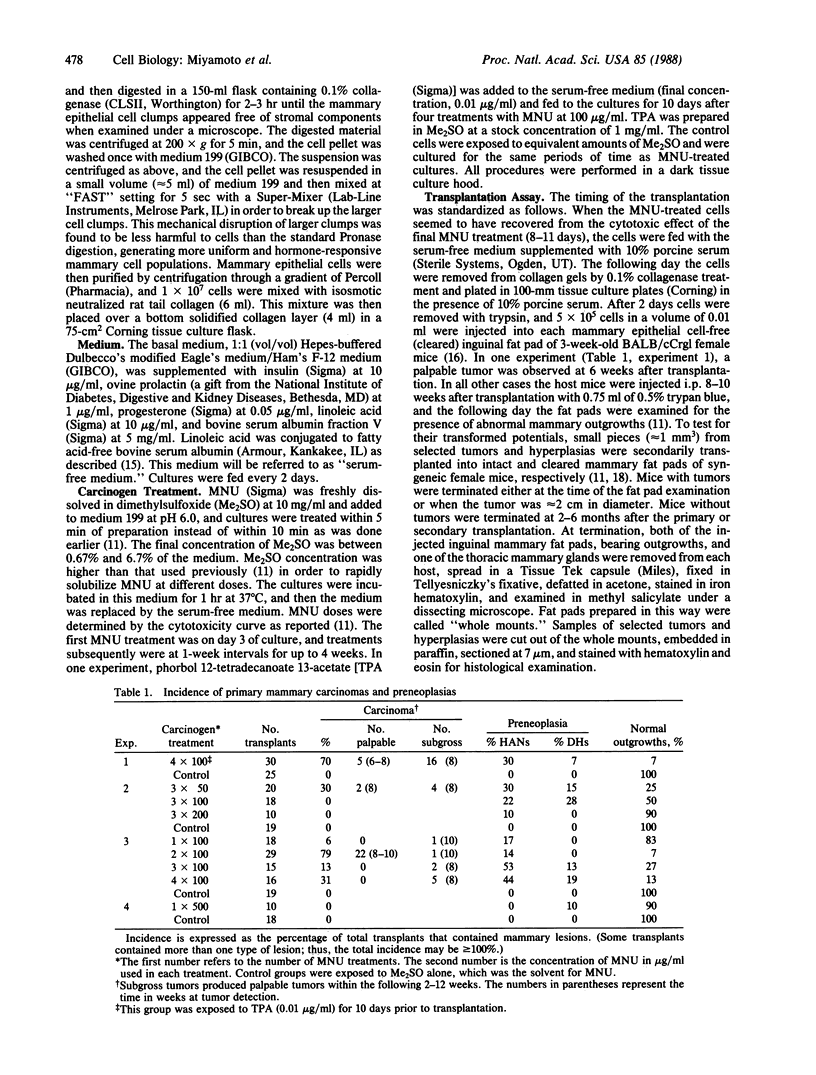
High-efficiency neoplastic transformation of mouse mammary epithelial cells in primary collagen gel culture was induced by N-methyl-N-nitrosourea (MNU). Mammary epithelial cells, isolated from virgin BALB/c mice, were embedded within collagen gels and grown in a serum-free medium containing prolactin, progesterone, and linoleic acid. The cells were then treated with MNU on day 3 of culture and subsequently at weekly intervals for up to 4 weeks. Eleven to 14 days after the final carcinogen treatment, the cells were removed from the collagen gels and injected into the cleared mammary fat pads of syngeneic hosts to assay for transformed cell populations. A single exposure or multiple exposures of these cells to MNU was effective in inducing tumorigenic cells that produced palpable tumors as early as 6 weeks after transplantation. Two treatments with MNU (100 micrograms/ml) were optimal for neoplastic transformation and produced tumors in 79% of the injected fat pads. All the tumors originated at the site of injection and had extensive central necroses. Histological examination indicated that the tumors were mammary carcinomas. Secondary transplantation of tumor pieces into intact mammary glands produced palpable carcinomas of the same histology within 1-8 weeks. Control cells cultured for the same periods of time as MNU-treated cells produced only ductal outgrowths that were morphologically similar to those found in the mammary glands of adult virgin hosts. This system provides a distinct means to study the mechanism of mammary neoplastic transformation at cellular and molecular levels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang S. E., Keen J., Lane E. B., Taylor-Papadimitriou J. Establishment and characterization of SV40-transformed human breast epithelial cell lines. Cancer Res. 1982 May;42(5):2040–2053. [PubMed] [Google Scholar]
- DEOME K. B., FAULKIN L. J., Jr, BERN H. A., BLAIR P. B. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959 Jun;19(5):515–520. [PubMed] [Google Scholar]
- Dotto G. P., Parada L. F., Weinberg R. A. Specific growth response of ras-transformed embryo fibroblasts to tumour promoters. Nature. 1985 Dec 5;318(6045):472–475. doi: 10.1038/318472a0. [DOI] [PubMed] [Google Scholar]
- Ethier S. P. Primary culture and serial passage of normal and carcinogen-treated rat mammary epithelial cells in vitro. J Natl Cancer Inst. 1985 Jun;74(6):1307–1318. [PubMed] [Google Scholar]
- Greiner J. W., DiPaolo J. A., Evans C. H. Carcinogen-induced phenotypic alterations in mammary epithelial cells accompanying the development of neoplastic transformation. Cancer Res. 1983 Jan;43(1):273–278. [PubMed] [Google Scholar]
- Guzman R. C., Osborn R. C., Bartley J. C., Imagawa W., Asch B. B., Nandi S. In vitro transformation of mouse mammary epithelial cells grown serum-free inside collagen gels. Cancer Res. 1987 Jan 1;47(1):275–280. [PubMed] [Google Scholar]
- Guzman R. C., Osborn R. C., Richards J. E., Nandi S. Effects of phorbol esters on normal and tumorous mouse mammary epithelial cells embedded in collagen gels. J Natl Cancer Inst. 1983 Jul;71(1):69–73. [PubMed] [Google Scholar]
- Guzman R. C., Osborn R. C., Yang J., DeOme K. B., Nandi S. Transplantation of mouse mammary epithelial cells grown in primary collagen gel cultures. Cancer Res. 1982 Jun;42(6):2376–2383. [PubMed] [Google Scholar]
- Hsiao W. L., Gattoni-Celli S., Weinstein I. B. Oncogene-induced transformation of C3H 10T1/2 cells is enhanced by tumor promoters. Science. 1984 Nov 2;226(4674):552–555. doi: 10.1126/science.6436974. [DOI] [PubMed] [Google Scholar]
- Hsiao W. L., Wu T., Weinstein I. B. Oncogene-induced transformation of a rat embryo fibroblast cell line is enhanced by tumor promoters. Mol Cell Biol. 1986 Jun;6(6):1943–1950. doi: 10.1128/mcb.6.6.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa W., Tomooka Y., Hamamoto S., Nandi S. Stimulation of mammary epithelial cell growth in vitro: interaction of epidermal growth factor and mammogenic hormones. Endocrinology. 1985 Apr;116(4):1514–1524. doi: 10.1210/endo-116-4-1514. [DOI] [PubMed] [Google Scholar]
- Imagawa W., Tomooka Y., Nandi S. Serum-free growth of normal and tumor mouse mammary epithelial cells in primary culture. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4074–4077. doi: 10.1073/pnas.79.13.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen E. M., LaPolla R. J., Kirby P. E., Haworth S. R. In vitro studies of chemical mutagens and carcinogens. I. Stability studies in cell culture medium. J Natl Cancer Inst. 1977 Sep;59(3):941–944. doi: 10.1093/jnci/59.3.941. [DOI] [PubMed] [Google Scholar]
- McCalla D. R., Reuvers A., Kitai R. Inactivation of biologically active N-methyl-N-nitroso compounds in aqueous solution: effect of various conditions of pH and illumination. Can J Biochem. 1968 Aug;46(8):807–811. doi: 10.1139/o68-122. [DOI] [PubMed] [Google Scholar]
- Medina D. Mammary tumorigenesis in chemical carcinogen-treated mice. I. Incidence in BALB-c and C57BL mice. J Natl Cancer Inst. 1974 Jul;53(1):213–221. doi: 10.1093/jnci/53.1.213. [DOI] [PubMed] [Google Scholar]
- Medina D. Preneoplastic lesions in murine mammary cancer. Cancer Res. 1976 Jul;36(7 Pt 2):2589–2595. [PubMed] [Google Scholar]
- Pai S. B., Steele V. E., Nettesheim P. Neoplastic transformation of primary tracheal epithelial cell cultures. Carcinogenesis. 1983;4(4):369–374. doi: 10.1093/carcin/4.4.369. [DOI] [PubMed] [Google Scholar]
- Richards J., Nandi S. Neoplastic transformation of rat mammary cells exposed to 7,12-dimethylbenz[alpha]anthracene or N-nitrosomethylurea in cell culture. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3836–3840. doi: 10.1073/pnas.75.8.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass B., Dunn T. B. Classification of mouse mammary tumors in Dunn's miscellaneous group including recently reported types. J Natl Cancer Inst. 1979 May;62(5):1287–1293. [PubMed] [Google Scholar]
- Schaefer F. V., Tonelli Q. J., Dickens M. S., Custer R. P., Sorof S. Nononcogenic hormone-independent alveoli produced by carcinogens in cultured mouse mammary glands. Cancer Res. 1983 Jul;43(7):3310–3315. [PubMed] [Google Scholar]
- Stampfer M. R., Bartley J. C. Induction of transformation and continuous cell lines from normal human mammary epithelial cells after exposure to benzo[a]pyrene. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2394–2398. doi: 10.1073/pnas.82.8.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telang N. T., Banerjee M. R., Iyer A. P., Kundu A. B. Neoplastic transformation of epithelial cells in whole mammary gland in vitro. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5886–5890. doi: 10.1073/pnas.76.11.5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch C. W. Host factors affecting the growth of carcinogen-induced rat mammary carcinomas: a review and tribute to Charles Brenton Huggins. Cancer Res. 1985 Aug;45(8):3415–3443. [PubMed] [Google Scholar]
- Yang J., Nandi S. Growth of cultured cells using collagen as substrate. Int Rev Cytol. 1983;81:249–286. doi: 10.1016/s0074-7696(08)62340-2. [DOI] [PubMed] [Google Scholar]