Abstract
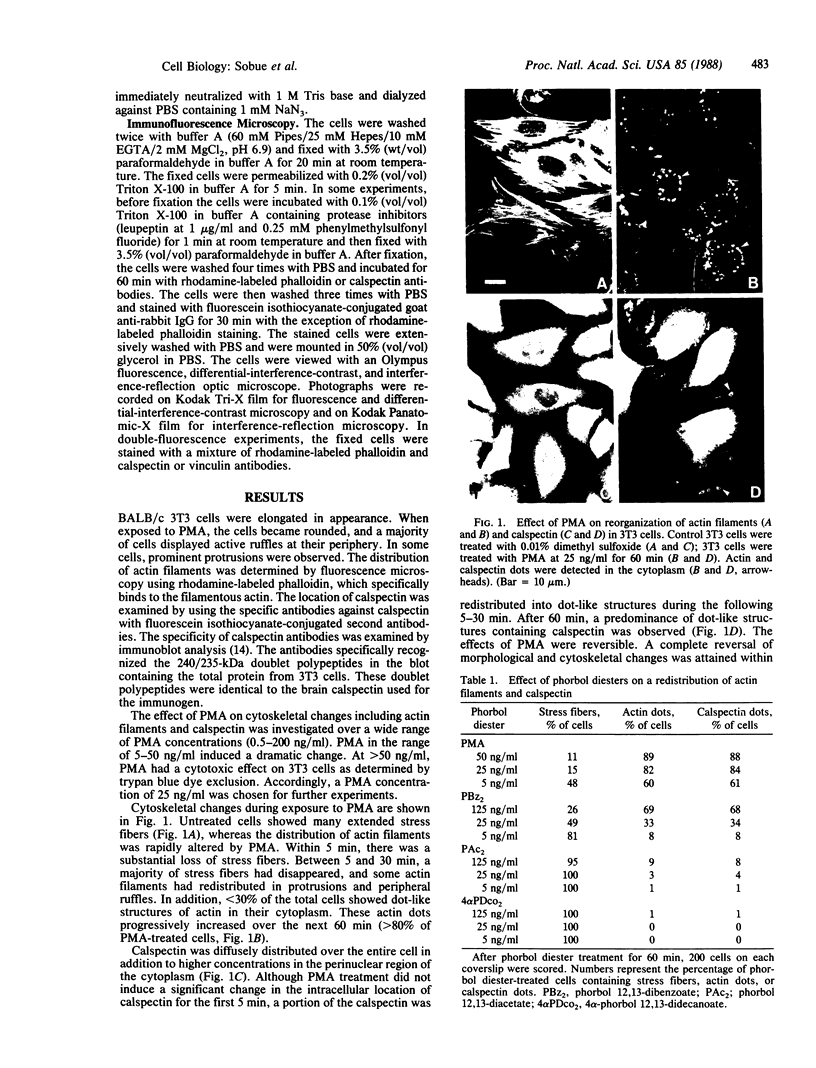
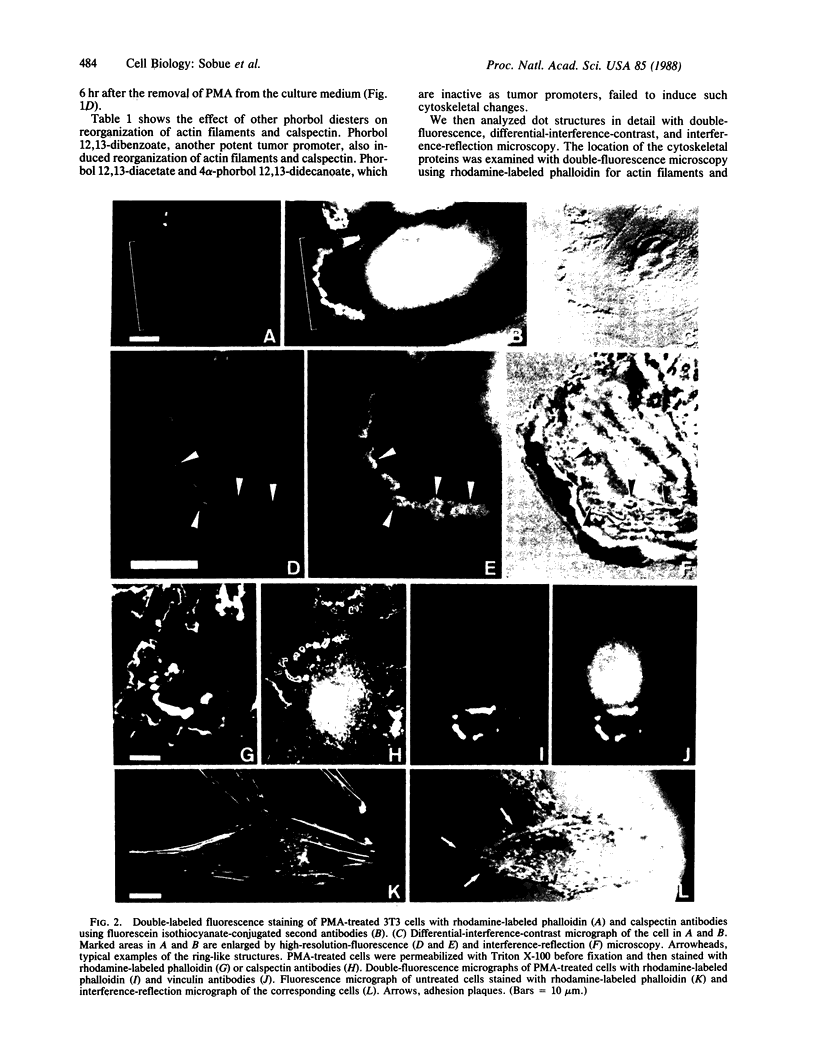
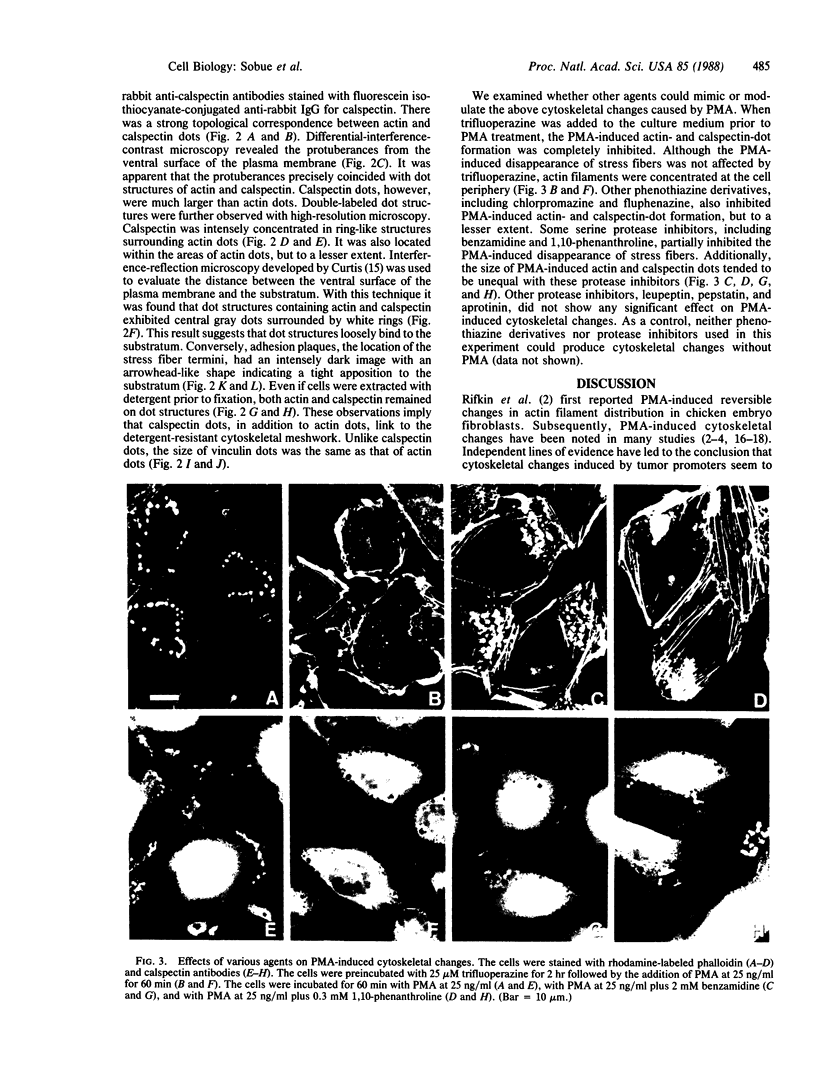
We have used immunofluorescence, differential-interference-contrast, and interference-reflection microscopy to examine the translocation of actin filaments and calspectin (fodrin or nonerythroid spectrin) in 3T3 cells induced by phorbol 12-myristate 13-acetate (PMA). The two cytoskeletal proteins were observed to localize in dot structures that corresponded to the cell-substratum contact sites (focal contact) of the cytoplasmic surface of the plasma membrane. The induction of these cytoskeletal changes was specific for tumor promoters. High-resolution microscopy revealed that calspectin was intensely concentrated in ring-like structures surrounding actin dots. It was also located within the areas of actin dots, but to a lesser extent. Trifluoperazine and other phenothiazine derivatives inhibited the formation of those dot structures that appeared after the addition of PMA. Some serine protease inhibitors were also demonstrated to influence cytoskeletal changes by PMA. Our results provide evidence that calspectin is closely associated with actin filaments in dot structures induced by PMA. Possible mechanisms for these cytoskeletal changes produced by PMA are discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop R., Martinez R., Nakamura K. D., Weber M. J. A tumor promoter stimulates phosphorylation on tyrosine. Biochem Biophys Res Commun. 1983 Sep 15;115(2):536–543. doi: 10.1016/s0006-291x(83)80178-8. [DOI] [PubMed] [Google Scholar]
- Boschek C. B., Jockusch B. M., Friis R. R., Back R., Grundmann E., Bauer H. Early changes in the distribution and organization of microfilament proteins during cell transformation. Cell. 1981 Apr;24(1):175–184. doi: 10.1016/0092-8674(81)90513-4. [DOI] [PubMed] [Google Scholar]
- Burridge K., Kelly T., Mangeat P. Nonerythrocyte spectrins: actin-membrane attachment proteins occurring in many cell types. J Cell Biol. 1982 Nov;95(2 Pt 1):478–486. doi: 10.1083/jcb.95.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS A. S. THE MECHANISM OF ADHESION OF CELLS TO GLASS. A STUDY BY INTERFERENCE REFLECTION MICROSCOPY. J Cell Biol. 1964 Feb;20:199–215. doi: 10.1083/jcb.20.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carley W. W., Barak L. S., Webb W. W. F-actin aggregates in transformed cells. J Cell Biol. 1981 Sep;90(3):797–802. doi: 10.1083/jcb.90.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casnellie J. E., Lamberts R. J. Tumor promoters cause changes in the state of phosphorylation and apparent molecular weight of a tyrosine protein kinase in T lymphocytes. J Biol Chem. 1986 Apr 15;261(11):4921–4925. [PubMed] [Google Scholar]
- Chen W. T., Chen J. M., Parsons S. J., Parsons J. T. Local degradation of fibronectin at sites of expression of the transforming gene product pp60src. Nature. 1985 Jul 11;316(6024):156–158. doi: 10.1038/316156a0. [DOI] [PubMed] [Google Scholar]
- Chen W. T., Olden K., Bernard B. A., Chu F. F. Expression of transformation-associated protease(s) that degrade fibronectin at cell contact sites. J Cell Biol. 1984 Apr;98(4):1546–1555. doi: 10.1083/jcb.98.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Pfeuty T., Singer S. J. Altered distributions of the cytoskeletal proteins vinculin and alpha-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6687–6691. doi: 10.1073/pnas.77.11.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L., O'Brien T. G., Baird W. M. Tumor promoters and the mechanism of tumor promotion. Adv Cancer Res. 1980;32:1–74. doi: 10.1016/s0065-230x(08)60360-7. [DOI] [PubMed] [Google Scholar]
- Fey E. G., Penman S. Tumor promoters induce a specific morphological signature in the nuclear matrix-intermediate filament scaffold of Madin-Darby canine kidney (MDCK) cell colonies. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4409–4413. doi: 10.1073/pnas.81.14.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P., Weber K. F-actin-binding and cross-linking properties of porcine brain fodrin, a spectrin-related molecule. J Biol Chem. 1982 Aug 25;257(16):9781–9787. [PubMed] [Google Scholar]
- Glenney J. Phospholipid-dependent Ca2+ binding by the 36-kDa tyrosine kinase substrate (calpactin) and its 33-kDa core. J Biol Chem. 1986 Jun 5;261(16):7247–7252. [PubMed] [Google Scholar]
- Grunberger G., Zick Y., Taylor S. I., Gorden P. Tumor-promoting phorbol ester stimulates tyrosine phosphorylation in U-937 monocytes. Proc Natl Acad Sci U S A. 1984 May;81(9):2762–2766. doi: 10.1073/pnas.81.9.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiuchi S., Sobue K., Fujita M. Purification of a 240 000 Mr calmodulin-binding protein from a microsomal fraction of brain. FEBS Lett. 1981 Sep 14;132(1):144–148. doi: 10.1016/0014-5793(81)80449-8. [DOI] [PubMed] [Google Scholar]
- Kanda K., Tanaka T., Sobue K. Calspectin (fodrin or nonerythroid spectrin)-actin interaction: a possible involvement of 4.1-related protein. Biochem Biophys Res Commun. 1986 Nov 14;140(3):1051–1058. doi: 10.1016/0006-291x(86)90741-2. [DOI] [PubMed] [Google Scholar]
- Kellie S., Holme T. C., Bissell M. J. Interaction of tumour promoters with epithelial cells in culture. An immunofluorescence study. Exp Cell Res. 1985 Oct;160(2):259–274. doi: 10.1016/0014-4827(85)90174-0. [DOI] [PubMed] [Google Scholar]
- Laszlo A., Bissell M. J. TPA induces simultaneous alterations in the synthesis and organization of vimentin. Exp Cell Res. 1983 Oct;148(1):221–234. doi: 10.1016/0014-4827(83)90201-x. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Nelson W. J. Expression of spectrin in nonerythroid cells. Cell. 1982 Dec;31(3 Pt 2):505–508. doi: 10.1016/0092-8674(82)90306-3. [DOI] [PubMed] [Google Scholar]
- Lehto V. P., Virtanen I., Paasivuo R., Ralston R., Alitalo K. The p36 substrate of tyrosine-specific protein kinases co-localizes with non-erythrocyte alpha-spectrin antigen, p230, in surface lamina of cultured fibroblasts. EMBO J. 1983;2(10):1701–1705. doi: 10.1002/j.1460-2075.1983.tb01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Binding of trifluoperazine to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. Mol Pharmacol. 1977 Jul;13(4):690–697. [PubMed] [Google Scholar]
- Levine J., Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 1981 Sep;90(3):631–642. doi: 10.1083/jcb.90.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchisio P. C., Cirillo D., Teti A., Zambonin-Zallone A., Tarone G. Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp Cell Res. 1987 Mar;169(1):202–214. doi: 10.1016/0014-4827(87)90238-2. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Phaire-Washington L., Silverstein S. C., Wang E. Phorbol myristate acetate stimulates microtubule and 10-nm filament extension and lysosome redistribution in mouse macrophages. J Cell Biol. 1980 Aug;86(2):641–655. doi: 10.1083/jcb.86.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin D. B., Crowe R. M., Pollack R. Tumor promoters induce changes in the chick embryo fibroblast cytoskeleton. Cell. 1979 Oct;18(2):361–368. doi: 10.1016/0092-8674(79)90055-2. [DOI] [PubMed] [Google Scholar]
- Rohrschneider L., Rosok M. J. Transformation parameters and pp60src localization in cells infected with partial transformation mutants of Rous sarcoma virus. Mol Cell Biol. 1983 Apr;3(4):731–746. doi: 10.1128/mcb.3.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury J. L., Condeelis J. S., Maihle N. J., Satir P. Calmodulin localization during capping and receptor-mediated endocytosis. Nature. 1981 Nov 12;294(5837):163–166. doi: 10.1038/294163a0. [DOI] [PubMed] [Google Scholar]
- Schliwa M., Nakamura T., Porter K. R., Euteneuer U. A tumor promoter induces rapid and coordinated reorganization of actin and vinculin in cultured cells. J Cell Biol. 1984 Sep;99(3):1045–1059. doi: 10.1083/jcb.99.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue K., Kanda K., Inui M., Morimoto K., Kakiuchi S. Actin polymerization induced by calspectin, a calmodulin-binding spectrin-like protein. FEBS Lett. 1982 Nov 8;148(2):221–225. doi: 10.1016/0014-5793(82)80811-9. [DOI] [PubMed] [Google Scholar]
- Sobue K., Okabe T., Kadowaki K., Itoh K., Tanaka T., Fujio Y. Cytosynalin: a Mr 35,000 cytoskeleton-interacting and calmodulin-binding protein. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1916–1920. doi: 10.1073/pnas.84.7.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarone G., Cirillo D., Giancotti F. G., Comoglio P. M., Marchisio P. C. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985 Jul;159(1):141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Goldberg A. R. Changes in microfilament organization and surface topogrophy upon transformation of chick embryo fibroblasts with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4065–4069. doi: 10.1073/pnas.73.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]