Abstract
Objective
Children with hypertension (HTN) are at increased risk for left ventricular hypertrophy (LVH). Increased LV mass by the process of remodeling in response to volume or pressure loading may be eccentric (increased LV diameter) or concentric (increased wall thickness). Our objective was to classify LV geometry among children with primary HTN and examine differences in ambulatory blood pressure (ABP).
Study design
Subjects aged 7-18 years with suspected HTN were enrolled in this cross-sectional study. ABP and LVM index (LVMI) and were measured within the same 24 hour period. LV geometry was classified as normal, concentric remodeling, concentric LVH or eccentric LVH.
Results
Children with LVH had significantly higher ambulatory systolic and diastolic BP levels and BMI z-score. Sixty-eight children had HTN based upon ABPM. Thirty-eight percent of the hypertensive subjects had LVH, with equal distribution in the concentric and eccentric groups. There were significant differences in the 24-hour diastolic BP (DBP) parameters when the eccentric LVH group was compared to the normal geometry and concentric LVH groups. Relative wall thickness was inversely associated with nighttime DBP parameters. These relationships persisted after controlling for BMI Z-score.
Conclusions
While the risk for LVH is associated with increased systolic BP and BMI Z-score, those with eccentric LVH had significantly higher DBP.
Keywords: Hypertension, left ventricular hypertrophy, concentric LVH, eccentric LVH, relative wall thickness
Hypertrophic growth of the heart is a response to ventricular wall stress, and has been associated with athletic training, neurohormonal activation, or chronically increased hemodynamic load.1 Left ventricular hypertrophy (LVH) is the most commonly assessed target organ effect of hypertension among children and adolescents. While the future impact of LVH detected during childhood is not known, LVH is associated with significantly increased cardiac risk among adults.2 The LV mass of children with hypertension (HTN) is greater than that of children with normal blood pressure (BP). In a cross sectional study of children with primary hypertension, the prevalence of LVH was 15.5 and 41.1% based upon adult and pediatric criteria, respectively.3 Several recent studies have emphasized the increased risk for LVH among children with hypertension and/or obesity.4-6
Hemodynamic loading by increased pressure (afterload) or volume (preload) results in changes in LV geometry. The former stimulates a concentric pattern characterized by the parallel addition of myofibrils that create LV wall thickening, whereas an eccentric pattern is characterized by the series addition of sarcomeres creating myofibril elongation and LV chamber enlargement.7, 8 Classification of these responses, in conjunction with the evaluation of relative wall thickness (LV wall thickness to internal chamber dimension ratio), has been extensively examined for prognostic importance in the diagnosis and treatment of hypertension and its related co-morbidities. Four geometric patterns have emerged.9, 10 Normal LV geometry is characterized by normal LV mass with normal relative wall thickness (RWT); concentric remodeling describes a pattern of normal LV mass in the presence of elevated RWT; concentric hypertrophy is identified by LVH in the presence of elevated RWT; and eccentric hypertrophy displays LVH with normal RWT (dilated LV chamber). Daniels et al. reported that 47% of children with primary hypertension had LVMI >95th percentile; within that cohort of 130 hypertensive children, 30% had eccentric hypertrophy and 17% had concentric hypertrophy, while an additional 9% displayed concentric remodeling.11
The aim of the current study was to assess LV geometry in a clinical cohort of untreated children with primary hypertension who underwent ambulatory blood pressure monitoring (ABPM) as part of their evaluation.
Subjects and Methods
Subjects were recruited from primary care clinics and from referrals to the University of Tennessee Medical Group nephrology or cardiology clinics for evaluation of elevated BP. Children ages 6-18 years with a casual systolic BP (SBP) or diastolic BP (DBP) ≥ 90th percentile or a first-degree relative on anti-hypertensive therapy or with known hypertension were eligible for the study; hypertension was defined as an SBP or DBP load >25%. Secondary causes of hypertension were excluded based upon recommendations of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents published in 2004.12 Children were excluded if they were on anti-hypertensive medications or had a diagnosis of renal or cardiac disease. Informed consent was obtained from a parent and assent obtained from the study subjects as appropriate. The research protocol was approved by the University of Tennessee Health Science Center Institutional Review Board and followed the guidelines for good clinical practice.
Study Procedures
During the 2-day study period, ABP and LVM were measured in each subject. Subject height and weight were measured using a balance beam scale and pediatric wall-mounted stadiometer. Height percentile was calculated using the CDC NHANES III data tables by age in months.13 Body mass index (BMI) was calculated and BMI Z-scores, which reflect the standard deviation score for the age- and gender-appropriate BMI distribution, were calculated using the same methods as used in the 2000 CDC Growth Charts for the United States.13 Ethnicity was categorized as that reported by the subject.
ABP monitoring was performed using the AM5600 ambulatory BP monitor (Advanced Biosensor, Columbia, SC).14 The monitors were programmed to measure the BP every 20 minutes for a 24-hour period using the auscultatory technique to detect SBP at Korotkoff phase I and DBP at Korotkoff phase V. After selection of the appropriate cuff size, the brachial artery of the non-dominant arm at the anticubital fossa was located. The microphone was taped to the subject's arm over the strongest impulse followed by placement of the cuff and electrodes for determination of heart rate. During the AM5600 “office check period”, with the subject in a seated position at least 5 minutes, a minimum of three readings were taken simultaneously by one of two study coordinators in the same arm with a mercury sphygmomanometer and stethoscope via a 3-way stopcock, and the AM5600, using the recorder's BP cuff. The calibration readings allowed for adjustments to be made in the recorder's microphone amplification and to establish a baseline for ABPM vs. manual SBP and DBP. The office check readings, described previously14, were not included in the 24-hour results; casual BP readings were determined by the mean of three readings by mercury sphygmomanometer and stethoscope at the study visit. The parent/guardian was asked to record the subject's bedtime and time of awakening; using the reported times, the asleep period was designated by the next reading after the time of sleep and the last reading prior to the time recorded for awakening. After 24 hours, the cuff and monitor were removed, and the data downloaded using AMPSI software. Subjects with fewer than 30 total readings or fewer than five nocturnal readings were excluded. Subjects were asked to refrain from sports activity but otherwise to continue usual activities including school.
The mean SBP and DBP were calculated separately for the 24-hour period and for awake (designated as daytime) and asleep (designated as nighttime) periods. The systolic and diastolic blood pressure index (BPI) for each subject were calculated by expressing the mean SBP or DBP as a ratio to the appropriate 95th percentile as reported by Soergel et al.15 When the BPI is greater than 1.0, the mean ambulatory SBP or DBP is above the 95th percentile. SBP and DBP standard deviation scores (SDS) were also calculated using the method of Wuhl et al.16 BP load was calculated for systolic and diastolic BP as the number of readings for day and night periods which were over the 95th percentile threshold for each time period divided by the total number of readings, and expressed as a percent. A 24-hour load ≥25% was defined as abnormal.17
Validation of the Instrument
Inter-method reliability between the mercury sphygmomanometer and the AM5600 ABPM system was compared using ANOVA, mixed effects model. The Pearson correlation coefficients were 0.992 and 0.979 for SBP and DBP, respectively, with coefficients of variation of 1.5 and 3.5%. The mean of the difference between the AM5600 and the mercury sphygmomanometer was 0.29 ± 3.5 and 0.045 ± 3.7 mmHg for systolic and diastolic BP, respectively; the monitor has been validated for use in children.14 Waveform analysis to detect artifactual readings was performed by a co-investigator (P. Richey) unfamiliar with the subjects. Artifactual readings were discarded based upon the waveform in the recording according to methods previously described.18 The accuracy of each reading was determined by inspection of the graphic display of the Korotkoff signals.
Measurement of Left Ventricular Mass
M-mode echocardiogram measurements of the interventricular septal thickness (IVS), posterior wall thickness (PWT), and left ventricular internal dimension (LVID) were performed at end diastole according to established standards of the American Society of Echocardiography (ASE). Left ventricular mass (LVM) was calculated using the formula by Devereux et al. according to the ASE guidelines: LV mass (g) = 0.81[1.04(IVS + PWT + LVID)3-(LVID)3] + 0.06.19 Echocardiography was performed by the same technician, and all measurements were performed in triplicate by the same cardiologist, who was unaware of the subject's BP. Left ventricular mass index was derived by dividing LVM in grams by the subject's height in meters raised to the 2.7 power.20, 21 Subjects were classified into 4 groups based upon LVMI and RWT.(11) LVMI was defined as < or ≥ the gender-based 95th percentile: 39.36 g/ht2.7 in males and 36.88 g/ht2.7 in females. RWT, defined as the ratio of LV wall thickness to LV internal dimension [(IVS + PWT) / LVID] was classified as < or ≥ 0.41. LV geometry was considered to be normal when LVMI was <95th percentile and RWT <0.41; concentric remodeling when LVMI was <95th percentile and RWT ≥ 0.41; concentric hypertrophy when LVMI was ≥95th percentile and RWT ≥0.41; and eccentric hypertrophy when LVMI was ≥ 95th percentile and RWT < 0.41. (11)
Statistical Analysis
SAS STAT v 9.1.3 (SAS Institute Inc., Cary, NC) was used for statistical analyses. Subjects were initially divided into four groups based upon LV geometry. BP levels were compared using either the t-test for two groups or ANOVA with Bonferroni method for multiple comparisons for four groups. Univariate analysis of continuous variables was performed using Pearson's correlation. Dichotomous variables were analyzed using the chi-square test; regression analysis was performed with LVMI as the dependent outcome of interest.
Results
A total of 101 participants had sufficient data for analysis. There were 68 children who met the definition of HTN based upon the presence of a SBP and/or DBP load ≥ 25%. Nineteen children had isolated systolic HTN and four had isolated diastolic HTN; the rest had combined systolic and diastolic HTN. Nine (8.9%) had masked HTN and 16 (15.8%) had white coat HTN. 22
Nearly 34% percent (34/101) of the subjects had LVH; of children with normal ABP, 8 of 33 (24%) had LVH. Subjects with LVH had significantly greater mean BMI z-score and mean ambulatory BP levels compared to those without LVH. These results are summarized in Table 1. There were no differences in pulse pressure or nocturnal blood pressure decline between those with or without LVH.
Table 1.
Characteristics of subjects according to left ventricular mass index: LVH versus normal.
| Normal LVMI | LVH | P value | |
|---|---|---|---|
| n | 67 | 34 | |
| Age (years) | 13.7 (0.34) | 13.7 (0.36) | 0.99 |
| Race (AA/Non-AA) | 48/19 | 26/8 | 0.60 |
| Gender | 44/23 | 22/12 | 0.92 |
| BMI z-score | 1.27 (0.13) | 2.00 (0.10) | <0.0001 |
| Casual SBPI | 1.00 (0.01) | 1.05 (0.02) | 0.68 |
| Casual DBPI | 0.90 (0.01) | 0.91 (0.02) | 0.07 |
| SBPI 24h | 0.968 (0.009) | 1.014 (0.014) | <0.01 |
| DBPI 24h | 0.913 (0.01) | 0.954 (0.018) | <0.05 |
| SBP load 24h (%) | 36.3 (3.3) | 51.0 (5.1) | <0.05 |
| DBP load 24h (%) | 26.4 (2.6) | 35.5 (4.3) | 0.061 |
| SBP SDS 24h | 0.838 (0.153) | 1.727 (0.276) | <0.01 |
| DBP SDS 24h | 0.451 (0.137) | 1.034 (0.236) | <0.05 |
| Pulse pressure | 52 (0.8) | 54 (1.2) | 0.105 |
| Nocturnal dip SBP | 12.7 (0.9) | 12.7 (1.4) | 0.71 |
| Nocturnal dip DBP | 9.4 (0.8) | 10.0 (1.4) | 0.98 |
Data represent mean (SEM). Comparisons of groups used the t-test. Abbreviations: SBPI, systolic blood pressure index; DBPI, diastolic blood pressure index; SDS standard deviation score; LVH, left ventricular hypertrophy defined as LVMI (left ventricular mass index) ≥ gender specific 95th percentile.
Regression analysis was performed with LVMI as the dependent outcome variable (Table 2). We used the standard deviation scores for 24 hour SBP and DBP, and included gender, race, BMI z-score, and pulse pressure in the multivariable analysis. BMI expressed as a z-score had a major effect on LVMI. Race and gender did not modify the relationship between LVMI and SBP. Although pulse pressure was not significantly associated with LVMI, it did affect the parameter estimate (β) for SBP (2.2 → 1.74). The best model included both BMI z-score and SBP.
Table 2.
Regression analysis of factors associated with LVMI among all subjects.
| Univariate model | Multivariable model | Best model | ||||
|---|---|---|---|---|---|---|
| β | P | β | P | β | P | |
| 24h SBP SDS | 2.21 | <0.01 | 2.05 | 0.31 | 1.44 | 0.036 |
| BMI Z-score | 4.14 | <0.001 | 4.28 | <0.0001 | ||
| Gender | −3.72 | 0.19 | ||||
| Race | −3.12 | 0.15 | ||||
| Pulse pressure | −0.09 | 0.77 | ||||
| 24h DBP SDS | −0.42 | 0.82 | ||||
Abbreviations: SBP SDS, systolic blood pressure standard deviation score; DBP SDS, diastolic blood pressure standard deviation score.
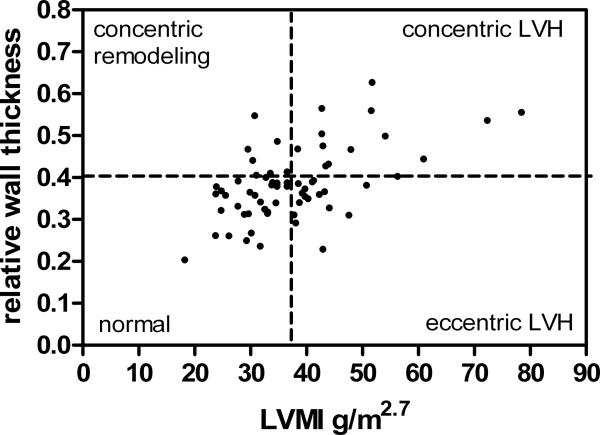
Hypertensive subjects were sub-divided into LV geometry groups based upon LVMI and RWT (Figure 1). Characteristics of subjects according to LV geometry are summarized in Table 3. The mean BMI Z-score was higher in the concentric geometry group as compared to the other groups. All subsequent analyses control for BMI Z-score. ABP according to LV geometry group is represented in Table 3. After correction for multiple comparisons mean SBP, but not SBP load, was significantly higher in the group with eccentric LVH as compared to the normal geometry group. There were significant differences in all DBP parameters when the eccentric LVH group was compared to the normal geometry and concentric LVH groups.
Figure 1.
Distribution of LVMI and RWT among hypertensive subjects. The 95th percentile LVMI for females is 36.88 and for males is 39.36; one line is drawn at 37.5 for figure but not for analysis.
Table 3.
Ambulatory blood pressure in hypertensive subjects according to left ventricular geometry group.
| Normal | Concentric remodeling | Concentric LVH | Eccentric LVH | |
|---|---|---|---|---|
| N, total = 68 | 35 (51.5%) | 7 (10.3%) | 13 (19.1%) | 13 (19.1%) |
| Age (years) | 13.4 (0.44) | 13.4 (1.07) | 13.4 (0.69) | 13.2 (0.50) |
| BMI Z-score | 1.51 (0.15) | 1.12 (0.59) | 2.13 (0.19) | 1.85 (0.18) |
| Race (AA/nonAA) | 25/10 | 5/2 | 9/4 | 11/2 |
| SBPI 24h | 1.004 (0.009) | 1.010 (0.015) | 1.028 (0.013) | 1.063 (0.021)§ |
| DBPI 24h | 0.946 (0.012) | 0.958 (0.029) | 0.924 (0.025) | 1.047 (0.021)† |
| SBP SDS 24h | 1.51 (0.16) | 1.55 (0.39) | 1.82 (0.24) | 2.72 (0.49)§ |
| DBP SDS 24h | 0.91 (0.15) | 1.16 (0.48) | 0.65 (0.26) | 2.27 (0.29)† |
| SBP load 24h (%) | 51.5 (3.7) | 56.6 (9.6) | 59.4 (6.0) | 66.7 (6.2) |
| DBP load 24h (%) | 36.1 (3.2) | 38.5 (9.9) | 28.5 (3.9) | 58.3 (5.4)† |
Data represent mean (SEM). Comparisons were performed by ANOVA with correction for repeated measures using the Bonferroni method.
Indicates significant difference (P<0.05) between the eccentric LVH group and normal geometry group.
Indicates P<0.05 between the eccentric LVH group compared to normal geometry and concentric LVH groups.
Abbreviations: SBPI, systolic blood pressure index; DBPI, diastolic blood pressure index; SDS standard deviation score.
Relative wall thickness was inversely associated with nighttime DBP parameters: DBPI (Pearson correlation coefficient −0.269, P <0.05), DBP SDS (Pearson correlation coefficient −0.266, P-<0.05) and DBP load (Pearson correlation coefficient −0.232, P=0.0572). This relationship persisted after controlling for BMI Z-score. Relative wall thickness was not significantly associated with age in our subjects (Pearson correlation coefficient= −0.137, P=0.23).
Discussion
Left ventricular hypertrophy, expressed as increased LVMI, represents the most prevalent end-organ effect reported among children with hypertension. While some studies have noted a relationship between ABPM and LVMI 23, 24, others have not.4 Previous studies which describe LV geometry in children with HTN have differed from the current study in their selection of subjects: they were retrospective, examined patients on anti-hypertensive therapy, and used casual BP readings to define hypertension.3, 4, 11 We classified our subjects based upon ABPM performed at the time of echocardiography. When subjects were divided based upon LVMI (LVH defined as ≥ the gender associated 95th percentile), those with LVH had significantly higher ABP but not casual BP. Among children with ambulatory HTN, we found that DBP was significantly higher in those with eccentric hypertrophy compared to those with normal geometry or concentric hypertrophy.
LV geometry among hypertensive youth reported by Daniels et al. showed no difference in BMI or casual BP between children with concentric and eccentric LVH. However, duration of HTN was shown to be significantly longer in the concentric LVH group.11 Our study was not designed to assess the duration of HTN. A multicenter, retrospective analysis reported by Hanevold et al. found that among treated and untreated hypertensive children, the prevalence of LVH using pediatric criteria was 41%.3 Distribution by LV geometry revealed concentric hypertrophy in 19%, eccentric hypertrophy in 22%, and concentric remodeling in 9%. The prevalence of the various geometric classifications was similar in our study: concentric hypertrophy 19%, eccentric hypertrophy 19% and concentric remodeling 10%. Although Hanevold et al. did not examine differences in BP levels or duration of HTN according to LV geometric pattern they did report that prevalence of LVH differed among the ethnic groups: 70% of Hispanic, 39% of African-American and 33% of Caucasian subjects had LVH by pediatric criteria. Specifically, they reported Hispanic and African-American children as being more likely to demonstrate concentric versus eccentric hypertrophy. We did not find any ethnic differences in the LV geometric pattern in our subjects, however, the number of subjects and predominance of AA may have affected the ability to ascertain ethnic differences in the geometric pattern.
Our previous findings, like others, demonstrate that children with ambulatory systolic hypertension have greater mean LVMI as compared to those with normal ABP and that increasing SBP levels are associated with increased risk for abnormal LV mass after controlling for BMI Z-score.23, 25 5 We further classified our hypertensive subjects according to LV geometry to examine the impact that elevated BP parameters might have on geometric adaptation of the LV. Diastolic BP is significantly different between children with concentric versus eccentric LVH after controlling for BMI Z-score. Given the nature of the diastolic load, which may be related to intravascular volume, an eccentric hypertrophy pattern characterized by normal RWT is predictable. Furthermore, one might postulate that increased intravascular volume results in higher diastolic BP and an eccentric pattern of LVH. To our knowledge, this observation in the pediatric population has not been previously reported. It is important to note that the majority of our subjects with diastolic HTN also had systolic HTN. Therefore, the differences observed between concentric and eccentric LVH may be a reflection of the severity of hypertension (i.e., both systolic and diastolic BP elevation) or may suggest an initial eccentric structural response preceding a concentric one that would act to normalize wall stress 26 if HTN continues over time. Further investigation in a larger hypertensive sample that includes more females and varied races is warranted to more precisely validate this observation. Concentric LVH has been found to have the greatest risk for serious cardiovascular events and to be associated with diastolic dysfunction whereas eccentric LVH is associated with worse systolic function. 1 Treatment with anti-hypertensive medication particularly ACE inhibitors has been shown to achieve regression of LVH. 1 The mechanisms for the beneficial effect of this class of agents on myocardial remodeling are postulated to extend beyond their effect on BP levels as they modify the angiotensin-aldosterone axis known to promote fibrosis. Studies which demonstrate regression of LVH or the impact of treatment on either concentric or eccentric LVH are lacking in youth. One would anticipate that ACE inhibitors or ACE inhibitors in combination with a diuretic would be beneficial for concentric and eccentric LVH, respectively.
Not all studies have demonstrated a significant relationship between BP-either casual or ambulatory-with left ventricular mass index or with presence of LVH. 11 4 27 Clearly, other factors such as duration of increased BP levels 11, presence or degree of obesity 3, 5, metabolic syndrome 28, and the level of activity of the renin-angiotensin-aldosterone system 29, 30 are also involved in the progression from normal to altered LV geometry.
The strengths of our study include the prospective design, the inclusion of untreated children with primary HTN, the quality control of the echocardiography data (one technician and one cardiologist), and the measurement of BP and definition of hypertensive based upon ABPM. Limitations of the study include insufficient information to assess duration of HTN, a relatively small sample size and a predominance of African-American and male subjects.
In conclusion, among hypertensive children with LVH, DBP is significantly higher in the group with eccentric versus concentric LVH. Increasing BMI and SBP are both associated with increased risk for LVH, but higher DBP is found among hypertensive children with eccentric LVH as compared to those with concentric LVH or normal geometric pattern.
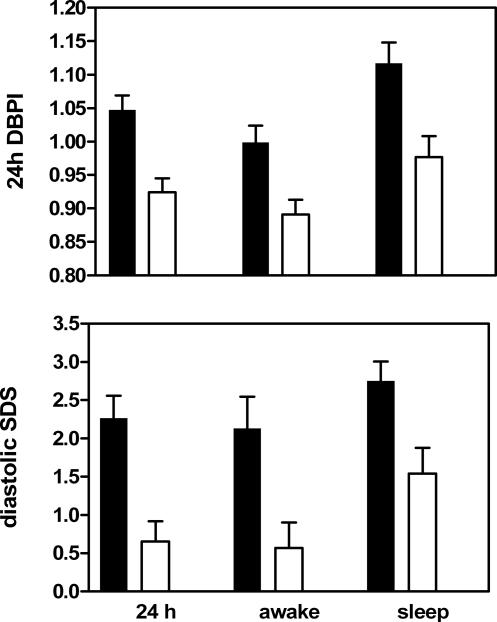
Figure 2.
Comparison of DBP parameters between subjects with eccentric LVH (solid bars) and subjects with concentric LVH. All comparisons were statistically significant by t-test, P<0.05.
Acknowledgment
Supported by a grant from the Children's Foundation Research Center at Le Bonheur Children's Medical Center, Memphis, TN, and the University of Tennessee Health Science Center, MO1 USPHS Grant RR-00211 and NIH NHLBI #5K23HL83910-2.
The authors would like to thank Sharon Myers for expert technical assistance.
Footnotes
Disclosure
The authors do not have conflicts of interest to declare.
References
- 1.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 3.Hanevold C, Waller J, Daniels S, Portman R, Sorof J. The effects of obesity, gender, and ethnic group on left ventricular hypertrophy and geometry in hypertensive children: a collaborative study of the International Pediatric Hypertension Association. Pediatrics. 2004;113:328–333. doi: 10.1542/peds.113.2.328. [DOI] [PubMed] [Google Scholar]
- 4.Brady TM, Fivush B, Flynn JT, Parekh R. Ability of blood pressure to predict left ventricular hypertrophy in children with primary hypertension. J Pediatr. 2008;152:73–78. 78, e71. doi: 10.1016/j.jpeds.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 5.Maggio AB, Aggoun Y, Marchand LM, Martin XE, Herrmann F, Beghetti M, Farpour-Lambert NJ. Associations among obesity, blood pressure, and left ventricular mass. J Pediatr. 2008;152:489–493. doi: 10.1016/j.jpeds.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 6.McNiece KL, Gupta-Malhotra M, Samuels J, Bell C, Garcia K, Poffenbarger T, Sorof JM, Portman RJ. Left ventricular hypertrophy in hypertensive adolescents: analysis of risk by 2004 National High Blood Pressure Education Program Working Group staging criteria. Hypertension. 2007;50:392–395. doi: 10.1161/HYPERTENSIONAHA.107.092197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roman MJ, Devereux RB. How does left ventricular hypertrophy develop in hypertension? Primary Cardiology. 1991;17:74–80. [Google Scholar]
- 8.Richey PA, Brown SP. Pathological versus physiological left ventricular hypertrophy: a review. Journal of Sports Sciences. 1996 doi: 10.1080/026404198366849. accepted for publication. [DOI] [PubMed] [Google Scholar]
- 9.Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. Journal of the American College of Cardiology. 1995;25:879–884. doi: 10.1016/0735-1097(94)00473-4. [DOI] [PubMed] [Google Scholar]
- 10.Verdecchia P, Angeli F, Achilli P, Castellani C, Broccatelli A, Gattobigio R, Cavallini C. Echocardiographic left ventricular hypertrophy in hypertension: marker for future events or mediator of events? Curr Opin Cardiol. 2007;22:329–334. doi: 10.1097/HCO.0b013e3280ebb413. [DOI] [PubMed] [Google Scholar]
- 11.Daniels SR, Loggie JM, Khoury P, Kimball TR. Left ventricular geometry and severe left ventricular hypertrophy in children and adolescents with essential hypertension. Circulation. 1998;97:1907–1911. doi: 10.1161/01.cir.97.19.1907. [DOI] [PubMed] [Google Scholar]
- 12.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 13.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 14.Jones DP, Richey PA, Aplert BS. Validation of the AM5600 Ambulatory Blood Pressure Monitor in Children and Adolescents. Blood Pressure Monitoring. 2008;13:349–351. doi: 10.1097/MBP.0b013e3283102cfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soergel M, Kirschstein M, Busch C, Danne T, Gellermann J, Holl R, Krull F, Reichert H, Reusz GS, Rascher W. Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial including 1141 subjects. J Pediatr. 1997;130:178–184. doi: 10.1016/s0022-3476(97)70340-8. [DOI] [PubMed] [Google Scholar]
- 16.Wuhl E, Witte K, Soergel M, Mehls O, Schaefer F. Distribution of 24-h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens. 2002;20:1995–2007. doi: 10.1097/00004872-200210000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Lurbe E, Sorof JM, Daniels SR. Clinical and research aspects of ambulatory blood pressure monitoring in children. J Pediatr. 2004;144:7–16. doi: 10.1016/j.jpeds.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 18.Richey PA, Jones CL, Harshfield GA, Somes GW, Johnson KC, Bailey JE, Soberman JE. The AM5600 ambulatory blood pressure recording system. Blood Press Monit. 1997;2:193–195. [PubMed] [Google Scholar]
- 19.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 20.Daniels SR, Meyer RA, Liang YC, Bove KE. Echocardiographically determined left ventricular mass index in normal children, adolescents and young adults. J Am Coll Cardiol. 1988;12:703–708. doi: 10.1016/s0735-1097(88)80060-3. [DOI] [PubMed] [Google Scholar]
- 21.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 22.Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, Mahoney L, McCrindle B, Mietus-Snyder M, Steinberger J, Daniels S. Ambulatory blood pressure monitoring in children and adolescents: recommendations for standard assessment: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension. 2008;52:433–451. doi: 10.1161/HYPERTENSIONAHA.108.190329. [DOI] [PubMed] [Google Scholar]
- 23.Richey PA, Disessa TG, Hastings MC, Somes GW, Alpert BS, Jones DP. Ambulatory blood pressure and increased left ventricular mass in children at risk for hypertension. J Pediatr. 2008;152:343–348. doi: 10.1016/j.jpeds.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorof JM, Cardwell G, Franco K, Portman RJ. Ambulatory blood pressure and left ventricular mass index in hypertensive children. Hypertension. 2002;39:903–908. doi: 10.1161/01.hyp.0000013266.40320.3b. [DOI] [PubMed] [Google Scholar]
- 25.Sorof JM. Prevalence and consequence of systolic hypertension in children. Am J Hypertens. 2002;15:57S–60S. doi: 10.1016/s0895-7061(01)02303-2. [DOI] [PubMed] [Google Scholar]
- 26.Grossman W. Cardiac hypertrophy: useful adaptation or pathologic process? American Journal of Medicine. 1980;69:576–584. doi: 10.1016/0002-9343(80)90471-4. [DOI] [PubMed] [Google Scholar]
- 27.Matteucci MC, Wuhl E, Picca S, Mastrostefano A, Rinelli G, Romano C, Rizzoni G, Mehls O, de Simone G, Schaefer F. Left ventricular geometry in children with mild to moderate chronic renal insufficiency. J Am Soc Nephrol. 2006;17:218–226. doi: 10.1681/ASN.2005030276. [DOI] [PubMed] [Google Scholar]
- 28.Litwin M, Sladowska J, Antoniewicz J, Niemirska A, Wierzbicka A, Daszkowska J, Wawer ZT, Janas R, Grenda R. Metabolic abnormalities, insulin resistance, and metabolic syndrome in children with primary hypertension. Am J Hypertens. 2007;20:875–882. doi: 10.1016/j.amjhyper.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Velagaleti RS, Gona P, Levy D, Aragam J, Larson MG, Tofler GH, Lieb W, Wang TJ, Benjamin EJ, Vasan RS. Relations of biomarkers representing distinct biological pathways to left ventricular geometry. Circulation. 2008;118:2252–2258. doi: 10.1161/CIRCULATIONAHA.108.817411. 2255p following 2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li R, Richey PA, DiSessa TG, Alpert BS, Jones DP. Relationship among Blood Aldosterone to Renin Ratio, Ambulatory Blood Pressure and Left Ventricular Mass in Children. Journal of Pediatrics. 2009 doi: 10.1016/j.jpeds.2009.02.029. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]