Abstract
Cytokine profiles in amniotic fluid, cord serum, and tracheal aspirate of premature infants suggest a shift toward a pro-inflammatory state. Cytokines also contribute to the pathogenesis of bronchopulmonary dysplasia (BPD). We hypothesize that the initiating events for BPD are reflected in the placenta and propose that placental expression of cytokines provide a blueprint of events leading to BPD. This is a retrospective, case controlled study of placental cytokines of premature infants with (n=49) and without (n=49) BPD, matched for gender, birthweight and year of birth at Women and Infants Hospital between 2003 and 2005. Cytokine expression, including IL-6 and IL-10, was determined by immunohistochemistry in membrane rolls, umbilical cords, and placentas. IL-6 was similarly expressed in all tissues of infants with and without BPD. In contrast, anti-inflammatory cytokine IL-10 was less prominent in the placenta of patients with BPD compared to those without BPD. IL-10 expression in the villous trophoblast layer was associated with a reduced odds ratio of developing BPD (adjusted Odds Ratio 0.08, 95% confidence interval 0.01–0.70, p=0.02). These results suggest that a placental balance between inflammatory and anti-inflammatory cytokines is crucial to normal lung organogenesis. Importantly, IL-10 appears to be protective against the development of BPD.
Bronchopulmonary dysplasia (BPD) is the most common major morbidity of prematurity and its frequency is higher with decreasing birth weight and gestational age. BPD is associated with longer neonatal hospitalizations, neurodevelopmental impairment, and high rates of re-hospitalization within the first 2 years of life (1–4). Despite improved survival of premature infants over the past 15 years, the incidence of BPD remains unchanged among infants with a BW <1250g, ranging between 12 % – 62 % (5).
The pathology of BPD has shifted from airway injury, inflammation, and parenchymal fibrosis (6) to a new BPD, characterized by an impairment of alveolar and vascular development (7). The pathogenesis of this new BPD is multifactorial, with environmental contributions from intrauterine infection, inflammation, and cytokine exposure as well as genetic heritability (8,9). Supportive data linking infection/inflammation to BPD include the higher rates of histological chorioamnionitis among infants with BPD compared to those without BPD (10,11). Several proinflammatory cytokines have been linked to BPD. In particular, elevated concentrations of IL-6 in umbilical cord blood (12), amniotic fluid (13,14), and tracheal aspirates (15) have been documented in preterm infants with BPD. In contrast, reduced levels of IL-10, a cytokine with potent anti-inflammatory properties, have been reported in tracheal aspirates of patients with BPD (16,17). It has been suggested that the susceptibility of preterm infants to BPD may in part reflect an inability to regulate inflammation through the decreased expression of IL-10 (18).
There has been little investigation of cytokine expression within the placenta and the potential association with BPD. The presence or absence of placental inflammation in maternal and fetal compartments can potentially provide further insight in understanding the pathogenesis of BPD. These changes in specific placental compartments may be linked to subsequent local organ inflammatory response and thereby serve as a blueprint to trigger events predisposing to the development of BPD. We specifically hypothesize that BPD among infants with a birth weight <1250g is associated with an imbalance between expression of “pro-inflammatory” and “anti-inflammatory” cytokines in specific compartments of the placenta.
Methods
This study was approved by the Institutional Review Board of Women and Infants' Hospital of Rhode Island (WIHRI). A waiver of consent was granted to perform this study.
Preliminary studies
Archived placental tissue of 10 infants, born between 24–26 weeks of gestation, was used to determine the adequacy of immunohistochemistry (IHC) staining of paraffin embedded tissue for IL-6, IL-8, TNF-α, and IL-10. An additional 19 placentas were stained to facilitate determination of sample size.
Study
This was a retrospective, case controlled study. Infants included were ≤1250g, born at WIHRI between January 2003 and June 2005, whose placental tissue had been collected at delivery. Pairs of infants with and without BPD were matched for gender, birthweight, and birth within 6 months of each other. Exclusion criteria were major congenital anomalies and death before 36 weeks post menstrual age (PMA). The NICU admission log was used to capture eligible infants.
Formalin fixed, paraffin embedded placental tissue blocks were retrieved from pathology archives. Sections were cut (5 μm thick) to provide the extra-placental membrane roll (for examination of the amnion and chorion), umbilical cord (for examination of the vein, artery and stroma), and full thickness placental disc (for examination of the amnion, chorion, decidua, villous trophoblast layer and stroma, and extravillous trophoblast).
Immunohistochemistry
Antigen retrieval of deparaffinized sections was performed using a citric acid based unmasking solution (Vector, Burlingame, CA) and microwave oven technique. Sections were blocked for 1 hour with 3% goat serum and incubated with the respective primary polyclonal rabbit antibody (1:100, Research and Diagnostic Systems, Inc.) in a humidified chamber for 1 hour in room air, followed by biotinylated secondary anti-rabbit antibody (VectaStain Elite Kit) for 45 minutes. Immunolabelling was performed by a standard Avidin-Biotin (ABC) technique. Labeling was developed with 0.05% 3,3'diaminobenzidine (DAB Sigma, St Louis, MO) and slides were counter stained with hematoxylin (Fisher Scientific, Kalamazoo, MI).
Positive controls were previously stained tissues that showed moderate to severe expression of the corresponding cytokine. Negative controls were standard rabbit IgG controls. Every batch of staining included 7 cases and their matched controls, as well as 2 positive and 2 negative controls.
Grading was done by 2 independent, blinded reviewers (SK and EM), with supervision by the third reviewer (SS). A categorical grading system was employed, 0 for no staining, 1 for mild staining, and 2 for intense staining. Due to potential grading subjectivity, interobserver variability was assessed (see data analysis).
Clinical Data
Clinical data was collected by chart review using predefined variables. Maternal data included demographics, and obstetric variables including antenatal steroid use, antibiotic use, diabetes, hypertension (chronic vs. pregnancy induced), prolonged rupture of membranes (>18 hours), pre-eclampsia, preterm labor, preterm premature rupture of membranes (PPROM), clinical chorioamnionitis (maternal temperature ≥ 37.8 °C and uterine tenderness ± malodorous vaginal discharge and maternal white blood cell count > 15,000), histological chorioamnionitis (any grade of inflammation documented on the placental report retrieved from pathology archives) and mode of delivery.
Neonatal data included birthweight (BW), gestational age (GA, based on Obstetric best estimate), race, gender, and delivery room data. BPD was defined by the use of supplemental oxygen to maintain oxygen saturation > 90% at 36 weeks PMA (19). Infant morbidities included number of days on ventilator support, air leaks, surfactant administration, late onset sepsis (LOS, culture positive), necrotizing enterocolitis (NEC, ≥ Bell's Stage II), patent ductus arteriosus (PDA, treated with indomethacin or ligation), intraventricular hemorrhage (IVH, ≥ grade 3), cystic periventricular leukomalacia (PVL), and retinopathy of prematurity (ROP, ≥ stage 3).
Sample size and data analysis
Based on our preliminary studies of trends in differences of IL-6 staining in placental compartments, a sample size of 64 patients (32 in each group) was needed to provide 80% power to reject the null hypothesis with a two sided α level of 0.05. In our study period, we analyzed 49 cases (with BPD) and 49 controls (without BPD).
Inter-observer agreement for grading of cytokine expression was analyzed using kappa values derived from the assessment of 11 different placental compartments. Group comparisons for maternal and infant data were analyzed by chi-square for dichotomous variables and Students' t-test for continuous variables. Associations between cytokine expression (in each placental compartment) and BPD were initially performed by univariate analysis using chi-squares. The latter analysis was done using data in matched pairs, instead of composite groups. To simplify the statistical analysis of the relationship between cytokines and BPD, we collapsed grades 1 and 2 staining into “any” staining. Therefore, grading was grouped as “none” (Grade 0) vs “any” (Grade 1 or 2). Conditional logistic regression was used on the matched data to determine associations between placental expression of cytokines and BPD, and was adjusted for GA and race. Results are expressed as an odds ratio (OR) with 95% confidence interval (CI). A p value of <0.05 was considered statistically significant.
Results
Our pilot experiments established the conditions for IHC analysis. Staining of the placental tissue of 29 infants indicated consistent staining for IL-6 and IL-10, but was not observed for TNF-α and IL-8. Based upon these results, staining for the main study was limited to IL-6 and IL-10 to facilitate a larger number of placental tissue block assessments. Grading of staining agreement between the two raters varied from Kappa values of 0.71 to 0.92 with an overall Kappa of 0.82.
There were 348 in-born patients, with a birthweight ≤1250g born between January 2003 and June 2005 with available placental tissue. Deaths occurred in 34 patients before 36 weeks PMA, leaving 314 patients (106 with BPD and 208 without BPD) available for this study. Using matching criteria, 98 infants were included in this study, 49 with BPD, and 49 without BPD.
Medical complications of pregnancy (diabetes, hypertension, pre-eclampsia,) were similar between the groups (see Table 1). There was no difference in the percentage of women with PPROM, prolonged rupture of membranes, nor histological and clinical chorioamnionitis (CA). The incidence of histologic CA was more than 2 times greater than clinical CA.
Table 1.
Maternal Data
| BPD N=47 * | No BPD N=49 | |
|---|---|---|
| Hypertension | 13% | 24% |
| Pre-eclampsia | 6% | 16% |
| PPROM | 34% | 26% |
| Prolonged rupture of membranes | 32% | 22% |
| Diabetes | 4% | 6% |
| Abruption | 19% | 20% |
| Chorioamnionitis | ||
| Clinical | 21% | 12% |
| Histological | 51% | 57% |
| Steroids (complete course) | 62% | 58% |
| Antibiotics (any prior to delivery) | 75% | 67% |
| Spontaneous Labor | 68% | 65% |
| Cesarean Delivery | 62% | 71% |
2 sets of twins in this group
Infants were matched for BW, gender, and interval of birth (see Table 2). In spite of the matching criteria, there was a small difference in gestational age; infants with BPD were almost 1 week less mature. There was a trend for a difference in distribution of race with a greater predominance of Caucasian patients, and less African Americans and Hispanic infants with BPD. Delivery room interventions were similar between groups. There was a difference between groups receiving surfactant (Table 3) but this was not felt to be clinically important. As expected, infants with BPD spent more days on ventilators and had more frequent postnatal steroid use. Morbidities that were higher in infants with BPD were PDA's requiring treatment and ROP ≥ stage III.
Table 2.
Infant Demographics and Delivery Room Data
| BPD N=49 | No BPD N=49 | |
|---|---|---|
| Birthweight (grams) | 814 ± 172 * | 879 ± 208 |
| Male | 55% | 55% |
| Gestational Age † | 25.9 ± 1.5 * | 26.7 ± 1.8 |
| Race ‡ | ||
| Caucasian | 80% | 60% |
| Hispanic | 10% | 22% |
| African American | 4% | 14% |
| Other | 6% | 4% |
| SGA | 10% | 14% |
| Intubated | 94% | 85% |
| Chest Compressions | 0% | 2% |
x ± SD
p=0.03
p=0.07
Table 3.
Infant Morbidities
| BPD N=49 | No BPD N=49 | P-value | |
|---|---|---|---|
| Surfactant Use | 100% | 90% | 0.02 |
| Air Leak | 20% | 10% | NS |
| IVH (≥ Grade 3) | 8% | 6% | NS |
| PDA treatment (indomethacin or ligation) | 49% | 14% | 0.0002 |
| Late Onset Sepsis (culture positive) | 33% | 25% | NS |
| NEC | 6% | 10% | NS |
| PVL | 10% | 6% | NS |
| Days on Ventilator | 42 (5–109d)* | 13 (0–55d) | 0.001 |
| Post Natal Steroid Use | 33% | 8% | 0.003 |
| ROP (≥ Stage 3) | 21% | 5% | 0.02 |
median (range)
IHC analysis of IL-6
IL-6 was widely expressed in all placental compartments. In our extraplacental membrane rolls, IL-6 was present in both amnion and chorion, and patterns were similar between newborns with and without BPD (Figure 1A, B). In patients with BPD, 82% (36/44) showed significant staining (Grade 1 or 2) in the amnion and 90% (43/48) had positive staining in the chorion. For patients without BPD, there was 68% (30/44) and 88% (39/48) positive staining for IL-6 in the amnion and chorion, respectively.
Figure 1.
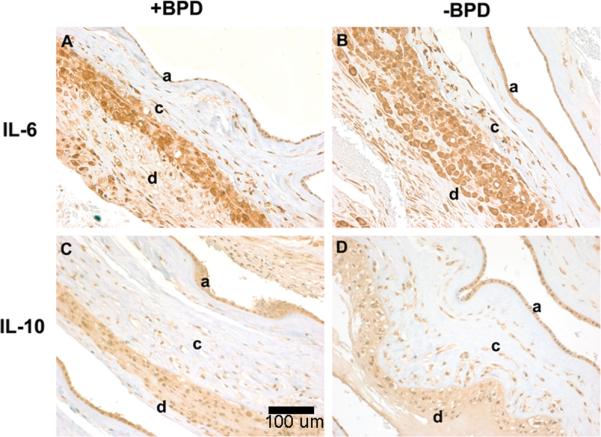
Representative immunostaining of IL-6 and IL-10 in extraplacental membrane rolls from patients with and without BPD, magnification 20x. A, Grade 2 (intense) staining of IL-6 is seen in amnion (a), chorion (c), and decidua (d) of the extraplacental membrane roll from patients with BPD. B, Grade 2 staining of IL- 6 is seen in the amnion, chorion, and decidua of the extraplacental membrane roll of patients without BPD. C, Grade 1 (mild) staining of IL-10 is seen in the amnion, chorion, and decidua of the extraplacental membrane roll from patients with BPD. D, Grade 1 staining of IL-10 seen in the amnion, chorion and decidua of the extraplacental membrane roll of patients without BPD.
IL-6 was also widely expressed in the villous trophoblasts (Figure 2a and b). The distribution of trophoblast staining for patients with BPD was as follows: Grade 0 (4%), Grade 1 (55%), and Grade 2 (40%). This distribution pattern was similar for villous trophoblasts of patients without BPD as well.
Figure 2.
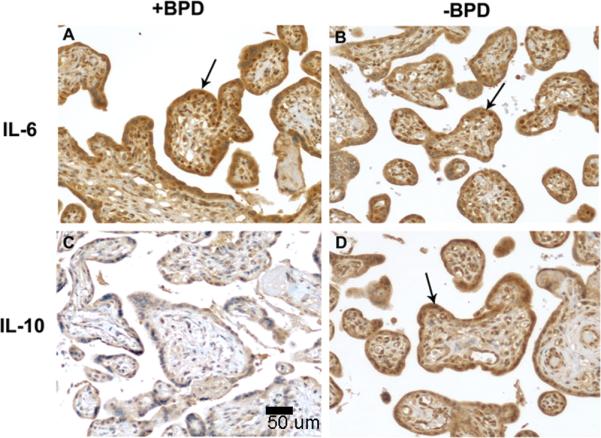
Representative immunohistochemical analysis of IL-6 and IL-10 in placental tissue from patients with or without BPD, magnification 20x. A. Grade 2 (intense) staining of IL-6 is seen in the placental villous trophoblasts in patients with BPD. B. Grade 2 staining of IL-6 is seen in the placental villous trophoblasts in patients without BPD. C. Grade 0 (no staining) of IL-10 is seen in the villous trophoblasts in patients with BPD. D. Grade 2 staining of IL-10 is seen in the villous trophoblasts in patients without BPD.
IL-6 staining in the umbilical cord vessels was not robust. In patients with BPD, only 27% (13/48) showed staining in the arterial endothelium and 31% (15/48) in the venous endothelium, and the staining was predominantly mild (Grade 1). This pattern was also similar for infants without BPD. There was more abundant staining in the cord stroma, with 75% of patients both with and without BPD demonstrating staining for IL-6 (data not shown).
IHC analysis of IL-10
Unlike IL-6, the overall staining for IL-10 was comparatively less prominent in all the placental compartments surveyed. For example, IL-10 staining was less intense in the extra-placental membrane roll (Figure 1C, D). In the amnion of patients with BPD 28% (10/36) had no staining, while 64% (23/36) had Grade 1 staining and the remaining 8% (3/56) had Grade 2. This distribution was similar for the chorion as well. A similar pattern of staining was present for infants without BPD. Interestingly, the adjoining decidual area showed no staining for IL-10. The umbilical cord tissue also showed little staining for IL-10, irrespective of the presence or absence of BPD.
There were differences in the staining for IL-10 in placental villous trophoblasts between patients with and without BPD (Figure 2C, D). 60% (23/38) of the patients with BPD had no staining, while only 40% (15/38) had Grade 1 staining. In contrast, for those patients without BPD, 63% (21/38) had Grade 1 or 2 IL-10 staining.
Associations between cytokines and BPD
There were no associations between IL-6 and BPD for all compartments examined. Similarly, there were no associations between IL-10 expression in the cord or membrane staining and BPD. However, as illustrated in Figure 2 and quantified in Figure 3, IL-10 expression in the trophoblast and stroma differed among infants with and without BPD. Specifically, trophoblast IL-10 expression was associated with a reduction in the odds of developing BPD (OR 0.08, 95% CI 0.01–0.64 p=0.017). IL-10 expression in the villous stroma was also associated with a decreased risk of BPD (OR 0.25, 0.07–0.89, p=0.03). Adjustment for race and GA did not change this relationship (OR 0.08, 0.01–0.70, p=0.02 and OR 0.12, 0.02–0.63, p=0.01) for villous trophoblasts and stroma respectively (Figure 3).
Figure 3.
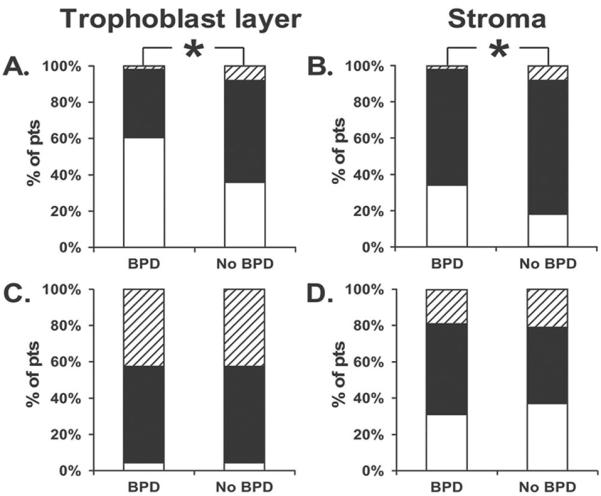
Grade distribution expressed as percent of patients (pts). Grade 0 as white bar, Grade 1 as black bar, Grade 3 as shaded bar of immunostaining in the placenta. A and B, The expression of IL-10 in patients without BPD is associated with a reduction in the odds of developing BPD in adjusted analyses for the villous trophoblast layer (OR 0.08,0.01–0.70, p=0.02) and stroma (0.12, 0.02 – 0.60, p= 0.01). C and D, A similar distribution of IL-6 staining grades seen in the trophoblast layer and stroma among infants with and without BPD.
Discussion
Higher concentrations of inflammatory cytokines have been documented in amniotic fluid, cord blood and tracheal aspirates of premature infants who subsequently developed BPD (12–17), whereas anti-inflammatory cytokines were found to be absent or in lower concentrations in bronchoalveolar lavage fluid of premature infants with BPD (17,18). These patterns may depend upon the expression of pro- and anti-inflammatory cytokines in multiple placental compartments. The results of this study support the hypothesis that placental tissues of infants who developed BPD manifest down regulation of IL-10 expression, while robustly express IL-6.
Our original hypothesis proposed an imbalance of cytokines among infants who develop BPD with an increase in pro-inflammatory cytokines and a decrease in anti-inflammatory cytokines in the placenta. Postulating a predominance of placental IL-6 staining relative to IL-10 is based upon the observations that IL-6 is elevated in amniotic fluid, cord blood and tracheal aspirates of premature infants who subsequently develop BPD (12–18). Furthermore, it is recognized that cytokines can be secreted from cells in the decidua and chorion, as well as from infiltrating macrophages (20,21). One mechanism responsible for the activation of an inflammatory response in the premature lungs may be direct damage, as ventilator induced injury can induce mRNA expression of IL-6 in the preterm lung (22). Inhalation of amniotic fluid by the fetus may favor the extension of the placental/amniotic fluid environment into the tracheobronchial tree, thus promoting inflammation, as proposed by Ghezzi et al, (14). Alternatively, placental expression of pro-inflammatory mediators may prompt a fetal systemic inflammatory response with increased endothelial permeability, capillary leakage and diffuse alveolar damage predisposing to BPD. Increased cord blood concentration of IL-6 among infants destined to develop BPD is supportive of this notion (12). The results of this study, in contrast, did not demonstrate differences in IL-6 staining in any of the placental compartments of infants with BPD compared to those without BPD. Similar expression of IL-6 in placental tissue with differences in IL-10 expression would certainly not preclude more prominent IL-6 production in other tissues and body fluid in response to inflammation and lung injury. We propose that in the absence of placental IL-10 in BPD cases, the inflammatory function of IL-6 remains unchecked.
It is interesting that we did not see a difference in the incidence of histological chorioamnionitis between newborns with and without BPD. Whereas some studies have found a positive association between chorioamnionitis and BPD (8,9), other studies have not supported the notion of chorioamnionitis as a risk factor for BPD (23,24). This conflicting data likely represents differences in definitions of chorioamnionitis and BPD, time periods studied, and antenatal factors, such as maternal steroid use. Chorioamnionitis is characterized by higher amniotic fluid and cord blood concentrations of IL-6 (25,26); however, no studies specifically examined the relationship between chorioamnionitis, as defined by neutrophil response, and placental cytokine expression. The relationship between the BPD pathology and cytokine response is likely more complex than we understand.
The results of this study support a critical anti-inflammatory and immunomodulatory role of IL-10 even before birth in the development of BPD. Recent reports have suggested that IL-10 placental expression is gestational age dependent (27) and we did not see prominent IL-10 staining in the cord, membranes, and placenta. Yet, in spite of this, the presence of placental IL-10 in the villous trophoblast was associated with a reduction in the odds of developing BPD. This relationship was present in unadjusted and adjusted analyses for differences in gestational age and race. In agreement with our findings, is the observation that the absence of IL-10 in tracheal aspirates in infants <27 weeks is associated with a higher risk of developing BPD (16). Similarly, poor IL-10 expression has been reported in preterm infants with hyaline membrane disease (18,28). While these latter studies did not specifically look at chronic lung disease, it may be that persistent and unchecked inflammation plays a role in the evolution of BPD.
Recent work has shown genetic heritability to BPD (8,9). Emerging data suggests that genetic variations in IL-10 gene regulation may ultimately affect the anti-inflammatory response. IL-10 regulation is complex and it is subject to genetic influence (29,30). The single-nucleotide polymorphism (SNP) at −1082 G allele has been associated with a higher IL-10 expression in certain cell types (31). Moreover, recent work has shown that preterm infants who carry two G alleles of the IL-10 (−1082) SNP were at a prominently reduced risk for neonatal cerebral, eye, and lung (specifically BPD) damage (32). Although it is plausible that polymorphic changes in the IL-10 promoter may be differentially utilized in different cell types in response to inflammatory triggers, our data suggest that IL-10 expression in the villous tissue is not uniquely influenced by chorioamnionitis. We propose that IL-10 down-regulation in the placenta of infants with BPD involves additional events, such as an excessive production of prostanoids or dysregulation of the hormonal cascade.
There are important limitations to this study that should be acknowledged. Although we matched for variables (sex, period of birth, and birthweight) that previously have shown to affect BPD (33–35), there may be unidentified variable influencing the results of a case controlled retrospective study. While we felt matching for the above variables was clinically relevant, we acknowledge potential bias in case selection in a retrospective analysis. The cohort of this study represents approximately 46% and 23% of BPD and no BPD infants, respectively, available from our NICU. Despite our matching of the above variables, there were group differences in gestational age and trends in differences in race. Racial differences in the development of BPD have been previously reported and matching on race would have made it impossible to attain a reasonable sample size (35). The lower gestational age and race were adjusted for in the logistic regressions. We also are aware that there are other criteria being used by some to define BPD, such as an oxygen challenge reduction test (36). This methodology was not being performed at the time period of our study and our definition of oxygen use at 36 weeks PMA is within the scope of the National Institute of Health consensus conference definition of BPD (37).
We also recognize the variability of immunohistochemical staining and the subjectivity of grading. To allow for a more accurate statistical analysis, each batch of staining consisted of patients with BPD and their matched controls, in addition to a standard reference control. The scoring system used for determining the extent of staining was qualitative rather than quantitative; ideally a system that determines extent of cytokine staining both in terms of placental area involved and degree of staining would be desirable. In spite of this, the analysis of inter-observer reliability was reassuring with a kappa value of 0.82. In addition, the categorical analysis of our data as either “no staining” or “any staining” also decreased subjectivity.
Our findings support a “cytokine balance” theory. Logistic regression analysis shows an independent association between IL-10 and BPD. This suggests that a baseline inflammation is present, but the absence of anti-inflammatory mediators in the placenta may initiate fetal inflammatory responses. We feel that this study is important as it presents a prenatal or antenatal environment that may ultimately affect the postnatal course of an infant. Further studies of placental examination may allow us to better identify those premature infants at increased risk for particular morbidities.
Acknowledgements
The authors thank Richard Tucker for his help with statistical analysis and Paul Monfils of the Rhode Island Hospital Core Research Laboratory for his help with immunohistochemical analysis.
Financial Support: This work was supported in part by the grant from NIH NCRR (P20RR018728), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS and a Subcontract WSU05056 under NICHD Contract #N01-HD-2-3342.
ABBREVIATIONS
- BPD
Bronchopulmonary dysplasia
- IHC
immunohistochemistry
References
- 1.Klinger G, Sirota L, Lusky A, Reichman B. Bronchopulmonary dysplasia in very low birth weight infants is associated with prolonged hospital stay. J Perinatol. 2006;26:640–644. doi: 10.1038/sj.jp.7211580. [DOI] [PubMed] [Google Scholar]
- 2.Furman L, Baley J, Borawski-Clark E, Aucott S, Hack M. Hospitalization as a measure of morbidity among very low birth weight infants with chronic lung disease. J Pediatr. 1996;128:447–452. doi: 10.1016/s0022-3476(96)70353-0. [DOI] [PubMed] [Google Scholar]
- 3.Short EJ, Klein NK, Lewis BA, Fulton S, Eisengart S, Kercsmar C, Baley J, Singer LT. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8 year outcomes. Pediatrics. 2003;112:e359. doi: 10.1542/peds.112.5.e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis BA, Singer LT, Fulton S, Salvator A, Short EJ, Klein N, Baley J. Speech and language outcomes of children with bronchopulmonary dysplasia. J Commun Disord. 2002;35:393–406. doi: 10.1016/s0021-9924(02)00085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, Lemons JA, Oh W, Papile LA, Shankaran S, Stevenson DK, Tyson JE, Poole WK, NICHD Neonatal Research Network Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147.e1–147.e8. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 6.O'Brodovich HM, Mellins R. Bronchopulmonary dysplasia. Unresolved neonatal acute lung injury. Am Rev Respir Dis. 1985;132:694–709. doi: 10.1164/arrd.1985.132.3.694. [DOI] [PubMed] [Google Scholar]
- 7.Coalson JJ. Pathology of bronchopulmonary dysplasia. Semin Perinatol. 2006;30:179–184. doi: 10.1053/j.semperi.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Bhandari V, Bizzarro MJ, Shetty A, Zhong X, Page GP, Zhang H, Ment LR, Gruen JR, Neonatal Genetics Study Group Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 2006;117:1901–1906. doi: 10.1542/peds.2005-1414. [DOI] [PubMed] [Google Scholar]
- 9.Lavoie PM, Pham C, Jang KL. Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the national institutes of health. Pediatrics. 2008;122:479–485. doi: 10.1542/peds.2007-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97:210–215. [PubMed] [Google Scholar]
- 11.Viscardi RM, Muhumuza CK, Rodriguez A, Fairchild KD, Sun CC, Gross GW, Campbell AB, Wilson PD, Hester L, Hasday JD. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res. 2004;55:1009–1017. doi: 10.1203/01.pdr.0000127015.60185.8a. [DOI] [PubMed] [Google Scholar]
- 12.Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim B, Jun JK. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1999;181:773–779. doi: 10.1016/s0002-9378(99)70299-1. [DOI] [PubMed] [Google Scholar]
- 13.Yoon BH, Romero R, Jun JK, Park JK, Park JD, Gezzi F, Kim B. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1997;177:825–830. doi: 10.1016/s0002-9378(97)70276-x. [DOI] [PubMed] [Google Scholar]
- 14.Ghezzi F, Gomez R, Romero R, Yoon BH, Edwin SS, David C, Janisse J, Mazor M. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol. 1998;78:5–10. doi: 10.1016/s0301-2115(97)00236-4. [DOI] [PubMed] [Google Scholar]
- 15.Bagchi A, Viscardi RM, Taciak V, Ensor JE, McCrea KA, Hasday JD. Increased activity of interleukin-6 but not tumor necrosis factor-α in lung lavage of premature infants is associated with the development of bronchopulmonary dysplasia. Pediatr Res. 1994;36:244–252. doi: 10.1203/00006450-199408000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Garingo A, Tesoriero L, Cayabyab R, Durand M, Blahnick M, Sardesai S, Ramanathan R, Jones C, Kwong K, Li C, Minoo P. Constitutive IL-10 expression by lung inflammatory cells and risk for bronchopulmonary dysplasia. Pediatr Res. 2007;61:197–202. doi: 10.1203/pdr.0b013e31802d8a1c. [DOI] [PubMed] [Google Scholar]
- 17.Oei J, Lui K, Wang H, Henry R. Decreased interleukin-10 in tracheal aspirates from preterm infants developing chronic lung disease. Acta Paediatr. 2002;91:1194–1199. doi: 10.1111/j.1651-2227.2002.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 18.Jones CA, Cayabyab RG, Kwong YC, Scotts C, Wong B, Hamden H, Minoo P, deLemos RA. Undetectable interleukin-10 and persistent interleukin-expression early in hyaline membrane disease:a possible developmental basis for the predisposition to chronic lung inflammation in preterm newborns. Pediatr Res. 1996;39:966–975. doi: 10.1203/00006450-199606000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;82:527–532. [PubMed] [Google Scholar]
- 20.Berner R, Niemeyer CM, Leititis JU, Funke A, Schwab C, Rau U, Richter K, Tawfeek MS, Clad A, Brandis M. Plasma levels and gene expression of granulocyte colony-stimulating factor, tumor necrosis factor-alpha, interleukin (IL)-1beta, IL-6, IL-8, and soluble intercellular adhesion molecule-1 in neonatal early onset sepsis. Pediatr Res. 1998;44:469–477. doi: 10.1203/00006450-199810000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Dudley DJ, Collmer D, Mitchell D, Trautman MS. Inflammatory cytokine mRNA in human gestational tissues: implications for term and preterm labor. J Soc Gynecol Investig. 1996;3:328–335. [PubMed] [Google Scholar]
- 22.Naik AS, Kallapur SG, Bachurski CJ, Jobe AH, Michna J, Kramer BW, Ikegami M. Effects of ventilation with different positive end-expiratory pressures on cytokine expression in the preterm lamb lung. Am J Respir Crit Care Med. 2001;164:494–498. doi: 10.1164/ajrccm.164.3.2010127. [DOI] [PubMed] [Google Scholar]
- 23.Redline RW, Wilson-Costello D, Hack M. Placental and other perinatal risk factors for chronic lung disease in very low birth weight infants. Pediatr Res. 2002;52:713–719. doi: 10.1203/00006450-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Kent A, Dahlstrom JE. Chorioamnionitis/funisitis and the development of bronchopulmonary dysplasia. J Paediatr Child Health. 2004;40:356–359. doi: 10.1111/j.1440-1754.2004.00366.x. [DOI] [PubMed] [Google Scholar]
- 25.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, Syn HC. Amniotic fluid interleukin 6: A sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960–970. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 26.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, Ghezzi F, Berry SM, Qureshi SM, Kim JC, Nadar N, Romero R. Funisitis and chorionic vasculitis:the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 27.Hanna N, Hanna I, Hleb M, Wagner E, Dougherty J, Balkundi D, Padbury J, Sharma S. Gestational age dependent expression of IL-10 and its receptor in human placental tissue and isolated cytotrophoblasts. J Immunol. 2000;164:5721–5728. doi: 10.4049/jimmunol.164.11.5721. [DOI] [PubMed] [Google Scholar]
- 28.Kwong KY, Jones CA, Cayabyab R, Lecart C, Khuu N, Rhandhawa I, Hanley JM, Ramanatha R, deLemos RA, Minoo R. The effects of IL-10 on proinflammatory cytokine expression (IL-1beta and IL-8) in hyaline membrane disease. Clin Immunol Immunopathol. 1998;88:105–113. doi: 10.1006/clin.1997.4510. [DOI] [PubMed] [Google Scholar]
- 29.Westendorp RG, Langermans JA, Huizinga TW, Elouali AH, Verweij CL, Boomsma DI, Vandenbrouke JP. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997;349:170–173. doi: 10.1016/s0140-6736(96)06413-6. [DOI] [PubMed] [Google Scholar]
- 30.Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphisms in the interleukin-10 gene promoter. Eur J Immunogenet. 1997;24:1–8. doi: 10.1111/j.1365-2370.1997.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 31.Schaaf BM, Boehmke F, Esnaashari H, Seitzer U, Kothe H, Maass M, Zabel P, Dalhoff K. Pneumococcal septic shock is associated with the interleukin-10-1082 gene promoter polymorphism. Am J Respir Crit Care Med. 2003;168:476–480. doi: 10.1164/rccm.200210-1164OC. [DOI] [PubMed] [Google Scholar]
- 32.Dordelmann M, Kerk J, Dressler F, Brinkhaus MJ, Bartels DB, Dammann CE, Dork T, Dammann O. Interleukin-10 high producer allele and ultrasound defined periventricular white matter abnormalities in preterm infants: a preliminary study. Neuropediatrics. 2006;37:130–136. doi: 10.1055/s-2006-924554. [DOI] [PubMed] [Google Scholar]
- 33.Oh W, Poindexter BB, Perritt R, Lemons JA, Bauer CR, Ehrenkranz RA, Stoll BJ, Poole K, Wright LL, Neonatal Research Network Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birthweight infants. J Pediatr. 2005;147:786–790. doi: 10.1016/j.jpeds.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 34.Costeloe K, Hennessy E, Gibson AT, Marlow N, Wilkinson AR. The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics. 2000;106:659–671. doi: 10.1542/peds.106.4.659. [DOI] [PubMed] [Google Scholar]
- 35.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, Wrage LA, Poole K, National Institute of Child Health and Human Development Neonatal Research Network Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 36.Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, Everette R, Peters N, Miller N, Muran G, Auten K, Newman N, Rowan G, Grisby C, Arnell K, Miller L, Ball B, McDavid G, National Institute of Child Health and Human Development Research Network Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114:1305–1311. doi: 10.1542/peds.2004-0204. [DOI] [PubMed] [Google Scholar]
- 37.Jobe AH, Bancalari E. Bronchopulmonary Dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]


