Abstract
Antimicrobial peptides are important effectors of innate immunity throughout the plant and animal kingdoms. In the mammalian small intestine, Paneth cell α-defensins are antimicrobial peptides that contribute to host defense against enteric pathogens. To determine if α-defensins also govern intestinal microbial ecology, we analyzed the intestinal microbiota in mice expressing a human α-defensin (DEFA5) and in mice lacking an enzyme required for processing of murine α-defensins. We detected significant α-defensin-dependent changes in microbiota composition, but not in total bacterial numbers, in these complementary models. Furthermore, DEFA5-expressing mice had striking losses of Segmented Filamentous Bacteria and fewer interleukin 17-producing lamina propria T cells. These data ascribe a new homeostatic role for α-defensins in regulating the makeup of the commensal microbiota.
INTRODUCTION
Humans and other animals exist in continual contact with a diverse array of microbes, and their mucosal surfaces colonized by a complex microbiota. These commensal microbiota are vital to many physiological and homeostatic functions, and play important roles in host defense through colonization resistance 1, 2, and by promoting the development and regulation of the acquired mucosal immune system 3, 4. Recent analysis using 16S rRNA technology demonstrated the complexity of the intestinal tract microbiota 5–7 and demonstrated that the host can influence this microbial ecosystem 8–13.
Although the intestinal microbiota contribute to host protection, the host must rely on multiple immune defense mechanisms to avert microbial disease. At the mucosal interface, the epithelium serves as a key arm of the immune system by providing a physical barrier, and by secreting various antimicrobial factors, including antimicrobial peptides14, 15. In the lumen of the small intestine of humans and other mammals, Paneth cell derived α-defensins (also called crypt defensins, cryptdins) are predominant antimicrobial factors 16 that are effective mediators of host defense against enteric bacterial pathogens 17–19. Mouse α-defensins are synthesized in inactive form and must be cleaved and activated by matrix metalloproteinase 7 (MMP7 (http://www.signaling-gateway.org/molecule/query?afcsid=A001477), also called matrilysin) 20. In addition to this host defense role, α-defensins may play an undocumented homeostatic role in establishing and maintaining the intestinal microbiota.
To examine this possibility, we used 16S rRNA technology to analyze the intestinal microbiota in two complementary mouse models: DEFA5 transgenic (tg) mice 18 express physiologically relevant amounts of the human Paneth cell α-defensin 5 (DEFA5) in mouse Paneth cells, and MMP7-deficient mice 17 lack the enzyme needed for processing and activation of murine Paneth cell α-defensins and thus serve as a model of α-defensin deficiency in the small intestine lumen. Our data provide direct evidence that Paneth cellα-defensins play a key role in shaping the composition of the small intestinal microbiota. Furthermore, we observed that α-defensin-dependent changes in the microbiota can modulate mucosal immune responsiveness. Given recent investigations demonstrating the involvement of Paneth cells 21, 22, and decreased expression of Paneth cell α-defensins in patients with ileal Crohn’s disease 23, our findings support the hypothesis that α-defensin deficiency may contribute to the host-microbe dysbiosis and enhanced inflammatory responsiveness associated with inflammatory bowel disease (IBD) pathogenesis.
RESULTS
Microbial composition of the small intestine
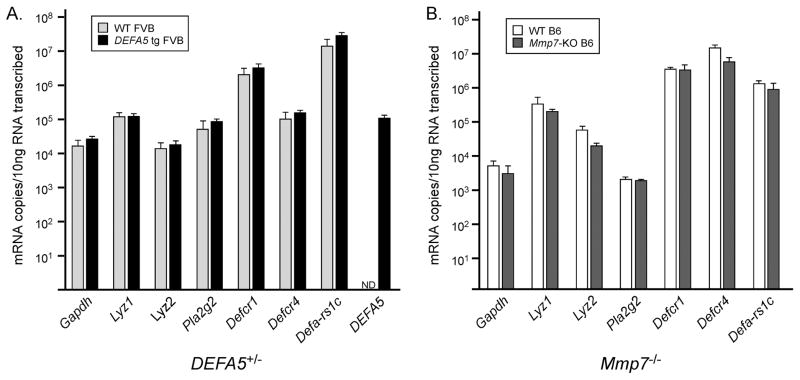
Studies of the intestinal microbiota are inherently complex, and often-neglected experimental variables such as husbandry, parental genotypes and environmental influences must be carefully controlled. Herein, mice expressing one copy of the DEFA5 tg (+/−) on an FVB genetic background were mated to produce mixed litters of mice expressing zero, one or two copies of the DEFA5 tg. Similarly, Mmp7+/− mice on a C57BL/6 (B6) background were mated to generate Mmp7+/+, Mmp7+/− and Mmp7−/−offspring. We detected comparable amounts of mRNA transcripts encoding Paneth cell effector molecules in the genetically altered mice and their wild-type counterparts (Fig. 1a,b), providing evidence that the genetic manipulation did not globally affect Paneth cell gene expression.
Figure 1. Expression of Paneth cell effector genes in Mmp7 −/−, DEFA5 tg (+/+) and wild-type (WT) mice.
a,b. The absolute mRNA copy numbers of transcripts encoding murine Paneth cell lysozyme (M and P isoforms Lyz1, Lyz2), secretory phospholipase A2 (Pla2g2), α-defensins (cryptdins) 1 (Defcr1) and 4 (Defcr4), cryptdin related sequence (Defa-rs1c), and DEFA5 were determined by quantitative real-time PCR using RNA isolated from the distal small intestine of (a) DEFA5 tg (+/+) (n=3) and wild-type (n=3) FVB mice or (b) Mmp7 −/− (n=4) and wild-type (n=4) B6 mice. The absolute mRNA copy numbers were determined using the same primers and standards for all mouse strains, except that the cryptdin 4 used strain specific primers (Supplementary Table 3). Mean values and standard deviations are presented. No statistically significant differences were identified.
To analyze the bacterial composition of the small intestinal microbiome, total genomic DNA was isolated from the distal 15 cm of the small intestine of each individual mouse. Full-length 16S rDNA sequences were amplified from these samples, generating a pool of PCR products representing the entire complex mixture of bacteria 24. Subclones were sequenced and analyzed using the Ribosomal Database Project II (RDP) classifier (Supplementary Tables 1a,b). To estimate microbial diversity, Shannon diversity indices (Table 1), rarefaction curves, and operational taxonomic unit (OTU) abundance (Supplementary Fig. 1a–d) were determined with the computer program DOTUR 25, at the phylum and class levels. The percentage of coverage in each genotype community was calculated by Good’s coverage (Table 1). This calculation revealed excellent coverage (≥ 98.5% in all groups) at the phylum level, consistent with the results of the rarefaction curves (Supplementary ig. 1a–d). Overall, the percentages for each class of bacteria revealed the predominance of two phyla, the Firmicutes and Bacteroidetes, consistent with previous investigations (Table 1) 8, 26, 27.
Table 1. Analysis of 16S rDNA subclones.
The 16S rDNA sequence was amplified from bacterial genomic DNA from the distal small intestine of individual mice. PCR products were subcloned and sequenced, and sequences were analyzed using the RDP classifier (version 9). Values for sequence abundance (%) were determined for each animal individually as specific bacterial class or phylum divided by total bacterial sequences per animal.
| Mmp7 −/− | Mmp7+/+ | Wild-type | DEFA5 tg (+/+) | |
|---|---|---|---|---|
| Samples | 8 | 9 | 7 | 4 |
| Total sequences | 764 | 863 | 616 | 366 |
| Good’s Coverage (phylum) | 99.0% | 98.8% | 98.5% | 99.5% |
| Shannon Index (phylum) | 2.13 | 2.20 | 1.69 | 1.36 |
| Bacteria (%) | ||||
| Firmicutes | 63.4 ± 4.4** | 41.1 ± 5.4** | 59.3 ± 11.3‡ | 25.5 ± 6.4‡ |
| -Bacilli | 11.3 ± 3.3 | 8.6 ± 3.1 | 20.7 ± 6.6 | 10.3 ± 3.5 |
| -Clostridia | 52.1 ± 5.2* | 32.6 ± 4.3* | 15.0 ± 7.6 | 3.0 ± 2.3 |
| -Erysipelotrichi | 0.3 ± 0.2 | 0.0 ± 0.0 | 23.6 ± 12.7 | 12.0 ± 4.6 |
| Tenericutes | 11.8 ± 2.9 | 7.3 ± 2.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Bacteroidetes | 17.5 ± 4.2*** | 40.9 ± 5.2*** | 34.9 ± 11.0‡ | 69.3 ± 6.1‡ |
| Actinobacteria | 2.0 ± 0.6 | 0.9 ± 0.3 | 0.3 ± 0.2 | 0.0 ± 0.0 |
| Proteobacteria | 4.8 ± 1.4 | 9.3 ± 2.1 | 2.4 ± 1.0 | 5.0 ± 0.9 |
| Deferribacteres | 0.3 ± 0.2 | 0.1 ± 0.1 | 3.0 ± 2.8 | 0.0 ± 0.0 |
Values are the mean ± s.e.m. of these percentages. Mann-Whitney test between Mmp7−/− and Mmp7+/+
P = 0.0152,
P = 0.0111,
P = 0.0037.
Mann-Whitney test between wild-type and DEFA5 tg (+/+)
P = 0.0424 for both comparisons.
Analysis of sequences from individual DEFA5 tg (+/+) mice compared to those from wild-type littermate controls showed a significant shift in microbial composition (Table 1). The percentage of Firmicutes was significantly lower and the percentage of Bacteroidetes was significantly higher in the DEFA5 tg (+/+) mice than in wild-type controls. In contrast, we detected a significant increase in the percentage of Firmicutes and a significant decrease in the percentage of Bacteroidetes in Mmp7−/− compared to Mmp7+/+ mice. The decrease in Firmicutes in DEFA5 tg mice was attributable to losses of Clostridia, Bacilli and Erysipelotrichi, whereas the increase in Firmicutes Mmp7−/− mice reflected a relatively selective increase in Clostridia. Significant differences between the microbial populations were also evident using the UniFrac computer program with a P-test and a UniFraC significance test (P ≤ 0.01). Community comparisons produced the clustering relationships and associated heat maps for the DEFA5 tg and wild-type (Fig. 2a) and Mmp7−/− and Mmp7+/+ mice (Fig. 2b). These data indicate that the composition of the endogenous microbiota is genotype-dependent in these two models, and that there are reciprocal differences in the defensin over-expressing and defensin-deficient mouse models.
Figure 2.
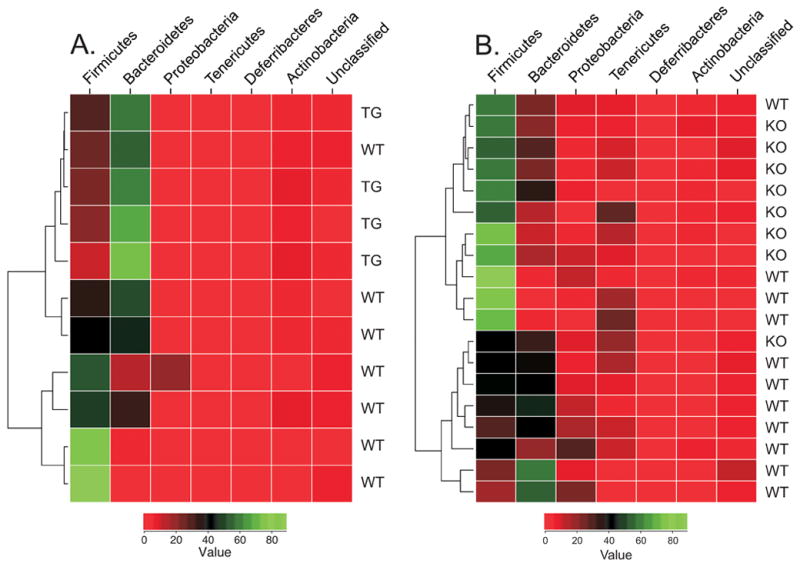
Community comparisons and phylogenetic analysis of bacterial composition of the distal small intestine. Genomic DNA was isolated from the distal small intestines of Mmp7 −/− (a) and DEFA5 tg (+/+) (b) mice and littermate controls (WT). 16S rRNA sequences were obtained by PCR amplification, subcloning, and sequencing. Using R-package software, the sequence data assigned by the RDP classifier were used for the comparison of microbial communities and the generation of heat maps. For each animal, the number of sequences in each phylum was normalized by the total number of sequences obtained for that animal, producing a percentage. The percentage was used as the matrix for generating the heat map. The color key values represent the percentage (0–100%) of sequences in each phylum.
An inversion of the Firmicute/Bacteroidetes ratio, with higher Firmicutes than Bacteroidetes, has been associated with obesity in both animals and humans 9, 26–28. In addition, blooms of Mollicute populations, a subclass of the Tenericutes, have been noted in obese animals28. We did not detect Mollicutes in DEFA5 tg or wild-type littermates, but Mmp7−/− and Mmp7+/+ littermates contained Mollicutes, with higher abundance in the Mmp7−/− mice. Nevertheless, we did not see evidence of obesity or increased weight in the Mmp7−/− mice compared to their Mmp7+/+ littermates (data not shown), as might be expected from the shifts in their Firmicute/Bacteroidetes/Tenericute phyla ratios. Likewise, we saw no weight differences between DEFA5 tg and wild-type littermates (data not shown). One caveat is that we analyzed bacteria specifically in the small intestines, whereas other studies analyzed the feces; and it is possible that the influences of changes in the microbiome on body weight are site-dependent.
A more detailed phylogenetic analysis was done on pooled clone sequences using ARB software. This provides further insight into differences between genotypes as well as between B6 and FVB wild-type strains (Supplementary Fig. 2a). A detailed analysis of the Bacteroidetes class shows a dominance of the Mouse Intestinal Bacteroides (MIB) group, a common Bacteroides strain found in the mouse intestinal tract 24, in all mouse models analyzed here. In fact, all Bacteroides identified by subclone sequence in DEFA5 tg mice belong exclusively to the MIB group (Supplementary Fig. 2b). However, a subset of the Firmicute/Clostridiae group, Candidatus arthromitis, commonly known as segmented filamentous bacteria (SFB) 29, was notably absent in the DEFA5 tg and wild-type FVB mice, while highly represented among the Mmp7−/− and Mmp7+/+ B6 mice (Supplementary Fig. 2c). In addition, within the Clostridiae group the E. rectale-C. coccoides (Erec) group, which is closely related to C. coccoides, dominated the DEFA5 tg and wild-type FVB mice whereas C. leptum is the dominant Clostridia species in Mmp7−/− and Mmp7+/+ B6 mice (Supplementary Fig. 2c).
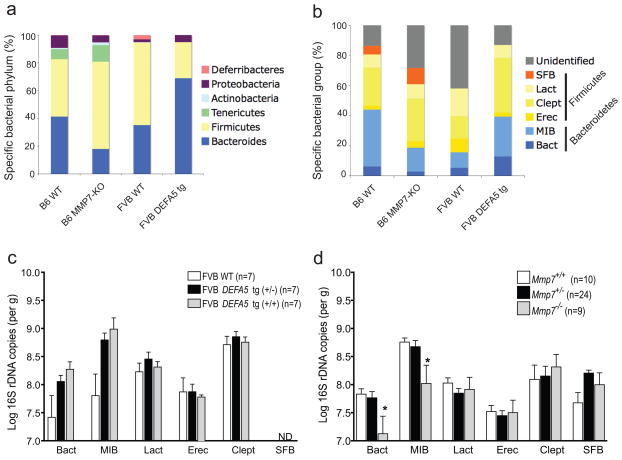
These analyses of 16S rRNA subclones demonstrated that there were significant reciprocal shifts in the abundance of specific bacterial populations in the two mouse models of altered Paneth cell α-defensin expression. Further support of this hypothesis was sought using a complementary approach that would more amenable to analysis of experimental variables. We employed quantitative real-time PCR (qPCR) 30 using both group-specific and kingdom-specific (Eubacterial) primers for 16S rRNA (Supplementary Table 2), targeting groups covering the dominant bacterial populations in the mouse intestinal tract 24. However, there are inherent limitations with this approach 30. For example, several of the bacterial groups analyzed by qPCR fall into the Firmicute phylum (SFB, Lactobacilli (Lact), C. leptum (Clept), E. rectales (Erec)), but primers that give complete coverage of this bacterial phylum are not available. Nevertheless, we observed similar differences in the proportions of bacterial groups present in mice of each genotype using sequence analysis of subclones and qPCR approaches (Fig. 3a,b). Specifically, we observed increases in the Bacteroides (Bact) and Mouse Intestinal Bacteroides (MIB) groups in DEFA5 tg mice as compared to wild-type FVB mice (Fig. 3a,c). Although these trends were entirely consistent with the shifts determined by subclone sequence analysis (Table 1, Fig. 3a), unlike the significant differences observed by subclone sequence analysis, the differences detected by qPCR did not achieve statistical significance. This likely reflects the noted limitations of relying on selective PCR primers to accurately capture a bacterial phylum.
Figure 3.
Quantitative analysis of intestinal bacterial groups. a,b. Bacterial composition of the distal small intestines of Mmp7 −/− and DEFA5 tg (+/+) mice was determined by analysis of subclone sequences (a) and qPCR (b). Stacked graphs show relative percentages of dominant bacterial groups in the distal small intestine of offspring from Mmp7+/− and DEFA5 tg (+/−) breeding pairs with total bacterial sequences (a) or total bacterial copies as determined by amplification with universal bacterial primers (b) used as the denominator for subclone and qPCR analyses, respectively. c,d. Log number of total copies of specific bacterial 16S rDNA in the distal small intestine of each mouse was measured by pPCR. In offspring of DEFA5 tg (+/−) breeding pairs, the presence of SFB was not detectable (ND) by the reliable limits of detection by this assay. * P < 0.05.
However, the effect of MMP7 deficiency on the microbiota was significant as measured by the qPCR approach. There were significantly lower proportions of both Bacteroides (Bact) and MIB groups in Mmp7−/− compared to Mmp7+/+ B6 mice (Fig. 3b,d). Mmp7+/− B6 mice contain enough MMP7 enzyme to process prodefensins and therefore have normal amounts of processed Paneth cell α-defensins 17. Consistent with this finding, the microbiota of Mmp7+/− B6 mice was equivalent to that of Mmp7+/+ B6 mice (Fig. 3d). While these data provide additional support for our hypothesis that the composition of the intestinal microbiota of mice is dependent on the presence or absence of mature Paneth cell α-defensins, a caveat must be noted. Along with its role in processing Paneth cell α-defensins that are secreted into the small intestinal lumen, MMP7 has been shown to have other extraintestinal and intestinal roles 20, 31, including epithelial repair and transepithelial influx of neutrophils 32, as well as activation of other enzymes. Although many of these roles are outside of the gut lumen and often associated with infection and inflammation, we cannot rule out that the loss of MMP7 has more obscure effects that could indirectly contribute to shifts in the biota.
Loss of SFB in DEFA5 tg mice
The most unexpected finding in the DEFA5 tg and wild-type FVB mice was observed within the Firmicutes, in a group of bacteria known as SFB. This morphologically distinct bacterium has been identified in the intestinal tract of several animal models, including mice 24, rabbits 33 and chickens 34, but is not cultivable. SFB is the only bacterial species that has been shown to directly contact the small intestinal epithelium in these animal models. Abnormal expansion of this bacterium has been noted in IgA deficient mice 13 and its continuous presence in the small intestine has been associated with the inability to produce immunoglobulins. 35. Quantitative PCR analysis revealed an absence of SFB from the distal small intestine of DEFA5 tg and wild-type FVB litters, with data consistently below the reliable limits of detection by this assay.
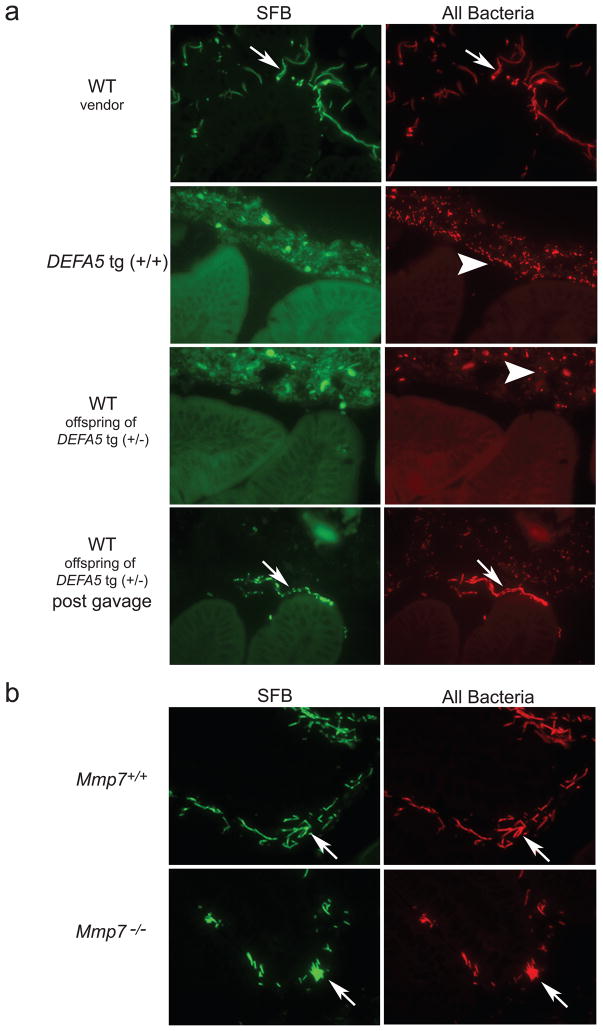
Initially we considered the possibility that mice of the FVB background might lack this organism. However analysis of the small intestines of litters from vendor-obtained FVB parents using fluorescence in situ hybridization (FISH) 36 demonstrated abundant SFB associated with the mucosa (Fig. 4a). In addition, isolated breeding colonies of wild-type FVB mice in our facility maintained consistent presence of SFB in the small intestine of parent and offspring mice. In contrast, FISH analysis of DEFA5 tg (+/+) mice and wild-type offspring from crosses of DEFA5 tg (+/−) parents revealed a complex mixture of morphologically diverse bacteria embedded within the intestinal mucus, closely approaching but not touching the epithelial cell layer. However, these mice completely lacked detectable SFB (Fig. 4a).
Figure 4.
FISH of adherent bacteria in mouse distal small intestine. a. Sections (3 micron) of distal small intestine from vendor-obtained wild-type (WT) mice or offspring from DEFA5 tg (+/−) breeding pairs were hybridized with a mixture of oligonucleotide probes recognizing SFB (6Fam-SFB) and total bacteria (TR-Bact338)36, and visualized by fluorescence microscopy23. Where indicated, mice were analyzed 14 days post-gavage with feces containing SFB. All mice were on a pure FVB background. b. Sections of distal small intestine (3 microns) from offspring from Mmp7+/− breeding pairs were hybridized and visualized as in (a). All mice in (b) were on a pure B6 background. Arrows point to SFB bacteria. Arrowheads point to non-SFB bacteria. Non-specific autofluorescence staining is noted in the mucus when viewing with FITC filters (SFB panels), and does not correlate with any specific bacteria (visualized using Texas Red filters-All Bacteria panels).
SFB colonization was reestablished in these wild-type offspring of DEFA5 tg (+/−) parents by oral gavage with feces containing SFB (Fig. 4a). This suggests that commensal biota may, in part, be established by caprophagy. However, similar gavage of DEFA5 tg (+/+) mice did not result in increased intestinal SFB colonization (data not shown). Interestingly, the wild-type offspring from crosses of DEFA5 tg (+/−) parents initially lacked detectable SFB at 5 weeks of age, but when interbred in cages separated from their DEFA5 tg-expressing parents and littermates these mice generated litters containing SFB. Offspring of crosses between DEFA5 tg-expressing offspring of DEFA5 tg (+/−) parents, however, did not contain SFB. Thus, in the absence of selective pressure imparted by ongoing DEFA5 tg intestinal expression, SFB eventually repopulates the intestinal tract in wild-type offspring. In contrast to the results in DEFA5 tg mice, the offspring of Mmp7+/− mice retained abundant SFB, irrespective of Mmp7 genotype (Fig. 4b).
Maternal transmission of SFB
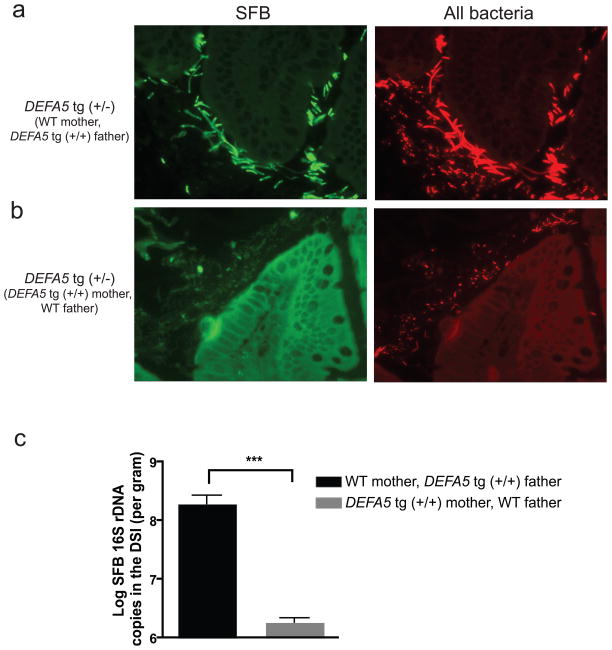
We hypothesized that the observed loss of SFB in all offspring of DEFA5 tg (+/−), which was irrespective of offspring genotype, was due to loss of maternal transmission of this bacterium. To test this idea, we set up breeding pairs of DEFA5 tg mice of varying genotypes. Males were removed from the cages of the pregnant dam to prevent exposure of the offspring to paternal stool bacteria by caprophagy. When DEFA5 tg (+/+) male mice were bred to wild-type female mice, the DEFA5 tg (+/−) offspring initially showed the presence of SFB (Fig. 5a). In contrast, when wild-type male mice were bred to DEFA5 tg (+/+) female mice, the DEFA5 tg (+/−) offspring lacked SFB (Fig. 5b). Quantitative analysis of SFB abundance by qPCR was consistent with the FISH findings, as SFB abundance decreased from 10% to 0.1% of the total distal small intestine bacteria when the mother’s genotype is DEFA5 tg (+/+) (Fig. 5c). Therefore, it is likely that the reduction of SFB in all offspring of the DEFA5 tg (+/−) parents was the result of a combination of both the gradual elimination of SFB in the parents in response to DEFA5, and reduced shedding of this bacterium. This lead to reduced maternal transmission and reduced exposure by caprophagy. Together these findings elucidate a direct relationship between the host expression of a single Paneth cell α-defensin and the fitness of a specific commensal bacterial species in the intestine. This also supports the view that Paneth cell α-defensins may exert their greatest affect on mucosa-associated bacteria as has been proposed for other Paneth cell antimicrobials 37.
Figure 5.
Influence of maternal exposure on SFB colonization in mouse distal small intestine. a,b. Sections of distal small intestine (3 microns) from 5-week-old offspring of indicated breeding pairs were analyzed by FISH as in Fig. 4 to detect epithelial associated SFB. Arrows point to SFB. Arrowheads point to non-SFB bacteria. Non-specific auto-fluorescence may be noted in the mucus in the FITC channel (SFB panels), but this does not correspond to any bacteria noted in the Texas Red channel (All Bacteria panels). c. Log numbers and percentages of SFB 16S rDNA in the distal small intestine were analyzed by qPCR. Only DEFA5 tg (+/−) offspring from indicated breeding pairs were analyzed (n=9–11 mice per litter). *** P=0.0002.
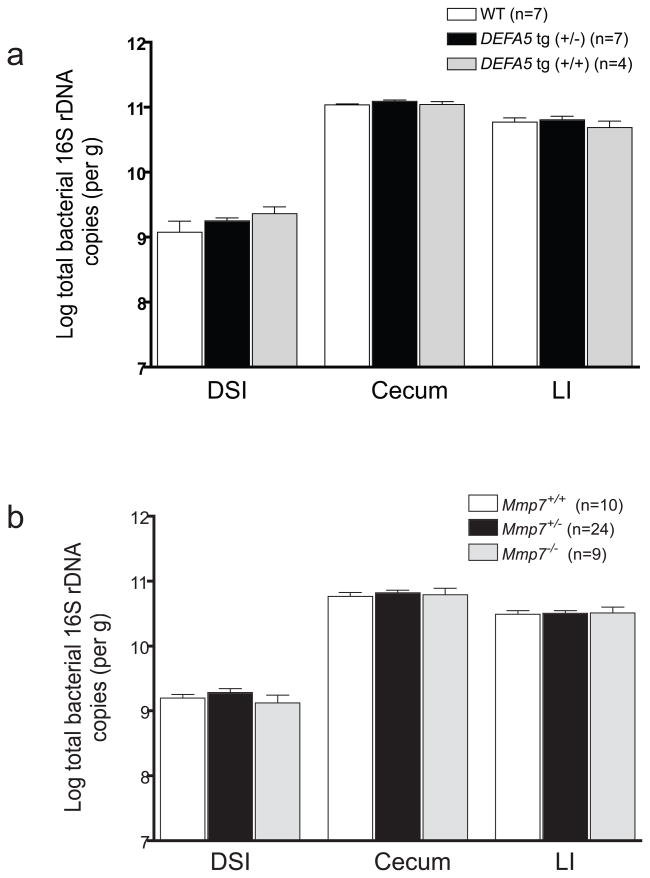
DEFA5 does not influence total bacteria numbers
Counter to our initial expectations, analysis of total bacterial numbers revealed equal amounts of bacteria in each mouse line at each anatomic site (Fig. 6a,b). Thus, Paneth cell α-defensins are important for regulating the composition of the microbiota, but not the absolute numbers of bacteria in the intestine. This is consistent with recent findings37 demonstrating that although Paneth cells were able to sense gut commensals via TLRs, Paneth cells were not involved in regulating total bacterial numbers in the gut. However, these data refute the commonly held assumption that Paneth cell α-defensins are responsible for the relative paucity of bacteria in the distal small intestine as compared to the cecum and large intestine.
Figure 6.
Quantitative comparison of total bacteria by intestinal segment. Log number of total copies of bacterial 16S rDNA were determined for distal small intestine (DSI), cecum, and large intestine (LI) of each mouse by qPCR, using universal bacterial primers, and quantified using a standard curve generated from Ruminococcus productus bacterial genomic DNA as a reference strain. Offspring from DEFA5 tg (+/−) (a) and Mmp7+/− (b) breeding pairs were analyzed.
Shift in Th17:Treg balance associated with DEFA5 expression and loss of SFB
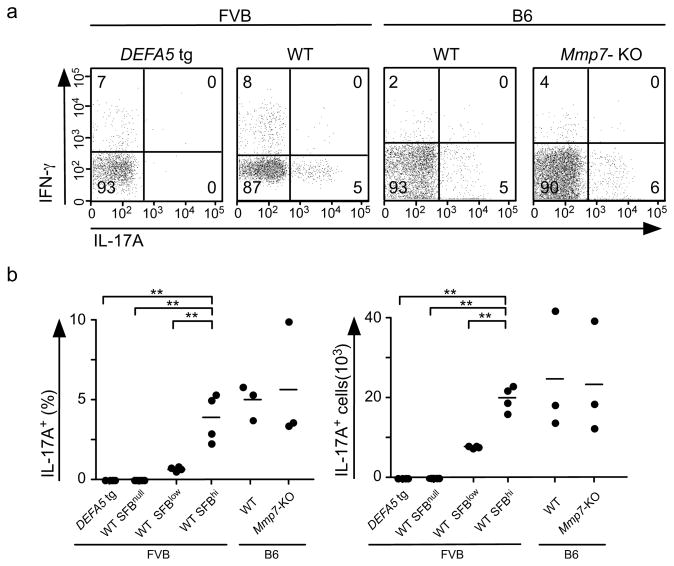
Recent work demonstrated that the composition of the microbiome can influence the differentiation of IL-17-producing T (TH17) cells in the small intestinal mucosa 38. To determine if Paneth cell α-defensins modulate mucosal T cell responses by regulating the composition of the microbiome, lamina propria lymphocytes (LPL) were isolated from the distal 15 cm of the small intestine of DEFA5 tg (+/+) and FVB wild-type mice. LPL were stimulated with PMA and ionomycin in vitro and intracellular expression of IL-17A and IFN-γ was analyzed by flow cytometry (Fig. 7A). CD4+ T cells from wild-type FVB but not DEFA5 tg (+/+) mice expressed IL17A. The fraction of CD4+ T cells expressing IFN-γ was similar in wild-type FVB but not in DEFA5 tg (+/+) mice. As the loss of SFB was the most striking finding in the DEFA5 tg (+/+) mice, we used the abundance of this bacterium as an indicator of α-defensin-dependent changes in microbiota and examined the impact of these microbial changes on TH17 cell frequency. We measured the abundance of SFB in the distal small intestine by qPCR in FVB wild-type mice, and determined the percent and number of TH17 cells in the lamina propria of these mice (Fig. 7B). Wild-type FVB mice with abundant SFB contained higher percentages and absolute numbers of CD4+ IL17A+ T cells than wild-type mice with little SFB (Fig. 7b,c). LPL from wild-type mice with undetectable SFB lacked CD4+ T cells expressing IL-17A, like DEFA5 tg (+/+) mice. Thus, the DEFA5-dependent composition of the microbiota as reflected by the SFB load is directly and significantly correlated with percent and number of CD4+ IL-17A+ T cells in the small intestine LP.
Figure 7.
TH17 profile of distal small intestinal LPL. (a) Representative flow cytometry analysis of IL-17A and IFN-γ expression in lamina propria CD4+ T cells from indicated mice after stimulation with PMA and ionomycin. (b) The percent and the number of CD4+ IL-17A+ T cells in LPL from distal small intestine of the indicated groups of mice. Each dot represents an individual mouse. ** P<0.001.
It is possible that SFB is directly responsible for the numbers of CD4+ IL-17A+ T cells in the LP, as other bacterial groups analyzed by qPCR in the wild-type mice did not show significant differences in abundance. However, we cannot exclude the possibility that other minor components of the biome influence CD4+ IL-17A+ T cell abundance. Similarly, we evaluated SFB and CD4+ IL-17A+ T cell abundance in litters of B6 Mmp7−/− and Mmp7+/+ mice. While the complete biome analysis by qPCR reflected the same shifts noted in previous experiments (Fig. 3d), B6 Mmp7−/− and Mmp7+/+ mice showed similar frequencies of SFB and CD4+ IL-17A+ T cell frequencies and numbers (Fig. 7b,c). It must be noted that these analyses were performed in unchallenged mice, and the impact of SFB on CD4+ IL-17A+ T cell numbers in infected or inflamed mice has yet to be determined. Together, these data are consistent with the conclusion that SFB are linked to the presence of Th17 cells in the LP.
DISCUSSION
The intestinal mucosa, whose epithelial surface consists of a single cell layer, serves as a critical interface between the host and a luminal environment that is teeming with bacteria. Many now-classic studies have established that intestinal microbes are vital to host physiology1. Several recent studies probed the impact of the intestinal bacteria on host gene expression 39, 40. Other recent investigations have demonstrated that hosts including mice 9, 37, drosophila 10, and zebrafish 8 are involved in establishing the composition of their intestinal microbiomes. Analyses of interactions between IgA and commensal bacteria have revealed a dynamic interaction between the microbiota and mucosal immune factors 13, 41, 42. Together, the emerging picture suggests that a dynamic and intimate interplay between host and microbiota facilitates mucosal homeostasis and normal host physiology 43. Here, we identify Paneth cell α-defensins, key effector molecules of intestinal innate immunity, as peptides that markedly alter the composition of the host commensal microbiota.
Previous studies by our group and others demonstrated that Paneth cell α-defensins have important effects on exogenous pathogenic bacteria in the intestinal lumen 17, 18. The current study is the first to directly demonstrate that alterations in the α-defensin constitution of Paneth cells result in distinct changes in the composition of the indigenous microbiota. The two genetic mouse models used in this study are strongly complementary, and have been integral to previous studies of in vivo Paneth cell α-defensin function 17, 18. In these models, the genetic manipulations that alter α-defensin expression do not affect expression of other Paneth cell effector molecules. However, it is possible that the absence of MMP7 in the small intestinal lumen may result in other veiled effects 20, 31, 32. Although we cannot rule out that some of these effects could contribute to the observations noted here, the presence of the DEFA5 transgene resulted in reciprocal effects as the absence of MMP7. In addition, although DEFA5 is not normally part of the mouse Paneth cell armamentarium 44, the DEFA5 transgene was expressed in physiologically relevant amounts for a mouse Paneth cell 18. This human α-defensin does not have a clear orthologue in mice and has an antimicrobial spectrum that is somewhat distinct from the murine crypt α-defensins 45. Nevertheless, the data generated using these two reciprocal mouse models strongly support the hypothesis that Paneth cell α-defensins have at least two key roles at the mucosal interface: protection from enteric pathogens and homeostatic control of the intestinal bacterial ecosystem.
This homeostatic role for Paneth cell α-defensins could have significant implications for the pathogenesis of inflammatory bowel disease. The chronic mucosal inflammation of Crohn’s disease is hypothesized to result from an inappropriate and ongoing activation of the immune system driven by the bacterial microbiota in genetically susceptible individuals 46. Several studies have characterized abnormalities in the colonizing microbiota in Crohn’s disease46, supporting the notion that an imbalance of the host-microbe homeostasis at the intestinal mucosa may underlie dysbiosis and disease pathogenesis. Several recent studies have focused attention on Paneth cells as having a primary role in IBD pathogenesis 21, 22 and intestinal homeostasis 37. Decreased amounts of Paneth cell α-defensins are associated with Crohn’s disease involving the ileum 23, and more significant decreases are seen in patients with a frameshift mutation in the intracellular receptor of muramyl dipeptide, nucleotide-binding oligomerization domain containing 2 (NOD2). Mutations in the gene encoding NOD2 underlie the genetic susceptibility to Crohn’s disease in approximately one third of patients, predominantly in those with disease of the ileum. Studies of NOD2-deficient mice have further supported the association between loss of NOD2 and decreased Paneth cell α-defensin expression 19.
Here we showed that alterations in Paneth cell α-defensin expression can have a significant impact on the bacterial composition of the microbiota, and that changes in the microbiota in response to DEFA5 expression are associated with changes in the numbers of IL-17A+ CD4+ T cells, consistent with a skewing of the mucosal immunologic response. Although SFB is likely the organism responsible for the skewing observed in the model system analyzed here, other members of the microbiome may drive similar responses in both mice and humans. Other studies demonstrated the role of the microbiota in shifting the TH17:regulatory T cell balance 38. Thus, loss of DEFA5, either specifically 23 or through global Paneth cell abnormalities21, 22, could skew mucosal responses towards a proinflammatory phenotype. In a unifying model linking these findings with ileal Crohn’s disease, a decrease in Paneth cell α-defensin expression could lead to alterations in the composition of the intestinal microbiota, resulting in or perpetuating the chronic intestinal inflammation associated with the disease.
METHODS
Animal experiments
FVB and B6 mice, used for breeding, were obtained from Taconic Laboratories. All experimental litters were bred and maintained under specific pathogen-free conditions in the Medical College of Wisconsin Biomedical Resource Center vivarium. The Institutional Animal Care and Use Committee at the Medical College of Wisconsin approved all animal experiments. Litters of DEFA5 tg (+/+) mice 18 on an FVB background were obtained by crossing DEFA5 tg (−/+) male and female parents. Litters of Mmp7 −/− knockout mice 17, on a B6 background, were obtained by crossing male and female Mmp7+/− parents. Mice were genotyped as described 17, 18. At weaning, offspring were separated and housed individually in cages, to prevent mouse-to-mouse caprophagy. Successive litters of individual breeding pairs were analyzed to control for maternal influence on the microbiota. Mice were euthanized at 5 weeks of age, and the intestinal tract removed for analysis.
Isolation of genomic DNA
The intestinal tract was excised from euthanized mice, and the distal small intestine (distal 15 cm of small intestine) was isolated, weighed and homogenized using a Polytron PT 10–35 homogenizer (Kinematica) in 2 ml sterile PBS. Genomic DNA was immediately isolated using the Qiagen Stool Kit according to the kit directions, using the optional high temperature step.
Subcloning, sequencing and phylogenetic analysis of bacterial 16S rDNA
For individual analyses of mouse small intestinal colonization, genomic DNA from each animal was sent to the Genome Center (Washington University, St. Louis). Methods for PCR amplication, subcloning, sequencing, sequence alignment, and construction of phylogenetic trees may be found in the Supplementary Methods. Aligned sequences from the paired genotypes were used to compare community difference by UNIFRAC (http://bmf2.colorado.edu/unifrac/index.psp) utilizing the P-test and UNIFRAC significance test. Assembled sequences were subsequently classified to genus level using the Ribosomal Database Project (RDP) classifier (version 9). The communities represented by each sample were analyzed further at the phylum level. The R-package software (version 2.8.1) was used to establish the clustering relationships and their associated heat maps based on Euclidean matrix for the different microbiome communities. The heatmaps were generated using the sequence data assigned by the RDP classifier. For each animal, the total number of sequences obtained for each individual phylum was dividing by the total number of sequences obtained for that animal, producing a percentage. The percentage was used as the matrix for generating the heatmap. The color key values, from red to green (0–100) represent the percentage (0–100%) of sequences in each phylum. Both weighted and unweighted UNIFRAC analyses were performed. To estimate microbial diversity, diversity indices, rarefaction curves, and OTU abundance were determined with DOTUR both at the phylum and class levels. The percentage of coverage in each genotype community was calculated by Good’s coverage: [1-(n/N)]*100, where n is the number of singletons predicted by DOTUR, and N is the total number of sequences.
Quantitative PCR for microbiota analysis
The abundance of specific intestinal bacterial groups was measured by qPCR using the MyiQ Single-Color Real-Time PCR Detection System (BioRad) using group specific 16S rDNA primers (Operon Technologies) (Supplementary Table 2) as previously described 30. Using genomic DNA from each sample, real time PCR reactions were completed using group specific primers to determine the amount of bacteria in each of the following major groups: Eubacterium rectal-Clostridium coccoides (Erec), Clostridium leptum (Clept), Lactobacillus sp. (Lact), Bacteroides sp. (Bact), Mouse Intestinal Bacteria (MIB), and Segmented Filamentous Bacteria (SFB). For complete methods see the Supplementary Methods section.
Fluorescence in situ hybridization
The composition of surface associated bacteria in the terminal 1.5 cm portion of the small intestine was analyzed by FISH using tissues prepared with Carnoy’s fixative (Ricca Chemical). Tissue was processed, paraffin embedded and sectioned at 3 um. FISH was done by the method of Swidsinski et al 36. As previously described for SFB 47 using a combination of 6Fam labeled SFB specific oligo (SFB1008 6Fam-GCGAGCTTCCCTCATTACAAGG, 29) and TR-labeled universal bacterial probe (Bact338 TR-GCTGCCTCCGTAGGAGT) (Operon Technologies). Slides were viewed using a Nikon E400 fluorescence microscope, equipped with Texas Red and FITC excitation-emission filter cubes. Images were captured using a Photometrics CoolSnap ES CCD camera (Photometrics) and analyzed using Metaview software (Universal Imaging Corporation, Molecular Devices).
Paneth cell effector expression
Tissue samples from the distal small intestine were isolated and homogenized in a guanidine thiocyanate buffer. RNA was isolated, quantified and reverse transcribed as previously described 48. Gene specific real-time PCR primers (Supplementary Table 3) were selected using MacVector software (MacVector, Inc.), and purchased from Invitrogen. Real-time PCR was performed on the tissue-specific cDNA as a template with specific oligonucleotide primer pairs as described previously 48. All samples were analyzed in duplicate, and variation between duplicates was < 10% for every reported value. For more complete methods, see Supplementary Methods section.
LPL isolation and analysis
The distal 15 cm of the small intestine was used as a source of LPL 49. Intraepithelial lymphocytes were removed by gently shaking 0.5 cm intestinal sections in buffer containing fetal calf serum (5%), dithiothreitol (1mM), and EDTA (5mM) for 30 minutes. Washed intestinal sections were digested with collagenase D (1 mg/ml), washed lymphocytes isolated on a Percoll gradient. Lymphocytes were counted and stimulated with PMA (10 ng/ml) and ionomycin (1 mM) in the presence of brefeldin A (BD Biosciences, 1 ml/ml) for 5 hours. Lymphocytes were stained for cell surface CD4 (RM4–5), intracellular IL-17A (TC11-4718H10.1) and intracellular IFN- γ (XMG1.2) in a buffer containing Brefeldin A as described 50. Flow cytometry data was collected on a BD™LSR II (BD Biosciences) and analyzed using FlowJo™ software.
Statistical analysis
Statistical analysis for microbiota qPCR studies was performed using a repeated measure model with unstructured covariance, testing for Genotype, Bacteria Type, and Genotype-by-Bacterial Type interaction. A 0.05 significance level was used for all tests. The analysis was performed using Proc Mixed in SAS version 9.1 (The SAS Institute). Comparisons of the percent Bacteroidetes and percent Firmicutes sequences from subcloning experiments were performed using Mann-Whitney tests. A 0.05 significance level was used for all tests.
Supplementary Material
Acknowledgments
We acknowledge the technical assistance of M. Hayward, D. Fish, P. Homolka, and A. Croswell. We acknowledge the Production Staff at The Genome Center for sequencing the 16S rRNA genes. We wish to acknowledge Jonathan A. Eisen and Amber L. Hartman for valuable discussions. This work was supported by grants from the National Institutes of Health AI057757 (N.H.S.) and AI/DK50843 and AI32738 (C.L.B.), the Crohn’s and Colitis Foundation of America (N.H.S.), and the Diabetes Foundation Netherlands (N.A.B). This work was begun while N.H.S. was at the Department of Microbiology, University of Pennsylvania, School of Medicine, Philadelphia, PA, USA.
Footnotes
Author contributions A collaboration between N.H.S. and C.L.B. resulted in the development of the DEFA5 transgenic mouse model and formulation of the underlying hypothesis. K.H. and N.H.S. developed the qPCR assays in collaboration with N.A.B., and M.S. K.H., P.T., M.H., and E.A. performed bacterial genomic isolations and qPCR microbiota assays. D.E. performed the statistical analysis of the data. Subcloning, sequencing, and clone analysis was performed by M.B., N.A.B., Y.Z., E.S., and G.M.W. D.H. and C.B.W. performed LPL isolation and analysis by flow cytometry. Paneth cell gene expression experiments were contributed by H.C, J.K.-S., and C.L.B. N.H.S. performed the fluorescence in situ hybridization studies and wrote the manuscript. N.H.S., K.H., E.A., C.L.B., and N.A.B. were responsible for interpretation of data. All authors discussed the results and commented on the manuscript.
Author Information The authors declare no competing financial interests.
References
- 1.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Reports. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stecher B, Hardt WD. The role of microbiota in infectious disease. Trends in Microbiology. 2008;16:107–114. doi: 10.1016/j.tim.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nature Reviews Immunology. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 4.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill SR, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckburg PB, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nature Reviews Microbiology. 2008;6:776–788. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawls JF, Mahowald MA, Ley RE, Gordon JI. Reciprocal Gut Microbiota Transplants from Zebrafish and Mice to Germ-free Recipients Reveal Host Habitat Selection. Cell. 2006;127:423–433. doi: 10.1016/j.cell.2006.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ley RE, et al. Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryu JH, et al. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008;319:777–782. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- 11.Nieuwenhuis EE, et al. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J Clin Invest. 2009 doi: 10.1172/JCI36509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrett WS, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. [see comment] Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki K, et al. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci USA. 2004;101:1981–1986. doi: 10.1073/pnas.0307317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 15.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nature Immunology. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 16.Porter EM, Bevins CL, Ghosh D, Ganz T. The multifaceted Paneth cell. Cell Mol Life Sci. 2002;59:156–170. doi: 10.1007/s00018-002-8412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson CL, et al. Regulation of intestinal α-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 18.Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi KS, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 20.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 21.Cadwell K, et al. A key role for autophagy and the autophagy gene Atg16L1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaser A, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. [see comment] Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wehkamp J, et al. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18129–18134. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salzman NH, et al. Analysis of 16S libraries of mouse gastrointestinal microflora reveals a large new group of mouse intestinal bacteria. Microbiology. 2002;148:3651–3660. doi: 10.1099/00221287-148-11-3651. [DOI] [PubMed] [Google Scholar]
- 25.Schloss PD, Handelsman J. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Applied & Environmental Microbiology. 2005;71:1501–1506. doi: 10.1128/AEM.71.3.1501-1506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 27.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 28.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-Induced Obesity is Linked to Marked but Reversible Alterations in the Mouse Distal Gut Microbiome. Cell Host & Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snel J, et al. Comparison of 16S rRNA Sequences of Segmented Filamentous Bacteria Isolated from Mice, Rats, and Chickens and Proposal of “Candidatus Arthromitus”. Int J Syst Bacteriol. 1995;45:780–782. doi: 10.1099/00207713-45-4-780. [DOI] [PubMed] [Google Scholar]
- 30.Barman M, et al. Enteric Salmonellosis disrupts the microbial ecology of the murine gasrointestinal tract. Infect Immun. 2008;76:907–915. doi: 10.1128/IAI.01432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. International Journal of Biochemistry & Cell Biology. 2008;40:1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swee M, Wilson CL, Wang Y, McGuire JK, Parks WC. Matrix metalloproteinase-7 (matrilysin) controls neutrophil egress by generating chemokine gradients. Journal of Leukocyte Biology. 2008;83:1404–1412. doi: 10.1189/jlb.0108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heczko U, Abe A, Finlay BB. Segmented Filamentous Bacteria Prevent Colonization of Enteropathogenic Escherichia coli 0103 in Rabbits. J Infect Dis. 2000;181:1027–1033. doi: 10.1086/315348. [DOI] [PubMed] [Google Scholar]
- 34.Klassen H, et al. Intestinal segmented filamentous bacteria in a wide range of vertebrate species. Lab Anim. 1993;27:141–150. doi: 10.1258/002367793780810441. [DOI] [PubMed] [Google Scholar]
- 35.Jiang HQ, Bos NA, Cebra JJ. Timing, Localization, and Persistence of Colonization by Segmented Filamentous Bacteria in the Neonatal Mouse Gut Depend on Immune Status of Mothers and Pups. Infect Immun. 2001;69:3611–3517. doi: 10.1128/IAI.69.6.3611-3617.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canny G, Swidsinski A, McCormick BA. Interactions of intestinal epithelial cells with bacteria and immune cells: methods to characterize microflora and functional consequences. Methods Mol Biol. 2006;341:17–35. doi: 10.1385/1-59745-113-4:17. [DOI] [PubMed] [Google Scholar]
- 37.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ivanov II, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host & Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: an new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4:269–273. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 40.Hooper LV, et al. Molecular Analysis of Commensal Host-Microbial Relationships in the Intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 41.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 42.Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host & Microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Salzman NH, Underwood MA, Bevins CL. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Seminars in Immunology. 2007;19:70–83. doi: 10.1016/j.smim.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Ouellette AJ, et al. Mouse Paneth cell defensins: primary structures and antibacterial activities of numerous cryptdin isoforms. Infect Immun. 1994;62:5040–5047. doi: 10.1128/iai.62.11.5040-5047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porter EM, van Dam E, Valore EV, Ganz T. Broad-spectrum antimicrobial activity of human intestinal defensin 5. Infect Immun. 1997;65:2396–2401. doi: 10.1128/iai.65.6.2396-2401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 47.Croswell A, Amir E, Teggatz P, Barman M, Salzman NH. Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric Salmonella infection. Infection & Immunity. 2009;77:2741–2753. doi: 10.1128/IAI.00006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wehkamp J, et al. Paneth cell antimicrobial peptides: topographical distribution and quantification in human gastrointestinal tissues. FEBS Letters. 2006;580:5344–5350. doi: 10.1016/j.febslet.2006.08.083. [DOI] [PubMed] [Google Scholar]
- 49.Weigmann B, et al. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nature Protocols. 2007;2:2307–2311. doi: 10.1038/nprot.2007.315. [DOI] [PubMed] [Google Scholar]
- 50.Haribhai D, Edwards B, Williams ML, Williams CB. Functional reprogramming of the primary immune response by T cell receptor antagonism. J Exp Med. 2004;200:1371–1382. doi: 10.1084/jem.20041226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.