Abstract
The current risk of infection in contemporary total knee arthroplasty (TKA) as well as the relative importance of risk factors remains under debate as a result of the rarity of the complication and temporal changes in the treatment and prevention of infection. We therefore determined infection incidence and risk factors after TKA in the Medicare population. The Medicare 5% national sample administrative data set was used to identify and longitudinally follow patients undergoing TKA for deep infections and revision surgery between 1997 and 2006. Cox regression was used to evaluate patient and hospital characteristics. In 69,663 patients undergoing elective TKA, 1400 TKA infections were identified. Infection incidence within 2 years was 1.55%. The incidence between 2 and up to 10 years was 0.46%. Women had a lower risk of infection than men. Comorbidities also increased TKA infection risk. Patients receiving public assistance for Medicare premiums were at increased risk for periprosthetic joint infection (PJI). Hospital factors did not predict an increased risk of infection. PJI occurs at a relatively high rate in Medicare patients with the greatest risk of PJI within the first 2 years after surgery; however, approximately one-fourth of all PJIs occur after 2 years.
Level of Evidence: Level II, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Periprosthetic joint infection (PJI) is a devastating complication of total joint arthroplasty [4]. Infection is currently the most frequently reported reason for revision in TKA [3]. Recent epidemiologic studies using the Nationwide Inpatient Sample suggest both the incidence and prevalence of PJI may be increasing over time in the United States [10, 11]. The reasons underlying these temporal trends in infection have not yet been fully elucidated.
Previous studies of infection risk have been single-institution reviews of retrospective clinical data starting as early as the 1970s [2, 16–19, 22, 23] or registry studies from Scandinavia [6, 7, 21] and thus may not be generalizable to the modern national population of joint arthroplasty recipients in the United States. A recent study related to antibiotic-related bone cement, drawn in part from the European registry experience, noted a lack of controlled clinical trials related to the treatment of infection [14]. Despite these limitations, observational studies conducted over the past three decades suggest multiple factors may be implicated in the infection risk for patients undergoing arthroplasty, including increased operative time, longer hospital stay, obesity, simultaneous bilateral joint arthroplasty, and diagnoses of rheumatoid arthritis, myocardial infarction, and atrial fibrillation, and the lack of use of certain infection countermeasures such as antibiotic bone cement [6, 14, 17, 18, 21, 23]. Thus, the current relative risk of infection in contemporary TKA as well as the relative importance of various risk factors remains under debate attributable both to the rarity of the complication, changes in the treatment and prevention of infection over time as well as the practical difficulties associated with conducting well-controlled clinical studies on this topic with long-term followup.
Administrative databases provide a mechanism for studying infection risk after joint arthroplasty at a national level. In the United States, where a joint arthroplasty registry has not yet been implemented, the Medicare claims data have been used effectively in longitudinal analysis of mortality, morbidity, and revision after joint arthroplasty [1, 11, 12]. Because of its comprehensive nationwide coverage, Medicare claims data have the potential to serve as a framework for longitudinal analysis of infections for the United States. Thus far, assessments of PJI risk using Medicare data have been limited to 3- to 6-month postoperative assessment and suggest procedure duration and volume may be important risk factors [8, 9, 12, 15].
We presumed PJI risk as well as revision risk after PJI diagnosis would be affected by procedure duration, patient factors, and hospital factors. We therefore (1) established the incidence of early-onset (less than 2 years) and late-onset (greater than 2 years) PJI in the Medicare population with up to 10 years followup; and (2) identified predisposing risk factors (age, race, gender, Medicare buy-in status, Census region, procedure duration, hospital location, hospital teaching status, hospital bed size, hospital ownership, and Charlson comorbidity scores) for PJI.
Patients and Materials
We used the Medicare 5% national sample administrative claims database to identify patients undergoing primary TKA between January 1, 1997, and December 31, 2006. Each year, this data set includes the healthcare records in inpatient, outpatient, Part B carrier, skilled nursing facility, hospice care, home health, and durable medical equipment analytic data files. To limit the study to the elective cohort, we excluded patients undergoing primary TKA who received their implant as a result of bone cancer, joint infection, or fracture using criteria identical to those used previously by Katz, Mahomed, and coworkers [9, 12]. Patients younger than 65 years old or health maintenance organization (HMO) enrollees were also excluded. An overall cohort of 82,362 patients undergoing primary TKA was initially identified between 1997 and 2006, from which 69,663 elective patients were included in our study. From this elective patient cohort, 1400 TKA infections were identified.
During the period that a patient is enrolled in Medicare, all the claims associated with the patient are collected in the database. Because each enrollee is assigned a unique, encrypted Medicare beneficiary identifier, this was used to follow each patient longitudinally by compiling all their claims throughout the 10-year study period. Patients’ Medicare entitlement status and mortality were tracked using a linked “denominator” file provided by the CMS that accompanied the analytic data sets. International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM: 81.54) and Current Procedural Terminology, 4th Edition (CPT-4: 27447) procedure codes were used to identify patients undergoing primary TKA.
PJI was identified with the ICD-9-CM diagnosis code 996.66 in any component of the Medicare data set. We also performed a post hoc analysis to determine the clinical setting (ie, inpatient versus outpatient care) in which the deep infection was diagnosed. Patients diagnosed with a PJI were classified as early onset if it appeared within eight quarters (2 years) of the primary procedure. Late infection was classified if it was diagnosed for the first time more than 2 years after implantation.
Kaplan-Meier survivorship curves were compiled with PJI as an end point, adjusted for comorbidities using the Charlson index, and censored appropriately for patient mortality during the study period. The Charlson comorbidity index was based on the implementation for administrative data sets recommended by Deyo and coworkers [5]. Based on the diagnosis and surgeries indicated from the claims records, a “weight” with values of 1, 2, 3, or 6 is assigned to each disease category and the final index is a composite value representing the overall degree of comorbidity. For our analysis, the scores were grouped into: 0 (none), 1 to 2 (low), 3 to 4 (moderate), and 5 or greater (high). Anesthesia time from the claims data was used as a proxy for procedure duration with categories of less than 120, 120 to 150, 150 to 180, 180 to 210, and 210 or greater minutes [20]. Cox regression was used to evaluate the effects of patient factors (age, race, gender, Medicare buy-in status), Census region, procedure duration, and hospital characteristics (urban versus rural location, teaching status, bed size, ownership) on the relative risk of PJI. The relative risks were computed by adjusting for these covariates. The Medicare buy-in status was an identifier of patients whose Medicare premiums and deductibles were subsidized by the state as a result of their financial status and was used as a proxy for the patient’s socioeconomic status. The classifications of the hospital characteristics were based on those denoted by CMS in the claims files. Statistical analyses were performed using SAS (Cary, NC).
Results
Of the 69,663 patients, 1080 and 320 patients were diagnosed with infection within 2 years and between 2 and up to 10 years, respectively, corresponding to an infection incidence (cumulative) within 2 years of 1.55% and between 2 and up to 10 years of 0.46%. The remaining 68,263 (98.0%) were not diagnosed with infection during the study period.
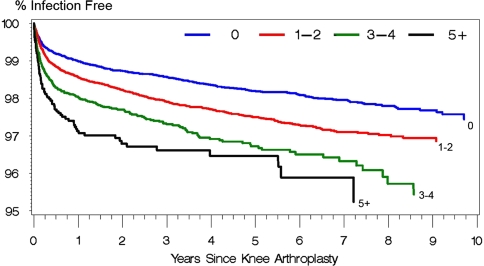
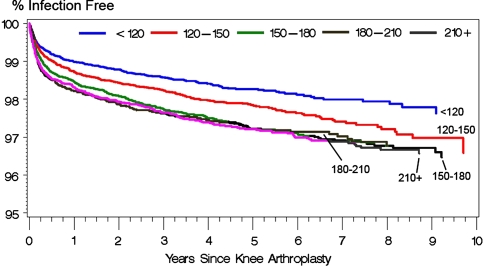
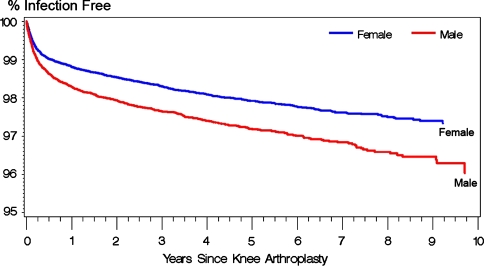
The presence of preexisting comorbidities, in terms of a nonzero Charlson index score, increased (p < 0.0001) the risk of PJI (adjusted hazard ratio for Charlson index of 5+ as compared with 0 = 2.16) (Table 1; Fig. 1). Longer duration procedures were at greater (p < 0.0001) risk of PJI (adjusted hazard ratio for 210+ minutes as compared with less than 120 minutes = 1.59) (Fig. 2). Patients receiving public assistance for Medicare premiums were at increased (p = 0.006) risk for PJI (adjusted hazard ratio: 1.27). Women had a lower (p = 0.077) risk of PJI compared with men (adjusted hazard ratio: 0.76) (Fig. 3). Hospital factors (location, teaching status, ownership, bed size) were not risk factors for infection.
Table 1.
Risk factors (p values) for infection as an end point (multivariate analysis)
| Factor | p Value |
|---|---|
| Age | 0.077 |
| Charlson index | < 0.0001 |
| Race | 0.943 |
| Gender | < 0.0001 |
| Census region | 0.372 |
| Ownership | 0.707 |
| Bed size | 0.573 |
| Medicare buy-in | 0.006 |
| Teaching status | 0.106 |
| Location | 0.469 |
| Procedure duration | < 0.0001 |
Fig. 1.
The effect of Charlson index on Kaplan-Meier survivorship after primary knee arthroplasty using diagnosis of a deep infection (996.66) as an end point.
Fig. 2.
The effect of procedure duration on Kaplan-Meier survivorship after primary knee arthroplasty using diagnosis of a deep infection (996.66) as an end point.
Fig. 3.
The effect of patient gender on Kaplan-Meier survivorship after primary knee arthroplasty using diagnosis of a deep infection (996.66) as an end point.
Discussion
Infection is currently the most frequently reported reason for revision in TKA [3]. However, the current risk of infection as well as the relative importance of various risk factors remains under debate as a result of the rarity of the complication, changes in the treatment and prevention of infection over time as well as the practical difficulties associated with conducting well-controlled clinical studies on this topic with long-term followup. Hence, we sought to establish the incidence of early-onset (less than 2 years) and late-onset (greater than 2 years) PJI in the Medicare population with up to 10 years followup. We also asked whether there were predisposing risk factors for PJI after primary TKA.
Our study is first limited by the use of an administrative database, which lacks clinical outcome measures such as the patient’s physical function status. We were also unable to investigate procedural or implant information such as the type of surgical approach or use of bone cement as a result of the administrative nature of the data. Second, the identification of PJI for each patient was based on the recording of the 996.66 ICD-9-CM diagnosis code in the claims records. The degree of miscoding is unclear, however, because the majority of these were diagnosed while the patient was hospitalized (66%) or by an orthopaedic surgeon or infection specialist (63%); thus, the incidence is likely reflective of true deep infections. Third, the findings were based on the elderly patient population; it is unclear if our findings extend to patients younger than 65 years old. Fourth, we were unable to identify the specific microorganism(s) implicated in the infection. Despite these limitations, our data suggest that although the incidence of PJI is the greatest within the first 2 years postoperatively, PJI may still present itself at up to 10 years followup.
The risk of PJI was the greatest within the first 2 years after TKA surgery; however, late presentation of PJI accounted for one-fourth of the cases in the study period. The majority of deep infections (approximately two-thirds) were diagnosed while the patient was hospitalized and most were diagnosed by an orthopaedic surgeon or infection specialists. This time-dependent risk of infection may help explain the variability of infection rates reported in the literature. When we limited our followup to 90 days or less, the risk of PJI was comparable to what was previously reported by Katz et al. [8]. In their study of the Medicare population from the first 9 months of 2000, Katz et al. [8] reported an overall rate of 0.4% with infection in the first 90 days after primary TKA. In a prospective analysis of 4185 patients undergoing primary TKA, Pulido et al. [17] reported an overall incidence of PJI of 1.1% with an average time to diagnosis of 1.2 years.
This study in a large cohort of patients with up to 10 years followup demonstrates deep prosthetic infection occurs at a relatively high rate in Medicare patients in a large national sample. Patient factors, especially degree of comorbidities and public assistance (eg, receiving public assistance for Medicare premium), were strong risk factors associated with deep infection. Procedure duration, a well-known risk factor of infection, was also confirmed by recent analysis of the Medicare data [13]. Longer operative time was also a predisposing factor for PJI from a cohort of 9245 patients undergoing total joint arthroplasty (4185 knees and 5060 hips) in an unadjusted univariate analysis [17].
In light of our longitudinal analysis of the incidence of PJI in the Medicare patient population, along with other followup studies [17] and cross-sectional analysis of national hospitalization records [3], emphasis should be placed on minimizing the risk of PJI after primary TKA. Because PJI is a challenging complication for patients, hospitals, surgeons, and payers [4], effective strategies such as stringent patient screening for risk factors or adequate antibiotic prophylaxis [17] should be developed and implemented to combat this complication.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
This work was performed at Exponent, Inc, Philadelphia, PA, USA.
References
- 1.Barrett J, Losina E, Baron JA, Mahomed NN, Wright J, Katz JN. Survival following total hip replacement. J Bone Joint Surg Am. 2005;87:1965–1971. doi: 10.2106/JBJS.D.02440. [DOI] [PubMed] [Google Scholar]
- 2.Blom AW, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total hip arthroplasty. The Avon experience. J Bone Joint Surg Br. 2003;85:956–959. doi: 10.1302/0301-620X.85B7.14095. [DOI] [PubMed] [Google Scholar]
- 3.Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2009 Jun 25 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 4.Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am. 2005;87:1746–1751. doi: 10.2106/JBJS.D.02937. [DOI] [PubMed] [Google Scholar]
- 5.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 6.Engesaeter LB, Espehaug B, Lie SA, Furnes O, Havelin LI. Does cement increase the risk of infection in primary total hip arthroplasty? Revision rates in 56, 275 cemented and uncemented primary THAs followed for 0–16 years in the Norwegian Arthroplasty Register. Acta Orthop. 2006;77:351–358. doi: 10.1080/17453670610046253. [DOI] [PubMed] [Google Scholar]
- 7.Espehaug B, Engesaeter LB, Vollset SE, Havelin LI, Langeland N. Antibiotic prophylaxis in total hip arthroplasty. Review of 10, 905 primary cemented total hip replacements reported to the Norwegian arthroplasty register, 1987 to 1995. J Bone Joint Surg Br. 1997;79:590–595. doi: 10.1302/0301-620X.79B4.7420. [DOI] [PubMed] [Google Scholar]
- 8.Katz JN, Barrett J, Mahomed NN, Baron JA, Wright RJ, Losina E. Association between hospital and surgeon procedure volume and the outcomes of total knee replacement. J Bone Joint Surg Am. 2004;86:1909–1916. doi: 10.1302/0301-620X.86B7.14358. [DOI] [PubMed] [Google Scholar]
- 9.Katz JN, Losina E, Barrett J, Phillips CB, Mahomed NN, Lew RA, Guadagnoli E, Harris WH, Poss R, Baron JA. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2001;83:1622–1629. doi: 10.1302/0301-620X.83B3.10487. [DOI] [PubMed] [Google Scholar]
- 10.Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984–991. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Kurtz SM, Ong KL, Schmier J, Mowat F, Saleh K, Dybvik E, Karrholm J, Garellick G, Havelin LI, Furnes O, Malchau H, Lau E. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(Suppl 3):144–151. doi: 10.2106/JBJS.G.00587. [DOI] [PubMed] [Google Scholar]
- 12.Mahomed NN, Barrett JA, Katz JN, Phillips CB, Losina E, Lew RA, Guadagnoli E, Harris WH, Poss R, Baron JA. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85:27–32. doi: 10.2106/00004623-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Ong KL, Lau E, Manley M, Kurtz SM. Effect of procedure duration on total hip arthroplasty and total knee arthroplasty survivorship in the United States Medicare population. J Arthroplasty. 2008;23:127–132. doi: 10.1016/j.arth.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop. 2008;79:335–341. doi: 10.1080/17453670710015229. [DOI] [PubMed] [Google Scholar]
- 15.Phillips CB, Barrett JA, Losina E, Mahomed NN, Lingard EA, Guadagnoli E, Baron JA, Harris WH, Poss R, Katz JN. Incidence rates of dislocation, pulmonary embolism, and deep infection during the first six months after elective total hip replacement. J Bone Joint Surg Am. 2003;85:20–26. doi: 10.1302/0301-620X.85B3.13201. [DOI] [PubMed] [Google Scholar]
- 16.Poss R, Thornhill TS, Ewald FC, Thomas WH, Batte NJ, Sledge CB. Factors influencing the incidence and outcome of infection following total joint arthroplasty. Clin Orthop Relat Res. 1984;182:117–126. [PubMed] [Google Scholar]
- 17.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87:844–850. doi: 10.1302/0301-620X.87B6.15121. [DOI] [PubMed] [Google Scholar]
- 19.Saleh K, Olson M, Resig S, Bershadsky B, Kuskowski M, Gioe T, Robinson H, Schmidt R, McElfresh E. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20:506–515. doi: 10.1016/S0736-0266(01)00153-X. [DOI] [PubMed] [Google Scholar]
- 20.Silber JH, Rosenbaum PR, Zhang X, Even-Shoshan O. Estimating anesthesia and surgical procedure times from Medicare anesthesia claims. Anesthesiology. 2007;106:346–355. doi: 10.1097/00000542-200702000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Smabrekke A, Espehaug B, Havelin LI, Furnes O. Operating time and survival of primary total hip replacements: an analysis of 31, 745 primary cemented and uncemented total hip replacements from local hospitals reported to the Norwegian Arthroplasty Register 1987–2001. Acta Orthop Scand. 2004;75:524–532. doi: 10.1080/00016470410001376. [DOI] [PubMed] [Google Scholar]
- 22.Surin VV, Sundholm K, Backman L. Infection after total hip replacement. With special reference to a discharge from the wound. J Bone Joint Surg Br. 1983;65:412–418. doi: 10.1302/0301-620X.65B4.6874711. [DOI] [PubMed] [Google Scholar]
- 23.Wilson MG, Kelley K, Thornhill TS. Infection as a complication of total knee-replacement arthroplasty. Risk factors and treatment in sixty-seven cases. J Bone Joint Surg Am. 1990;72:878–883. [PubMed] [Google Scholar]