Abstract
Patients with medical comorbidities that necessitate chronic anticoagulation therapy frequently present as candidates for total knee arthroplasty (TKA). We asked whether it was necessary to stop warfarin preoperatively to avoid postoperative bleeding complications. We retrospectively reviewed 77 preoperatively anticoagulated patients undergoing TKA. Thirty-eight of these 77 patients were maintained on their routine therapeutic warfarin regimen throughout the perioperative period. The remaining 39 patients had their routine preoperative warfarin regimen discontinued preoperatively and then restarted after surgery. We compared rates of comorbid illness, blood transfusions, wound complications, and reoperations. The demographic data and the ratio of primary to revision arthroplasties were similar in the two groups. The age-adjusted risk ratios for blood transfusions, wound complications, and reoperations were 0.61, 0.29, and 0.43, respectively. The data presented suggest maintaining a therapeutic warfarin regimen throughout the perioperative period for high-risk patients is not associated with an increase risk of complications after TKA.
Level of Evidence: Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
In 2005, there were over 511,000 TKAs performed in the United States. It has been estimated that by 2030, this number will exponentially rise to 3.48 million annually. This represents a 673% increase [17, 18]. With this projected increase in number of TKAs performed, it is expected there will be an associated increase in patients presenting as candidates for arthroplasty who require chronic anticoagulation secondary to medical comorbidities. The reasons for this chiefly include a history of previous deep venous thrombosis (DVT), pulmonary embolism (PE), atrial fibrillation, artificial heart valve, thrombophilia, and cardiac bypass surgery or stent procedures.
Consensus guidelines for the perioperative management of these high-risk patients recommend discontinuance of warfarin preoperatively, bridging with low-molecular-weight heparin while the prothrombin time normalizes, then resuming therapy after surgery [12]. A concern with this practice has been our clinical observation of increased perioperative blood loss and also hematomas, which may or may not be related to impaired wound healing and the resulting additional surgery to drain major hematomas. In addition, after stopping warfarin perioperatively, we have noted large fluctuations in the international normalized ratio (INR) while trying to regain the therapeutic level, which may pose a separate set of risks.
Juxtaposed is the concern regarding morbidity after TKA secondary to DVT and PE. For patients receiving no thromboprophylaxis after major orthopaedic surgery, the rate of venographically detectable DVT has been documented at 40% to 60% [10, 14]. Although thromboprophylaxis is now the standard of care in the setting of arthroplasty, symptomatic PE is still reported at 1.3% to 10% during the first 3 months after surgery [2, 6, 8, 11, 14, 19, 20, 26, 27, 35, 36, 38]. Fatal PE has been reported at 0.09–0.15% [20, 26, 27].
We therefore asked whether there were any differences in blood transfusions, wound complications, and reoperations during the postoperative period between patients who continued their established therapeutic dosage of warfarin throughout the perioperative period and those who stopped their warfarin preoperatively and then restarted it again after surgery.
Patients and Methods
We identified preoperatively 83 anticoagulated patients (86 knees) who had a TKA between 2000 and 2008 (when the switch was made to electronic medical records and at the beginning of the anticoagulation service at our institution). The anticoagulation service database was queried for Current Procedural Terminology codes of TKA and revision TKA, which was then cross-referenced for International Classification of Diseases, 9th Revision (ICD-9) codes of DVT, PE, atrial fibrillation, heart valve, thrombophilia, cerebrovascular accident, and congestive heart failure. Using ICD-9 codes to search the database allowed us to ascertain why the patients in this study were on warfarin. Nine patients (9 knees) from this initial cohort were excluded from further analysis because of variance regarding intraoperative management with respect to tourniquet letdown around the time of wound closure and drain usage. The remaining 74 patients who underwent TKA and were also on warfarin for an unrelated medical comorbidity were included in this data analysis. Thirty-seven patients (38 knees) were maintained on their warfarin through surgery and 39 patients (39 knees) had their warfarin withheld. The anticoagulation service queried patients as to whether or not their warfarin was held preoperatively. For uniformity of comparison, we established an INR of greater than or equal to 1.5 on the day of surgery as verification that warfarin therapy was continued. In the continued warfarin cohort, there was no preoperative adjustment of patients’ warfarin regimen. We had prior Institutional Review Board approval.
All surgeries were performed through a standard medial parapatellar approach under tourniquet control. The tourniquet was released and hemostasis obtained before wound closure in all cases and the drain was removed on postoperative day one. All patients wore venous foot pumps bilaterally, TED hose on the nonoperative leg, and participated in the standard physical therapy protocol for knee reconstruction. For the discontinued warfarin cohort, warfarin was stopped 1 week before surgery and they were bridged with low-molecular-weight heparin (LMWH) through the evening before the procedure. This was then resumed on the night of surgery along with their regular dose of warfarin. The INR was checked preoperatively and each day postoperatively until entering the therapeutic range. LMWH was discontinued when the INR was above 1.5.
Once these two groups were established, key demographic data and the primary end points for comparison were collected. The protocol on the adult reconstruction service at the authors’ institution is for the patient to receive a red blood cell transfusion if the hematocrit falls below 27% and is accompanied by changes in vital signs or urine output. To ensure completeness of data collection, the entire 90-day global period was reviewed for each medical record regardless of outcomes identified. Importantly, there was a risk associated with the use of general anesthetic that these patients were subjected to because they could not receive a spinal anesthetic. This was addressed preoperatively with each patient during the informed consent period. The senior authors (AAH, HKD) believed the risk of general anesthetic was justified when considering the thrombotic risk posed to these high-risk patients. We thus avoided spinal anesthesia in both groups thereby mitigating the potential complication of epidural hemorrhage.
For categorical patient characteristic variables, we compared the study arms with the chi square test or Fisher’s exact test, as appropriate. For categorical variables with many categories, the Fisher-Freeman-Halton test was used, which is the Fisher’s exact test extended to greater than 2 × 2 crosstabulation tables [7]. For continuous variables, we compared the study arms using the independent samples t test. For change in INR from preoperatively to postoperatively, the groups were compared postoperatively while controlling for preoperatively in an analysis of covariance fashion. We recorded gender, age, procedure, diagnosis necessitating warfarin therapy, and pre- and postoperative INR levels (Table 1).
Table 1.
Patient characteristics
| Patient characteristics | Coumadin continued (n = 38) | Coumadin discontinued preoperatively (n = 39) | p Value |
|---|---|---|---|
| Male, number (%) | 18 (47) | 15 (38) | 0.43 |
| Age, mean ± SD | 73 ± 13 | 65 ± 12 | 0.004 |
| Minimum–maximum | 39–96 | 33–87 | |
| Procedure, number (%) | 0.68 | ||
| TKA | 27 (71) | 26 (67) | |
| Revision TKA | 11 (29) | 13 (33) | |
| Diagnosis for Coumadin, number (%) | < 0.001 | ||
| Congestive heart failure | 1 (3) | — | |
| Cerebrovascular accident | 1 (3) | — | |
| Deep vein thrombosis | 1 (3) | — | |
| Pulmonary embolus | 6 (16) | 2 (5) | |
| Pulmonary embolus/atrial fibrillation | 1 (3) | — | |
| Pulmonary embolus/arrhythmia | 1 (3) | — | |
| Systemic lupus erythematosus | 1 (3) | — | |
| Atrial fibrillation | 15 (39) | 11 (28) | |
| Atrial fibrillation/pulmonary embolus | 1 (3) | — | |
| Arrhythmia | 2 (5) | — | |
| Artificial valve | 4 (11) | 2 (5) | |
| Pacemaker | 1 (3) | — | |
| Recent hip surgery | — | 1 (3) | |
| Recent knee surgery | — | 5 (13) | |
| Thrombophilia | 1 (3) | — | |
| None | — | 11 (28) | |
| Unknown | 1 (3) | — | |
| Preoperative international normalized ratio | < 0.001 | ||
| Mean ± SD | 2.1 ± 0.6 | 1.2 ± 0.1 | |
| Postoperative international normalized ratio | < 0.001 | ||
| Mean ± SD | 3.3 ± 1.5 | 2.0 ± 0.8 | |
| Change in international normalized ratio from pre- to postoperatively | 0.16 | ||
| Mean ± SD | 1.2 ± 1.5 | 0.9 ± 0.8 | |
| Median (IQR)* | 0.8 (0.4–1.6) | 0.6 (0.4–1.2) | |
* IQR = interquartile range (25th–75th percentiles); SD = standard deviation.
Three patients in the study required reoperation. To assess if this number of repeated measurements required mixed effect models to account for lack of independence in the observations, mixed effect models were fitted to the outcome variables, which were complications and transfusions. The intraclass correlation coefficients were zero, suggesting there was no need for a mixed effects modeling approach, because all 77 surgery outcomes were sufficiently independent. Therefore, we used standard statistical tests and models for all comparisons.
To model the complication outcome, all complications were combined into a binary variable (1 = any complication occurred, 0 = no complication occurred). The severity of the complications was not accounted for with this approach, so the approach is conservative. That is, any other approach would make continuing warfarin appear even more protective against complications, so no bias was introduced in favor of the study hypothesis by taking this binary approach. To compare the outcomes of presence of a complication (binary) and need for blood transfusion (binary) between the two study groups, an exact Poisson regression model was fitted controlling for age at surgery as a covariate. The modified Poisson regression approach permits the direct calculation of a risk ratio in followup studies and so is preferred to logistic regression, which estimates odds ratios. To compare the outcome of the number of units of blood transfused, we again used exact Poisson regression. In this form of the Poisson model, the number of rare events, or units transfused, was modeled, and the risk ratio represented the ratio of the mean number of units required for each study group. Because most patients required zero units, linear regression was not appropriate, because the data were too nonnormally distributed, even if transformation was applied. The exact version of Poisson regression is not affected by overfitting, where unreliable associations can be introduced from having too many predictors for the number of outcomes. These models were appropriate, then, even with the small number of outcome events observed and the inclusion of age as a covariate.
Results
The mean age at time of surgery was older (p = 0.004) in the group continued on warfarin than the discontinued warfarin group (mean ± SD, 73 ± 13 and 65 ± 12; Table 1), so age was controlled for in the regression models. The warfarin-continued group required a transfusion less often than the warfarin discontinued preoperatively group while controlling for age at the time of surgery (adjusted risk ratio, 0.61; 95% confidence interval, 0.02–1.86; Table 2). As well, the warfarin-continued group required fewer transfusion units than the warfarin-discontinued preoperatively group while controlling for age at the time of surgery (adjusted ratio of mean number of units, 0.50; 95% confidence interval, 0.23–1.06; Table 2). Similarly, the warfarin-continued group experienced a wound complication less often than the warfarin-discontinued preoperatively group while controlling for age at the time of surgery (adjusted risk ratio, 0.29; 95% confidence interval, 0.01–4.26; Table 2). All wound complications occurred in separate patients.
Table 2.
Study outcomes
| Study outcomes | Coumadin continued (N = 38) | Coumadin discontinued (N = 39) | Unadjusted risk ratio* (95% confidence interval) | Age-adjusted risk ratio† (95% confidence interval) |
|---|---|---|---|---|
| Additional procedure type, number (%) | ||||
| I & D | 1 (3) | 1 (3) | ||
| PRS flap | — | 1 (3) | ||
| Additional procedure required, number (%) | ||||
| Yes | 1 (3) | 2 (5) | 0.51 | 0.43 |
| No | 37 (97) | 37 (95) | (0.01–9.86) | (0.01–9.18) |
| Complication type, number (%) | ||||
| Dehiscence | — | 1 (3) | ||
| Hematoma | 1 (3) | 1 (3) | ||
| Infected open wound | — | 1 (3) | ||
| None | 37 (97) | 36 (92) | ||
| Complication occurred, number (%) | ||||
| Yes | 1 (3) | 3 (8) | 0.34 | 0.29 |
| No | 37 (97) | 36 (92) | (0.01–4.26) | (0.01–4.26) |
| Transfusion (units), number (%) | ||||
| 0 | 30 (79) | 29 (74) | ||
| 1 | 1 (3) | 2 (5) | ||
| 2 | 7 (18) | 6 (15) | ||
| 4 | — | 2 (5) | ||
| Transfusion required, number (%) | ||||
| Yes | 8 (21) | 10 (26) | 0.82 | 0.61 |
| No | 30 (79) | 29 (74) | (0.28–2.31) | (0.20–1.86) |
| Transfusion (units) | ||||
| Mean ± SD | 0.4 ± 0.8 | 0.6 ± 1.1 | 0.70 | 0.50 |
| Median (IQR) | 0 (0–0) | 0 (0–1) | (0.34–1.41) | (0.23–1.06) |
* Risk ratio is warfarin continued postoperative proportion, or mean, over warfarin preoperative only proportion, or mean, from an exact Poisson regression model; †risk ratio is from an exact Poisson regression model that includes age (continuous variable) as a covariate; I & D = irrigation and debridement; PRS = plastic and reconstructive surgery; SD = standard deviation; IQR = interquartile range.
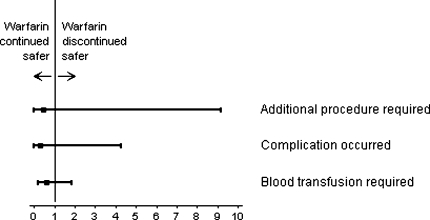
After controlling for age at the time of surgery, the patients in the two groups required similar numbers of additional procedures: one of 38 (3%) in the group with warfarin continued and two of 39 (5%) in the group with warfarin discontinued (adjusted risk ratio, 0.43; 95% confidence interval, 0.01–9.18; Table 2). Although not statistically significant, for each of the three outcome variables, the trends were in favor of a reduced risk for the cohort continued on their regular dose of warfarin through the perioperative period (Fig. 1).
Fig. 1.
Age-adjusted relative risk ratios and two-sided 95% confidence intervals from the Poisson regression models for the three study outcomes of reoperations, wound complications and blood transfusions in patients either continued or discontinued on their preoperative warfarin dose are shown.
Discussion
Patients presenting for TKA frequently have comorbid illnesses requiring chronic anticoagulation therapy. The authors of this investigation endeavored to determine if maintaining these patients on their regular therapeutic warfarin regimen throughout the perioperative period led to increased complications in the first 90 days after surgery. We obtained our results by comparing the aforementioned cohort with a group that had their therapy discontinued preoperatively and then restarted after surgery. Outcome measures were blood transfusions, wound complications, and reoperations.
We acknowledge some limitations to our study. First, it is a retrospective study that is primarily observational: inaccurate charting in the medical record or observer bias could potentially confound the results. On the other hand, the ability to retrieve complete data sets from the electronic record allowed accurate comparison of the cohorts with respect to the outcome variables. Second, patients in the discontinued warfarin preoperatively cohort received bridge therapy with LMWH. This confounding variable could potentially account for some of the complications seen in that group. However, our objective was to compare a previously unreported strategy for managing warfarin therapy in the perioperative period of TKA against the consensus guidelines for high-risk patients. Second, we did not account for severity of the complications and rather treated them as binary variables during the data analysis. While this is a conservative approach, it may make meaningful determination of the implications for these complications more difficult. Third, interpretation of the study outcomes from our data could possibly be of concern as the study may be underpowered. Maintaining high-risk patients on warfarin throughout the perioperative period of TKA has been the practice in this community for over twenty-five years. We unfortunately only had data on these patients for the last eight years coinciding with the database created by our anticoagulation service. Our inability to analyze a larger number of patients does not diminish our long-term and continued view that this is a safe approach when caring for patients with this risk profile. Finally, we acknowledge concern over subjecting the patients in this study to a general anesthetic. The decision to continue a patient on their therapeutic warfarin regimen throughout the perioperative period of TKA forces the use of general anesthesia because of the risk of epidural hematoma with spinal anesthesia. While some early studies demonstrated an increase in mortality and VTE in patients receiving general as opposed to spinal or regional anesthesia, recent large randomized controlled trials do not support this decrease in mortality or VTE events [4, 24, 25, 28]. This is thought to be due to improvements in perioperative care and the use of more effective thromboprophylactic strategies. So while the necessity of avoiding spinal or regional anesthesia in these patients is real, the risk of general anesthesia appears overstated.
We found no difference in postoperative blood transfusions between these two high-risk cohorts. Sutherland and Schurman reported on a series of 47 patients considered “high risk” for the development of thromboembolic events [32]. In their study, they gave a 10-mg dose of warfarin the night before surgery and no warfarin the day of surgery. Further dosing was titrated to an INR target value of 1.5 [32]. They reported a 4% postoperative blood transfusion rate (two of 47 patients). This study is cited because it is the only identifiable study looking at warfarin therapy before TKA.
Warfarin selectively inhibits vitamin K-dependent Factors II, VII, IX, and X as well as Protein C and S. Unfortunately, its action on the blood clot-preventing Protein C and S has a prothrombotic systemic effect, and this usually occurs before inhibition of the vitamin K-dependent clotting factors [31, 37]. The relevance to the current study question is clinically important. Interruption of anticoagulation in patients on chronic warfarin therapy poses a major risk of morbidity and mortality [5, 15, 29]. One study suggests there is a greater risk of DVT or PE when oral anticoagulation is manipulated compared with the background risk without anticoagulation [3]. In addition to the loss of DVT and PE protection caused by perioperative manipulation of the anticoagulation regimen the patient is normally on, restoring the patient to their preoperative steady-state INR is associated with added costs [34].
Similarly, we found no difference in the occurrence of postoperative wound complications for the two groups in this study. In a retrospective review of patients undergoing open inguinal herniorrhaphy whose medical conditions required chronic warfarin therapy, McLemore et al. concluded continuation of warfarin therapy perioperatively may be a safe alternative to discontinuation of warfarin [21]. In fact, McLemore et al. found no major difference in postoperative complications between the patients who were continued on warfarin versus those who were temporarily stopped [21]. The only statistically significant finding was there was a longer hospital stay and, thus, added cost in the discontinued-warfarin with bridge therapy group [21].
We also found reoperations during the global period were similar for the two cohorts. In another retrospective study of patients undergoing hand surgery, Wallace et al. reported only two of 39 patients experienced minor bleeding-related complications, neither of whom required reoperation [34].
Several other studies suggest uninterrupted chronic anticoagulation therapy appears safe in cataract surgery, dental surgery, arthrocentesis, and diagnostic endoscopy, yet there are no studies of which the authors are aware looking at this issue in elective TKA [9, 13, 21, 33]. There is obvious controversy surrounding the proper prophylaxis management of patients who do not have medical comorbidities or thromboembolic risk factors in the setting of elective TKA. This study does not attempt to address that patient population; rather, it specifically addresses the high-risk patient subset on chronic warfarin therapy. The current chest guidelines recommend postoperative LMWH, fondaparinux, or warfarin therapy for a goal INR of 2.5 in the setting of elective TKA for all patients [16]. This implies there are three different clinical scenarios the treating surgeon can consider with a patient on chronic warfarin: (1) discontinue the warfarin several days preoperatively and then restart after surgery; (2) discontinue the warfarin several days preoperatively and bridge with LMWH until surgery and then start warfarin postoperatively; or (3) continue the patient’s steady-state warfarin dosing perioperatively.
The decision to discontinue warfarin preoperatively is common; however, as stated earlier, the interruption of anticoagulation in patients on chronic warfarin therapy poses a major risk of morbidity and mortality [5, 15]. Discontinuing the warfarin and bridging with a LMWH preoperatively is also a reasonable alternative; however, it has been our clinical experience that there is much more difficulty in trying to coordinate the discontinuation of warfarin and then reaching a steady state again postoperatively. Frequently, the senior authors of this study have observed large fluctuations in the perioperative INR while trying to regain the therapeutic level, which may pose a separate set of risks. Consequent to the inception of the anticoagulation service at this institution our data confirm that large fluctuations in postoperative INR have decreased in both groups. However, many smaller institutions do not have access to this resource and the surgeon managing warfarin levels postoperatively may consider the strategy described here in caring for these patients. The scenarios listed are what led the investigators of this study to operate through the warfarin.
Our data provide another argument for continuing warfarin throughout the perioperative period in high-risk patients at their established steady-state doses before elective surgery [1, 13, 15, 21–23, 30, 33, 34]. We observed no increased risks or complications in the cohort continued on their regular warfarin dose through the perioperative period of TKA, which is our community’s standard of care for this high-risk patient population.
Acknowledgments
We thank Roy Bloebaum, PhD, for assistance with editing the manuscript and Greg Stoddard, MS, for assistance with the statistical analysis.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Alcalay J. Cutaneous surgery in patients receiving warfarin therapy. Dermatol Surg. 2001;27:756–758. doi: 10.1046/j.1524-4725.2001.01056.x. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DR, Wilson SJ, Blundell J. Comparison of a nomogram and physician-adjusted dosage of warfarin for prophylaxis against deep-vein thrombosis after arthroplasty. J Bone Joint Surg Am. 2002;84:1992–1997. doi: 10.2106/00004623-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Blacker DJ, Wijdicks EFM, McClelland RL. Stroke risk in anticoagulated patients with atrial fibrillation undergoing endoscopy. Neurology. 2003;61:964–968. doi: 10.1212/01.wnl.0000086817.54076.eb. [DOI] [PubMed] [Google Scholar]
- 4.Brinker MR, Reuben JD, Mull JR. Comparison of general and epidural anesthesia in patients undergoing primary unilateral THR. Orthopedics. 1997;20:109–115. doi: 10.3928/0147-7447-19970201-06. [DOI] [PubMed] [Google Scholar]
- 5.British Thoracic Society. Optimum duration of anticoagulation for deep vein thrombosis and pulmonary embolism. Research Committee of the British Thoracic Society. Lancet. 1992;340:873–876. [PubMed]
- 6.Colwell CW, Collis DK, Paulson R. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty: evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am. 1999;81:932–940. doi: 10.1302/0301-620X.81B5.10316. [DOI] [PubMed] [Google Scholar]
- 7.Conover WJ. Practical Nonparametric Statistics. 2nd Ed. New York, NY: John Wiley & Sons; 1980:165–169.
- 8.Dahl OE, Gudmundsen TE, Haukeland L. Late occurring clinical deep vein thrombosis in joint-operated patients. Acta Orthop Scand. 2000;71:47–50. doi: 10.1080/00016470052943883. [DOI] [PubMed] [Google Scholar]
- 9.Dunn AS, Turpie AG. Perioperative management of patients receiving oral anticoagulants: a systematic review. Arch Intern Med. 2003;163:901–908. doi: 10.1001/archinte.163.8.901. [DOI] [PubMed] [Google Scholar]
- 10.Freedman KB, Brookenthal KR, Fitzgerald RH, Williams S, Lonner JH. A meta-analysis of thromboembolic prophylaxis following elective total knee arthroplasty. J Bone Joint Surg Am. 2000;82:929–938. doi: 10.2106/00004623-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Gangireddy C, Rectenwald JR, Upchurch GR. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45:335–342. doi: 10.1016/j.jvs.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 12.Geerts WH, Pineo GF, Heit JA. Prevention of venous thromboembolism: the Seventh ACCP Conference on antithrombotic and thrombolytic therapy. Chest. 2004;126:338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 13.Hall DL, Steen WH, Jr, Drummond JW, Byrd WA. Anticoagulants and cataract surgery. Ophthalmic Surg. 1988;19:221–222. [PubMed] [Google Scholar]
- 14.Heit JA, Elliott CG, Trowbridge AA. Ardeparin sodium for extended out-of-hospital prophylaxis against venous thromboembolism after total hip or knee replacement: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2000;132:853–861. doi: 10.7326/0003-4819-132-11-200006060-00002. [DOI] [PubMed] [Google Scholar]
- 15.Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med. 1997;336:1506–1511. doi: 10.1056/NEJM199705223362107. [DOI] [PubMed] [Google Scholar]
- 16.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed) Chest. 2008;133(Suppl):454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 17.Kurtz SM, Ong KL, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005–2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 18.Kurtz SM, Ong KL, Schmier J, Mowat F, Saleh K, Dybvik E, Karrholm J, Garellick G, Havelin LI, Furnes O, Malchau H, Lau E. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(Suppl):144–151. doi: 10.2106/JBJS.G.00587. [DOI] [PubMed] [Google Scholar]
- 19.Leclerc JR, Gent M, Hirsh J. The incidence of symptomatic venous thromboembolism during and after prophylaxis with enoxaparin: a multi-institutional cohort study of patients who underwent hip or knee arthroplasty. Arch Intern Med. 1998;158:873–878. doi: 10.1001/archinte.158.8.873. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman JR, Wollaeger J, Dorey F. The efficacy of prophylaxis with low-dose warfarin for prevention of pulmonary embolism following total hip arthroplasty. J Bone Joint Surg Am. 1997;79:319–325. doi: 10.2106/00004623-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 21.McLemore EC, Harold KL, Cha SS, Johnson DJ, Fowl RJ. The safety of open inguinal herniorraphy in patients on chronic warfarin therapy. Am J Surg. 2006;192:860–864. doi: 10.1016/j.amjsurg.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 22.Morris A, Elder MJ. Warfarin therapy and cataract surgery. Clin Experiment Ophthalmol. 2000;28:419–422. doi: 10.1046/j.1442-9071.2000.00350.x. [DOI] [PubMed] [Google Scholar]
- 23.Otley CC, Fewkes JL, Frank W, Obricht SM. Complications of cutaneous surgery in patients who are taking warfarin, aspirin, or nonsteroidal anti-inflammatory drugs. Arch Dermatol. 1996;132:161–166. doi: 10.1001/archderm.132.2.161. [DOI] [PubMed] [Google Scholar]
- 24.Park YW, Thompson J. Effect of epidural anesthesia and analgesia on perioperative outcome. A randomized, controlled veterans affairs cooperative study. Ann Surg. 2001;234:560–571. doi: 10.1097/00000658-200110000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peyton PJ, Myles PS, Silbert BS. Perioperative epidural analgesia and outcome after major abdominal surgery in high-risk patients. Anesth Analg. 2003;96:548–554. doi: 10.1097/00000539-200302000-00046. [DOI] [PubMed] [Google Scholar]
- 26.Pelligrini VD, Donaldson CT, Farber DC. Prevention of readmission for venous thromboembolic disease after total hip arthroplasty. Clin Orthop Relat Res. 2005;441:56–62. doi: 10.1097/01.blo.0000194726.55372.4d. [DOI] [PubMed] [Google Scholar]
- 27.Pelligrini VD, Donaldson CT, Farber DC. The Mark Coventry Award: prevention of readmission for venous thromboembolic disease after knee arthroplasty. Clin Orthop Relat Res. 2006;452:21–27. doi: 10.1097/01.blo.0000229357.19867.84. [DOI] [PubMed] [Google Scholar]
- 28.Rigg J, Jamrozik K, Myles P. Epidural anesthesia and analgesia and outcome of major surgery: a randomized trial. Lancet. 2002;359:1276–1282. doi: 10.1016/S0140-6736(02)08266-1. [DOI] [PubMed] [Google Scholar]
- 29.Seyfer AE, Seaber AV, Dombrose FA, Urbaniak JR. Coagulation changes in elective surgery and trauma. Ann Surg. 1981;193:210–213. doi: 10.1097/00000658-198102000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smit A, Hooper G. Elective hand surgery in patients taking warfarin. J Hand Surg Br. 2004;29:206–207. doi: 10.1016/j.jhsb.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Srinivasan AF, Rice L, Bartholomew JR, Rangaswamy C, LaPerna L, Thompson JE, Murphy S, Baker K. Warfarin-induced skin necrosis and venous limb gangrene in the setting of heparin-induced thrombocytopenia. Arch Intern Med. 2004;164:66–70. doi: 10.1001/archinte.164.1.66. [DOI] [PubMed] [Google Scholar]
- 32.Sutherland CJ, Schurman JR. Complications associated with warfarin prophylaxis in total knee arthroplasty. Clin Orthop Relat Res. 1987;219:158–162. [PubMed] [Google Scholar]
- 33.Wahl MJ. Myths of dental surgery in patients receiving anticoagulant therapy. J Am Dent Assoc. 2000;131:77–81. doi: 10.14219/jada.archive.2000.0024. [DOI] [PubMed] [Google Scholar]
- 34.Wallace DL, Latimer MD, Belcher HJ. Stopping warfarin therapy is unnecessary for hand surgery. J Hand Surg Br. 2004;29:203–205. doi: 10.1016/j.jhsb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Warwick D, Whitehouse S. Symptomatic venous thromboembolism after total knee replacement. J Bone Joint Surg Br. 1997;79:780–786. doi: 10.1302/0301-620X.79B5.7761. [DOI] [PubMed] [Google Scholar]
- 36.Warwick D, Williams MH, Bannister GC. Death and thromboembolic disease after total hip replacement. J Bone Joint Surg Br. 1995;77:6–10. [PubMed] [Google Scholar]
- 37.Weiss P, Soff GA, Halkin H, Seligsohn U. Decline in proteins C and S and Factors II, VII, XI and X during the initiation of warfarin therapy. Thromb Res; 1987;45:783–790. doi: 10.1016/0049-3848(87)90088-0. [DOI] [PubMed] [Google Scholar]
- 38.White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost. 2003;90:446–455. doi: 10.1160/TH03-03-0152. [DOI] [PubMed] [Google Scholar]