Abstract
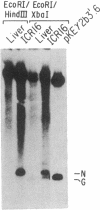
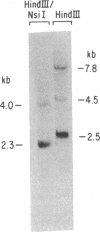
Previous studies from this laboratory have provided evidence that the duplication of the IgG2a heavy chain constant region gene (C gamma 2a) in the murine myeloma cell line MPC-11 occurred via unequal sister chromatid exchange. We now report the determination of the DNA sequences of the germ-line regions in which this exchange has occurred. The two donor sequences have long regions of virtual identity. Furthermore, both chromatids contain stretches of T-C and T-G dinucleotides; the latter has the potential to form Z-DNA. Localization of the actual breakpoints via genomic Southern blot analysis suggests a novel mechanism for the recombination and strongly implicates the simple sequence in the process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel T., Austin B., Catcheside D. G. Regulation of recombination at the his-3 locus in Neurospora crassa. Aust J Biol Sci. 1970 Dec;23(6):1229–1240. doi: 10.1071/bi9701229. [DOI] [PubMed] [Google Scholar]
- Bullock P., Miller J., Botchan M. Effects of poly[d(pGpT).d(pApC)] and poly[d(pCpG).d(pCpG)] repeats on homologous recombination in somatic cells. Mol Cell Biol. 1986 Nov;6(11):3948–3953. doi: 10.1128/mcb.6.11.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaganti R. S., Schonberg S., German J. A manyfold increase in sister chromatid exchanges in Bloom's syndrome lymphocytes. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4508–4512. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou H. S., Anderson S. J., Louie M. C., Godambe S. A., Pozzi M. R., Behlke M. A., Huppi K., Loh D. Y. Tandem linkage and unusual RNA splicing of the T-cell receptor beta-chain variable-region genes. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1992–1996. doi: 10.1073/pnas.84.7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower N. A., Stahl F. W. Chi activity during transduction-associated recombination. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7033–7037. doi: 10.1073/pnas.78.11.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt L. A., Tilley S. A., Lang R. B., Marcu K. B., Birshtein B. K. DNA rearrangements in MPC-11 immunoglobulin heavy chain class-switch variants. Proc Natl Acad Sci U S A. 1982 May;79(9):3006–3010. doi: 10.1073/pnas.79.9.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Efstratiadis A. Sequence-dependent S1 nuclease hypersensitivity of a heteronomous DNA duplex. J Biol Chem. 1986 Nov 5;261(31):14771–14780. [PubMed] [Google Scholar]
- Feigon J., Wang A. H., van der Marel G. A., van Boom J. H., Rich A. Z-DNA forms without an alternating purine-pyrimidine sequence in solution. Science. 1985 Oct 4;230(4721):82–84. doi: 10.1126/science.4035359. [DOI] [PubMed] [Google Scholar]
- Gutz H. Site Specific Induction of Gene Conversion in SCHIZOSACCHAROMYCES POMBE. Genetics. 1971 Nov;69(3):317–337. doi: 10.1093/genetics/69.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Petrino M. G., Kakunaga T. A novel repeated element with Z-DNA-forming potential is widely found in evolutionarily diverse eukaryotic genomes. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6465–6469. doi: 10.1073/pnas.79.21.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbers K., Soriano P., Müller U., Jaenisch R. High frequency of unequal recombination in pseudoautosomal region shown by proviral insertion in transgenic mouse. Nature. 1986 Dec 18;324(6098):682–685. doi: 10.1038/324682a0. [DOI] [PubMed] [Google Scholar]
- Hayes T. E., Dixon J. E. Z-DNA in the rat somatostatin gene. J Biol Chem. 1985 Jul 5;260(13):8145–8156. [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Kenter A. L., Birshtein B. K. Chi, a promoter of generalized recombination in lambda phage, is present in immunoglobulin genes. Nature. 1981 Oct 1;293(5831):402–404. doi: 10.1038/293402a0. [DOI] [PubMed] [Google Scholar]
- Kmiec E. B., Angelides K. J., Holloman W. K. Left-handed DNA and the synaptic pairing reaction promoted by Ustilago rec1 protein. Cell. 1985 Jan;40(1):139–145. doi: 10.1016/0092-8674(85)90317-4. [DOI] [PubMed] [Google Scholar]
- Kmiec E. B., Holloman W. K. Homologous pairing of DNA molecules by Ustilago rec1 protein is promoted by sequences of Z-DNA. Cell. 1986 Feb 28;44(4):545–554. doi: 10.1016/0092-8674(86)90264-3. [DOI] [PubMed] [Google Scholar]
- Kmiec E. B., Holloman W. K. Synapsis promoted by Ustilago rec1 protein. Cell. 1984 Mar;36(3):593–598. doi: 10.1016/0092-8674(84)90338-6. [DOI] [PubMed] [Google Scholar]
- Kobori J. A., Winoto A., McNicholas J., Hood L. Molecular characterization of the recombination region of six murine major histocompatibility complex (MHC) I-region recombinants. J Mol Cell Immunol. 1984;1(2):125–135. [PubMed] [Google Scholar]
- Lam S. T., Stahl M. M., McMilin K. D., Stahl F. W. Rec-mediated recombinational hot spot activity in bacteriophage lambda. II. A mutation which causes hot spot activity. Genetics. 1974 Jul;77(3):425–433. doi: 10.1093/genetics/77.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean M. J., Blaho J. A., Kilpatrick M. W., Wells R. D. Consecutive A X T pairs can adopt a left-handed DNA structure. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5884–5888. doi: 10.1073/pnas.83.16.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesfeld R., Krystal M., Arnheim N. A member of a new repeated sequence family which is conserved throughout eucaryotic evolution is found between the human delta and beta globin genes. Nucleic Acids Res. 1981 Nov 25;9(22):5931–5947. doi: 10.1093/nar/9.22.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Leder P. Organization and complete sequence of identical embryonic and plasmacytoma kappa V-region genes. J Biol Chem. 1980 Apr 25;255(8):3691–3694. [PubMed] [Google Scholar]
- Nordheim A., Lafer E. M., Peck L. J., Wang J. C., Stollar B. D., Rich A. Negatively supercoiled plasmids contain left-handed Z-DNA segments as detected by specific antibody binding. Cell. 1982 Dec;31(2 Pt 1):309–318. doi: 10.1016/0092-8674(82)90124-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S. H., Slightom J. L., Smithies O. A history of the human fetal globin gene duplication. Cell. 1981 Oct;26(2 Pt 2):191–203. doi: 10.1016/0092-8674(81)90302-0. [DOI] [PubMed] [Google Scholar]
- Shimizu A., Hamaguchi Y., Yaoita Y., Moriwaki K., Kondo K., Honjo T. Japanese wild mouse, Mus musculus molossinus, has duplicated immunoglobulin gamma 2a genes. Nature. 1982 Jul 1;298(5869):82–84. doi: 10.1038/298082a0. [DOI] [PubMed] [Google Scholar]
- Shimizu A., Takahashi N., Yamawaki-Kataoka Y., Nishida Y., Kataoka T., Honjo T. Ordering of mouse immunoglobulin heavy chain genes by molecular cloning. Nature. 1981 Jan 15;289(5794):149–153. doi: 10.1038/289149a0. [DOI] [PubMed] [Google Scholar]
- Smith G. R. Chi hotspots of generalized recombination. Cell. 1983 Oct;34(3):709–710. doi: 10.1016/0092-8674(83)90525-1. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Stephan D., Fischer Lindahl K. Gene organization and recombinational hotspots in the murine major histocompatibility complex. Cell. 1986 Mar 28;44(6):895–904. doi: 10.1016/0092-8674(86)90012-7. [DOI] [PubMed] [Google Scholar]
- Szostak J. W., Wu R. Unequal crossing over in the ribosomal DNA of Saccharomyces cerevisiae. Nature. 1980 Apr 3;284(5755):426–430. doi: 10.1038/284426a0. [DOI] [PubMed] [Google Scholar]
- Tartof K. D. Unequal mitotic sister chromatin exchange as the mechanism of ribosomal RNA gene magnification. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1272–1276. doi: 10.1073/pnas.71.4.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D., Trick M., Dover G. A. Cryptic simplicity in DNA is a major source of genetic variation. Nature. 1986 Aug 14;322(6080):652–656. doi: 10.1038/322652a0. [DOI] [PubMed] [Google Scholar]
- Thomas J. R., Bolla R. I., Rumbyrt J. S., Schlessinger D. DNase I-resistant nontranscribed spacer segments of mouse ribosomal DNA contain poly(dG-dT).poly(dA-dC). Proc Natl Acad Sci U S A. 1985 Nov;82(22):7595–7598. doi: 10.1073/pnas.82.22.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley S. A., Birshtein B. K. Unequal sister chromatid exchange. A mechanism affecting Ig gene arrangement and expression. J Exp Med. 1985 Aug 1;162(2):675–694. doi: 10.1084/jem.162.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treco D., Arnheim N. The evolutionarily conserved repetitive sequence d(TG.AC)n promotes reciprocal exchange and generates unusual recombinant tetrads during yeast meiosis. Mol Cell Biol. 1986 Nov;6(11):3934–3947. doi: 10.1128/mcb.6.11.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treco D., Thomas B., Arnheim N. Recombination hot spot in the human beta-globin gene cluster: meiotic recombination of human DNA fragments in Saccharomyces cerevisiae. Mol Cell Biol. 1985 Aug;5(8):2029–2038. doi: 10.1128/mcb.5.8.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkel-Meiman K., Keil R. L., Roeder G. S. Recombination-stimulating sequences in yeast ribosomal DNA correspond to sequences regulating transcription by RNA polymerase I. Cell. 1987 Mar 27;48(6):1071–1079. doi: 10.1016/0092-8674(87)90714-8. [DOI] [PubMed] [Google Scholar]
- Yu H., Eckhardt L. A. DNA rearrangement causes a high rate of spontaneous mutation at the immunoglobulin heavy-chain locus of a mouse myeloma cell line. Mol Cell Biol. 1986 Dec;6(12):4228–4235. doi: 10.1128/mcb.6.12.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]