Abstract
The alignment of the components of unicompartmental knee arthroplasty (UKA) reportedly influences outcomes and durability. A novel robotic arm technology has been developed with the expectation that it could improve the accuracy of bone preparation in UKA. During the study period, we compared the postoperative radiographic alignment of the tibial component with the preoperatively planned position in 31 knees in 31 consecutive patients undergoing UKA using robotic arm-assisted bone preparation and in 27 consecutive patients who underwent unilateral UKA using conventional manual instrumentation to determine the error of bone preparation and variance with each technique. Radiographically, the root mean square error of the posterior tibial slope was 3.1° when using manual techniques compared with 1.9° when using robotic arm assistance for bone preparation. In addition, the variance using manual instruments was 2.6 times greater than the robotically guided procedures. In the coronal plane, the average error was 2.7° ± 2.1° more varus of the tibial component relative to the mechanical axis of the tibia using manual instruments compared with 0.2° ± 1.8° with robotic technology, and the varus/valgus root mean square error was 3.4° manually compared with 1.8° robotically. Further study will be necessary to determine whether a reduction in alignment errors of these magnitudes will ultimately influence implant function or survival.
Level of Evidence: Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Unicompartmental knee arthroplasty (UKA) can provide durable pain relief and functional improvement in greater than 90% of patients with focal arthritis or osteonecrosis of the medial or lateral compartments of the knee [3]. It is used in approximately 10% of knees in the United States managed with arthroplasty [12]. Despite the success of the procedure, the functional outcomes and survival of UKA are influenced by a variety of factors, including the underlying diagnosis, patient selection, prosthesis design, polyethylene quality, and implant alignment and fixation [1–3, 5, 7–9, 11]. Tibial and femoral component and/or limb malalignment is poorly tolerated in UKA and can jeopardize long-term survival [5, 7–9, 14]. Swienckowski and Page reported that coronal malalignment of the tibial component beyond 3° predisposed to failure [15]. Hernigou and Deschamps reported a propensity to aseptic failure when the tibial component was implanted with a posterior slope greater than 7°, particularly with a deficient or lax anterior cruciate ligament [9]. Others have reported that the mechanical alignment of the limb, a surrogate of component alignment, can influence the durability of the prosthesis after UKA, although the ideal range of alignment in UKA, and whether under- or overcorrection is better tolerated, remains a source of debate [3, 5, 7, 8].
Using a minimally invasive approach with conventional instrumentation, it is difficult to accurately align the tibial component in UKA on a consistent basis [5–7, 10]. As many as 40% to 60% of components may be malaligned more than 2° from the preoperative plan with conventional methods [4, 10]. Although the precise range of coronal and sagittal component alignment and mechanical axis is not entirely known, there is agreement that variance beyond a safe range can predispose to aseptic loosening of the prosthesis components [3, 5, 8, 9]. Additionally, the range of component alignment varies considerably, even in the hands of skilled knee surgeons [5]. A study by Collier et al. [5] found that of 245 medial UKAs, the mean tibial component varus was 8° ± 3° (range, −5° to +21°) and the mean posterior tibial component slope was 9° ± 4° (range, −2° to +21°). The problem is compounded when using minimally invasive surgical approaches, which is how most contemporary UKAs are likely performed [6, 7]. One study analyzing the results of 221 UKAs performed through a minimally invasive approach reported a large range of tibial component alignment with a mean of 6° (± 4 standard deviation) and a range from 18° varus to 6° valgus relative to the mechanical axis of the tibia [7]. In a series by Fisher et al. there was a statistically significant difference in the coronal alignment of the tibial component in UKA performed with a minimally invasive technique compared with a standard open technique with a medial parapatellar arthrotomy (84.6° ± 2.8° [range, 78°–97°] compared with 85.9° ± 2.1° [range, 80°–92°], respectively; p = 0.001) [6]. Whether or not the mean difference (1.3°) is clinically important, the outlier malaligned components are at increased risk of early failure [5, 8, 9].
Computer navigation was introduced to reduce the number of outliers and improve the accuracy of UKA compared to a preoperative plan. However, even with computer navigation, the number of outliers (beyond 2° of the preoperatively planned implant position) may approach 15% [10]. Robotic guidance was therefore introduced to capitalize on the improvements seen with computer navigation, but also to further refine and enhance the accuracy of bone preparation, even with minimally invasive techniques [4, 13].
The purpose of this study was to determine the error and variance in tibial prosthesis alignment in UKA performed using a minimally invasive approach with either the assistance of a contemporary robotic arm system that integrates the preoperative plan with bone preparation or conventional manual instrumentation when compared with the preoperative plan.
Materials and Methods
In this pilot study, we prospectively followed one surgeon’s (JHL) initial 31 patients who underwent unilateral medial UKA with robotic arm-assisted bone preparation using the Tactile Guidance System (TGS™; MAKO Surgical Corp, Ft Lauderdale, FL). The system consists of patient-specific preoperative planning using a three-dimensional computed tomography scan of the involved knee and limb, intraoperative soft tissue balancing, and haptic-guided burring to remove the predefined volume of cartilage and bone. There were 16 women and 15 men with an average age of 64 years (range, 46–82 years), average height of 170 ± 13 cm (67 ± 5 inches), and average weight of 86 ± 17 kg (189 ± 38 pounds) for an average body mass index of 30 ± 5 kg/m2. For comparison, we retrospectively examined 27 consecutive UKAs performed by the same surgeon with conventional manual instrumentation that immediately preceded the start of the use of the robotic arm-assisted technique for bone preparation. In this group, there were 10 women and 17 men with an average age of 57 years (range, 36–80 years). The average height was 173 ± 8 cm (68 ± 3 inches) and average weight of 85 ± 16 kg (187 ± 36 pounds) for an average body mass index of 28 ± 4 kg/m2. There was no difference between the two groups in height (p = 0.18), weight (p = 0.85), or body mass index (p = 0.19).
All surgeries were performed through a minimally invasive surgical approach using an arthrotomy that extended from the proximal pole of the patella to the tibial tubercle. All components were cemented into place. In the robotic-assisted group, 28 all-polyethylene inlay-design and three metal-backed onlay-design tibial components were implanted. All robotic-assisted UKAs performed by the treating surgeon (JHL) since the 30th have used a metal-backed onlay-style tibial component, which achieves cortical support. Each of the 27 UKAs in the manual group was metal-backed onlay-design tibial components. We reviewed the radiographs at 2 or 6 weeks postoperatively to compare differences in the preoperatively planned positions and postoperatively achieved positions (error) for both the robotically and manually implanted tibial components to discern differences in error between the two groups. We received Institutional Review Board approval for this study.
Weightbearing anteroposterior, lateral, and sunrise radiographs of the knee were taken for all patients pre- and postoperatively. One of the authors (TKJ, not the treating surgeon) measured the natural posterior slope and varus of the medial tibial plateau on preoperative radiographs. The coronal and sagittal alignment of the tibial component relative to the mechanical axis of the tibia was measured postoperatively by the same author (TKJ). In general, the preoperative goal with an inlay-style tibial component is to match the patient’s anatomic varus and slope of the medial plateau. The goal of alignment with the onlay tibial component is to reproduce the native posterior sagittal slope and make a coronal resection at 90° relative to the mechanical tibial axis. Regardless of the style of tibial implant, the preoperative plan was determined in advance and the postoperative coronal and sagittal alignment compared with the three-dimensional preoperative plan and preoperative radiographs to provide an error measurement. We did not determine the reliability of the radiographic measurements. The radiographs were all taken by the same technician with the same specifications with the knee fully extended and the knee and foot directed anteriorly. In addition to this, we recorded preoperatively the natural posterior slope and varus of the medial tibial plateau, because those values were used as targets. For the postoperative measurements, we had multiple radiographs to choose from to make radiographic measurements and selected the films that most closely matched the preoperative films from the perspective of limb rotation as determined by the proximal tibia-fibular overlap.
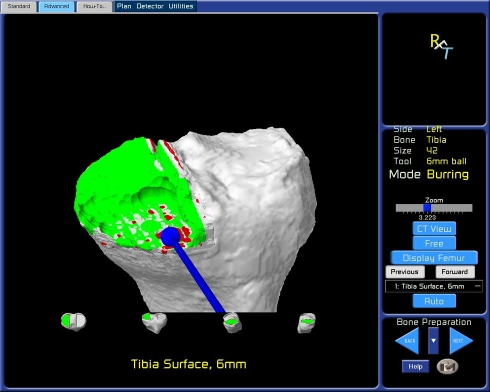
The robotic system provides a stereotactic interface, which constrains the surgeon in the preparation of the femur and the tibia. Stereotactic boundaries, ie, virtual walls, are created by the software and implemented through the robotic arm hardware to restrict the tips of burrs of varying sizes to within a predefined resection volume. Real-life manipulation of the robotic arm and burr with gross inspection and real-time observation of the procedure on the virtual image are used to prepare the bone based on the preoperative plan (Fig. 1). After completing bone preparation, an optical probe can be used to trace the surfaces of recipient bone to ensure the volume of bone removed and the orientation of bone preparation match the preoperative plan. Alternatively, the trial components can be provisionally inserted after preparing the bones and the optical probe applied to the articulating surfaces of the implant with a similar goal of corroborating accurate bone preparation and implant position (Fig. 2).
Fig. 1.
Tibial bone preparation with a robotic arm and 6-mm burr is seen in a real-time virtual image.
Fig. 2.
The tip of an optical probe (represented by the crosshairs on the screen) is moved across the articulating surface of the implanted trial tibial component to demonstrate how it matches up with the preoperatively planned position. In this case, the position matches perfectly with the preoperative plan.
The measured error of each technique of bone preparation was determined by comparing the preoperatively planned position for each tibial component with the postoperatively achieved coronal and sagittal alignment of each tibial component. To avoid skewing results as a result of averaging positive and negative errors, the root mean square, or the quadratic mean, was used to quantify alignment errors. The variance of alignment for each method of bone preparation was also calculated as a measure of statistical dispersion. Statistical significance of the root mean square error and variance was determined using unpaired Student’s t-tests.
Results
The root mean square (RMS) error of the tibial slope was 3.1° with the conventional manual technique compared with 1.9° robotically. In addition, the variance using manual instruments was 2.6 times greater (p = 0.02) than the robotic arm-guided bone preparation method. In the coronal plane, the average error of tibial alignment was 2.7° ± 2.1° more varus using manual instruments compared with 0.2° ± 1.8° when bone preparation was performed with robotic arm assistance (p < 0.0001), and the varus/valgus RMS error was 3.4° manually compared with 1.8° robotically (Figs. 3, 4).
Fig. 3.
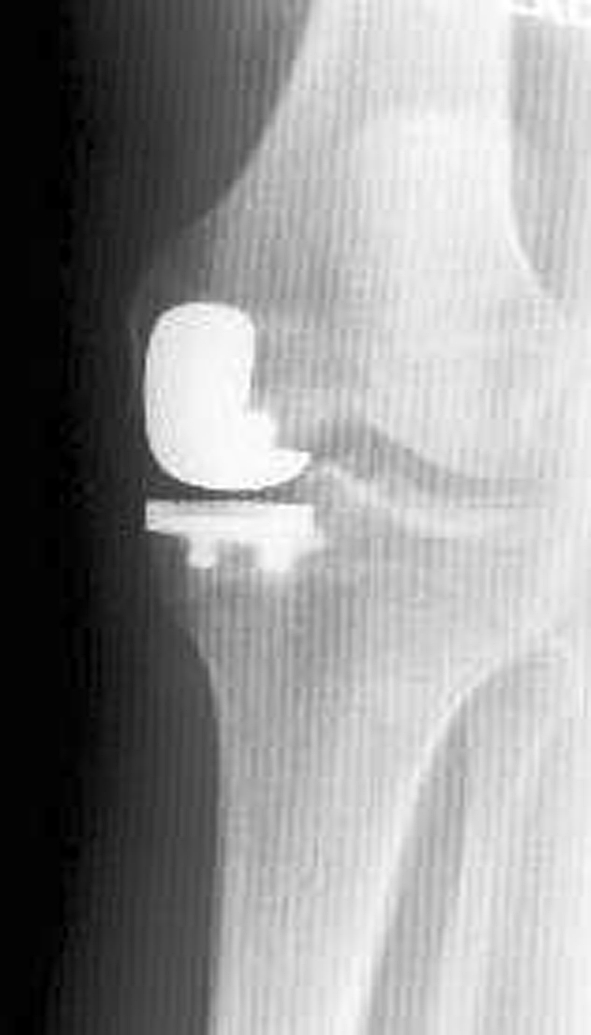
Anteroposterior radiograph of a unicompartmental knee arthroplasty performed with manual instrumentation demonstrates the tibial component is in 84° relative to the anatomic axis of the tibia (6° of relative varus).
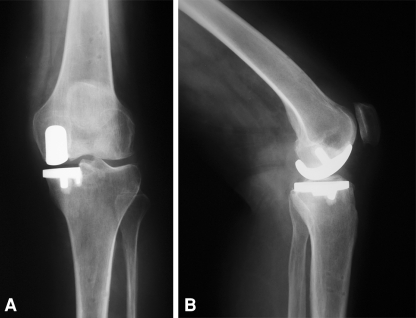
Fig. 4A–B.
(A) Anteroposterior radiograph demonstrates coronal alignment of the tibial implant within a fraction of a degree of the preoperative plan after unicompartmental knee arthroplasty performed with robotic arm assistance. (B) Lateral radiograph shows posterior slope of the tibial implant within a fraction of a degree of the preoperative plan after unicompartmental knee arthroplasty performed with robotic arm assistance.
Of the 28 all-polyethylene inlay-style tibial components in the robotically assisted group, there has been one case of isolated tibial loosening in a patient who had been doing well at the 6-week postoperative visit but who developed pain at 3 months and had tibial subsidence. Analysis of this patient’s radiographs demonstrated the tibial component alignment was within 0.1° of the preoperative plan in the coronal plane and 1° in the sagittal plane. No additional cases of loosening have occurred in the two cohorts.
Discussion
The alignment of the components of unicompartmental knee arthroplasty (UKA) influences outcomes and durability. While most UKAs are reasonably aligned, virtually all studies report outliers. Even with computer navigation, the number of outliers (beyond 2° of the preoperatively planned implant position) was as high as 13% in one study [10]. A novel robotic arm technology has been developed with the expectation that it could improve the accuracy of bone preparation in UKA and reduce the risk of outliers. We hypothesized that compared with the preoperative plan, there would be less error and variance in the tibial component alignment in UKA performed with contemporary robotic arm assistance than when conventional manual instrumentation is used.
We note some limitations of this study. First, mid- and long-term followup is not available given the recent adoption of this technology. Further followup will be necessary to determine whether the reduction in alignment errors that we observed with robotic arm-assisted bone preparation will ultimately influence implant function or survival. One cannot discern from the literature the ideal individual prosthesis component and limb alignment after UKA or how precision (or error) of component alignment influences function and survivorship after UKA. However, surgeons will have preferences regarding individual prosthesis component and limb alignment in UKA and achieving that alignment with minimal error and variance is desirable. Our data demonstrate a reduction in both error and variance of the alignment of the tibial component using robotic arm assistance through a minimally invasive surgical approach. Second, we did not make a digital preoperative plan from a computed tomography (CT) scan protocol in the retrospective cohort of patients who underwent UKA with a conventional manual approach. However, the preoperative plan was determined based on preoperative plain radiographs and we presume there would be little error in measurements of the posterior tibial slope and coronal alignment on standard radiographs comparing preoperative and postoperative radiographs. Third, although we did not determine the reproducibility of the radiographic measurements, the radiographs were all taken by the same technician with the same specifications, and as accurately as possible, the postoperative measurements were made on films that most closely matched the preoperative films from the perspective of limb rotation. Although this may potentially represent the greatest weakness in the process, we believe there was acceptable consistency in radiographic techniques and analysis and the interpretation and conclusions were not compromised. Fourth, we did not use CT scans to measure postoperative alignment, but we believe plain radiographs are suitable for measuring implant alignment, particularly when comparing preoperative and postoperative studies. We looked only at tibial component alignment and did not address femoral component alignment, because the senior author (JHL) believes determining femoral component coronal alignment in UKA with plain radiographs is difficult. Fifth, we did not assess overall limb alignment, because we were unable to get full-length radiographs of the entire limb in all patients. Future studies may address overall limb alignment as well as CT scan determination of femoral component alignment after UKA with conventional and robotic instrumentation. Sixth, although we surmise that the robotic-assisted bone preparation accounts for the improved accuracy of bone preparation and tibial component alignment, a third group would be useful to determine what role the computer assistance has independent of the robotic bone preparation. Additionally, although the two groups studied were matched for height, weight, body mass index, and deformity, a randomized, controlled study may be the most effective way to truly identify whether one technique of bone preparation is more accurate than the other. Finally, we did not perform a cost-benefit analysis. However, given the initial capital equipment cost or lease of up to $800,000 as well as operational costs, longer-term clinical followup will be necessary to determine if the investment in this technology is worthwhile vis-à-vis its impact on implant function and durability [14]. We do not believe these limitations compromise our conclusions given that the study is limited to an analysis of variance and error in radiographic alignment
We presumed radiographic alignment of the tibial component would have less error and variability when the bone was prepared with robotic arm assistance than when conventional manual instrumentation was used for UKA through a minimally invasive approach. Notwithstanding the potential limitations of this study, the use of robotic technology improved the accuracy of the position of the tibial component in the coronal and sagittal planes. The data and results presented in the current series are similar to those reported in other studies of UKA with robotic bone preparation. In a comparison of matched groups undergoing unicompartmental arthroplasties using minimally invasive approaches with either standard manual instrumentation or the robotic arm-guided bone preparation system, Coon et al. (unpublished data) reported the RMS error of the posterior tibial slope was 2.5 times greater and the variance was 2.8 times greater with a manual-instrumented technique than with the same robotic arm-guided technique used in the current series. In the coronal plane, the average error was 3.3° ± 1.8° additional varus using manual instruments compared with 0.1° ± 2.4° when using robotic arm assistance [13]. Roche et al. (unpublished data) evaluated their first 43 patients who underwent UKA using robotic assistance. Of the 344 radiographic measurements that were analyzed, less than 1% were believed to be outliers [13]. These studies, which reviewed the authors’ early experience with a robotic arm technique, show the accuracy of bone preparation is substantially improved, even early after technology adoption. Future studies will address the duration of surgery and the impact of the learning curve on component alignment. Cobb et al. compared the results of 13 robotic-assisted UKAs (Acrobot Co Ltd, London, UK) with 15 UKAs that were performed using conventional techniques in a randomized prospective study [4]. Unlike our study, the authors used postoperative CT scans to determine postoperative alignment. They measured coronal plane tibiofemoral alignment and individual component alignment and reported all patients treated with robotic bone preparation had coronal plane tibiofemoral alignment within 2° of the planned position with a mean of 0.65° (standard deviation [SD] 0.59; range, −1.6° to 0.3°), whereas only 40% in the conventional group achieved this level of accuracy with a mean of −0.84° (SD, 2.75; range, −4.2° to +4.2°).
Although these data show that in UKA, tibial component alignment is more accurate and less variable using robotic arm assistance with the tactile guidance system compared with manual, jig-based instrumentation through minimally invasive approaches, further followup will be necessary to establish whether the improved accuracy will influence longer-term outcomes.
Footnotes
One or more of the authors (JHL, MAC) have received financial support from Mako Surgical Corp, Ft Lauderdale, FL. JHL has received payment as a consultant and is a stock holder. MAC receives compensation as Director of Clinical Research.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research. A waiver for informed consent was granted on February 2, 2009.
This work was performed at Pennsylvania Hospital, Philadelphia, PA, USA.
References
- 1.Berend KR, Lombardi AV, Jr, Adams JB. Obesity, young age, patellofemoral disease, and anterior knee pain: identifying the unicondylar arthroplasty patient in the United States. Orthopedics. 2007;30(Suppl):19–23. [PubMed] [Google Scholar]
- 2.Berend KR, Lombardi AV, Jr, Mallory TH, Adams JB, Groseth KL. Early failure of minimally invasive unicompartmental knee arthroplasty is associated with obesity. Clin Orthop Relat Res. 2005;435:171–180. doi: 10.1097/01.blo.0000187062.65691.e3. [DOI] [PubMed] [Google Scholar]
- 3.Borus T, Thornhill T. Unicompartmental knee arthroplasty. J Am Acad Orthop Surg. 2008;16:9–18. doi: 10.5435/00124635-200801000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Cobb J, Henckel J, Gomes P, Harris S, Jakopec M, Rodriguez F, Barrett A, Davies B. Hands-on robotic unicompartmental knee replacement. J Bone Joint Surg Br. 2006;88:188–197. doi: 10.1302/0301-620X.88B2.17220. [DOI] [PubMed] [Google Scholar]
- 5.Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(Suppl):108–115. doi: 10.1016/j.arth.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Fisher DA, Watts M, Davis KE. Implant position in knee surgery: a comparison of minimally invasive, open unicompartmental, and total knee arthroplasty. J Arthroplasty. 2003;18(Suppl):2–8. doi: 10.1016/S0883-5403(03)00291-2. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton WG, Collier MB, Tarabee E, McAuley JP, Engh CA, Jr, Engh GA. Incidence and reasons for reoperation after minimally invasive unicompartmental knee arthroplasty. J Arthroplasty. 2006;21(Suppl):98–107. doi: 10.1016/j.arth.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res. 2004;423:161–165. doi: 10.1097/01.blo.0000128285.90459.12. [DOI] [PubMed] [Google Scholar]
- 9.Hernigou P, Deschamps G. Posterior slope of the tibial implant and the outcome of unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2004;86:506–511. doi: 10.2106/00004623-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Keene G, Simpson D, Kalairajah Y. Limb alignment in computer-assisted minimally-invasive unicompartmental knee replacement. J Bone Joint Surg Br. 2006;88:44–48. doi: 10.1302/0301-620X.88B1.16266. [DOI] [PubMed] [Google Scholar]
- 11.Kozinn SC, Scott RD. Unicondylar knee arthroplasty. J Bone Joint Surg Am. 1989;71:145–150. [PubMed] [Google Scholar]
- 12.Riddle DL, Jiranek WA, McGlynn FJ. Yearly incidence of unicompartmental arthroplasty in the United States. J Arthroplasty. 2008;23:408–412. doi: 10.1016/j.arth.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Sinha RK. Outcomes of robotic arm assisted unicompartmental arthroplasty. Am J Orthop. 2009;38(Suppl):20–22. [PubMed] [Google Scholar]
- 14.Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop. 2009;38(Suppl):32–36. [PubMed] [Google Scholar]
- 15.Swienckowski J, Page BJ. Medial unicompartmental arthroplasty of the knee. Use of the L-cut and comparison with the tibial inset method. Clin Orthop Relat Res. 1989;239:161–167. [PubMed] [Google Scholar]