Abstract
Quadriceps muscle strength is an important predictor of functional abilities in patients having TKA. However, because several daily activities are characterized by a limited time to generate force, it has been suggested that rate of force development (RFD) could better predict functional difficulties than maximal strength. We therefore hypothesized the side-to-side asymmetry would be larger for RFD than for maximal strength, and RFD asymmetry relates to subjective symptoms and/or functional daily living activities. We studied 31 subjects (17 women, 14 men) 6 ± 1 months after undergoing TKA for unilateral osteoarthritis. Symptoms and limitations during activities of daily living were quantified using the knee outcome survey-activities of daily living scale (KOS-ADLS). Quadriceps maximal strength and RFD at different times (50 to 200 ms from contraction onset) were quantified during unilateral maximal voluntary isometric actions. Side-to-side asymmetries (involved versus uninvolved side) were larger for RFD (approximately 36%) than for maximal strength (approximately 24%). Subjective knee function related to all RFD asymmetry variables, but not to maximal strength asymmetry. In addition to maximal strength, quadriceps RFD in the first 100 to 200 ms from contraction onset provides an alternative functional outcome measure for individuals undergoing TKA.
Introduction
Studies based on self-reported data show TKA reduces pain and improves perceived function relative to preoperative levels [9]. However, despite postoperative improvements in objective performance-based measures, patients who have TKAs experience considerable impairments in lower limb muscle strength, particularly the quadriceps [5, 10, 11, 16, 19–22, 34], and in functional measures such as walking and stair climbing even years after surgery [15, 19]. Such residual limitations have become an important focus of postoperative care [34].
In patients undergoing TKA, the maximal quadriceps force-generating capacity generally recovers to levels only slightly better than preoperative values and then plateaus 6 to 12 months after surgery [20]. For example, maximal strength side-to-side asymmetries (involved versus uninvolved) of 15% to 32% have been reported 6 months after TKA [10, 16, 19, 20]. Quadriceps strength deficits appear even larger when comparing patients who have had TKA with healthy age-matched subjects: 19% to 44% [10, 19] as much as 13 years postsurgery [11]. We questioned why it is so important to quantify quadriceps strength in patients who have TKAs. Quadriceps muscle weakness after TKA, which predominantly is attributable to neuromuscular activation failure [21], is an important predictor of functional abilities and a likely contributor to persistent disability in patients having TKAs: quadriceps strength of the involved and uninvolved sides is related more strongly to functional outcomes than knee flexion range of motion or bodily pain [20]. Preoperative and postoperative quadriceps strength are determinants of the ability to perform daily activities, such as walking and stair-climbing [20, 34] in patients undergoing TKA. Quadriceps force-generating capacity therefore is regarded as an essential functional outcome measure, and a prime factor to consider when examining disability following TKA [22].
Quadriceps strength generally is quantified using short burst isometric tests of 3 to 5 seconds or slow (60–120°/second) isokinetic concentric tests [28]; in these tests the maximal voluntary contraction (MVC) strength is achieved 400 to 600 ms after the onset of the contraction [33, 34]. However, some studies suggest the RFD, which is obtained from the slope of the isometric force-time curve at different intervals [1] and strongly depends on fast neuromuscular activation [8], could better predict functional difficulties of daily activities than maximal strength [1, 6, 13, 17, 30, 31]. Several daily activities such as descending stairs [24], walking fast [31], or preventing a fall after a sudden postural perturbation [26] are characterized by a limited time to generate force (50 to 200 ms, depending on the activity), and in which the ability to produce force rapidly could be viewed as an essential functional parameter [1, 13, 30]. The only indirect evidence in favor of such an assumption was provided by Suetta et al. [31], who observed maximal walking speed was noteworthy related to quadriceps RFD but not to MVC strength in patients who had THAs. Gapeyeva et al. [10] observed considerable side-to-side asymmetries in quadriceps RFD (40%) and MVC strength (32%) 6 months after surgery in 10 women who had TKAs. However, RFD was determined exclusively at 50% of MVC strength, and no attempt was made to relate it to subjective knee function.
We therefore hypothesized (1) RFD would show larger side-to-side differences compared with MVC strength (construct validity), and (2) RFD asymmetry would correlate with subjective symptoms (eg, pain, stiffness, weakness) and/or functional limitations (eg, difficulty in walking, stair ascending and descending) during daily living activities.
Materials and Methods
From our postoperative lists and medical files we retrospectively identified approximately 150 subjects who had TKAs. We included patients who: (1) had a unilateral primary TKA for osteoarthritis (Kellgren/Lawrence [14] Grade 3 or greater) of the knee 6 ± 1 months before testing, (2) had the same type of prosthesis implanted using the same surgical approach (see below), (3) had Kellgren/Lawrence [14] Grade 2 or less of the uninvolved knee (as assessed by bilateral radiographs), (4) were asymptomatic (ie, pain free) on the uninvolved side, and (5) were able to walk without aid. We performed assessments approximately 6 months after TKA because this corresponds to the postoperative period when quadriceps strength asymmetry plateaus at approximately 20% [19] and maximal tests are pain-free. We excluded patients who had any disease or disorder that could have affected hip and low back function. Based on a Cohen’s large effect size of 0.80, a level of significance of 0.05, and a power of 80%, a sample size of 16 patients was required to detect a side-to-side asymmetry of 30% (or more) in quadriceps RFD 6 months after TKA [10]. Seventeen women and 14 men (Table 1) met the inclusion criteria and volunteered to participate in the study. Before participation in the study, we obtained informed consent from all participants. The study was conducted according to the Declaration of Helsinki and the protocol was approved by the local ethics committee.
Table 1.
Subject characteristics and self-reported scores (n = 31)
| Variable | Mean ± SD | Range |
|---|---|---|
| Age (years) | 66.3 ± 7.1 | 51–78 |
| Height (cm) | 168.9 ± 9.8 | 153–192 |
| Body mass (kg) | 78.4 ± 17.3 | 54–118 |
| BMI (kg/m2) | 27.2 ± 3.6 | 19–34 |
| KOS-ADLS total score (0–100) | 79.4 ± 13.2 | 49–100 |
| KOS-ADLS symptoms (0–100) | 82.0 ± 12.8 | 53–100 |
| KOS-ADLS functional limitations (0–100) | 78.0 ± 15.5 | 45–100 |
| Subjective rating score (0–100) | 83.9 ± 12.2 | 50–100 |
BMI = body mass index; KOS-ADLS = knee outcome survey-activities of daily living scale.
All patients had implantation of a mobile-bearing posterior cruciate ligament-retaining total knee prosthesis (Innex; Zimmer, Warsaw, IN) using the same surgical technique. No patellar replacement was performed. All surgeries were performed by one of three surgeons (UM, TP, SP), with standardized Zimmer instrumentation. The standard medial parapatellar approach and the same method of bony resection were used by the three surgeons. Regional anesthesia was used in all patients in the form of a spinal anesthetic. The procedures were performed under tourniquet control, which was released once implantation was complete. A median skin incision was followed by a tibial cut, anteroposterior femoral cuts, distal femoral cut, chamfer cuts and trochlea recess, trial assembly, and final implantation. The overall duration of the surgery ranged from 70 to 80 minutes.
We instituted continuous passive motion and isometric exercises approximately 2 days after surgery. The mean length of hospital stay was 4.4 days. Rehabilitation guidelines given to the patients after discharge included weightbearing and exercise recommendations [7, 23]. Patients were allowed to weightbear as tolerated during the first 4 postoperative weeks, with the goal of walking without crutches after this period. Walking and range of motion exercises were performed in the pool under guidance of a physical therapist. Isometric strengthening of the thigh muscles (mainly knee extensors and knee flexors), cycling on an ergometer at a self-selected comfortable intensity, and balance exercises on a foam pad (first in double, than in single-limb support) also were supervised by a qualified physical therapist. We asked patients to complete this rehabilitation program with two physical therapy sessions per week (duration approximately 30 minutes) and one pool session per week (approximately 20 minutes) for the first 4 postoperative weeks.
All patients participated in a standard routine followup which included visits at approximately 6 weeks and 6 months postoperative. Followups mainly consisted of clinical examinations performed by the orthopaedic surgeon who operated on the patient. Additionally, all patients completed a questionnaire (5–10 minutes) and had quadriceps function assessment (20–30 minutes) at the 6-month postoperative visit.
Subjects completed the KOS-ADLS, which measures the symptoms and functional limitations resulting from the knee during activities of daily living [4, 12]. Subjects assessed their symptoms with six questions regarding pain, stiffness, swelling, giving way, weakness, and limping. Functional limitations were quantified using eight questions comprising difficulty walking on level surfaces, ascending and descending stairs, standing, kneeling, squatting, sitting, and rising from a chair. The highest possible total score for the 14 questions is 70 points. For clarity, however, total score and subscores were converted to a 0 to 100 scale, in which 100 represents no difficulty or symptoms during activities of daily living. Finally, we asked subjects to self-rate their overall knee function using a supplementary question, as proposed by Bizzini and Gorelick [4]. They had to determine a value between 100 (level of function comparable to function before onset of knee problems) and 0 (complete loss of function).
We quantified unilateral quadriceps MVC strength and RFD in isometric conditions using an adjustable dynamometer chair instrumented with a strain-gauge system (linear range, 0–1961 N; sensitivity, 5.1 mV/N; Good Strength; Metitur, Jyvaskyla, Finland) [27]. The same testing procedure was followed for the involved and uninvolved sides (randomly presented). Subjects were seated comfortably on the dynamometer chair with the hip at approximately 90°. The knee of the tested limb was fixed at 60° flexion, which is the angle of maximal isometric quadriceps force generation [32]. We attached the distal shin pad of the dynamometer 3 to 5 cm proximal to the lateral malleolus by using a strap. To minimize extraneous body movements, straps were applied across the chest, pelvis, and midthigh. We asked participants to position their arms across the chest during the testing procedure. Subjects warmed up by performing a series of submaximal (20% to 80% of the estimated maximum effort) isometric contractions of the knee extensor and knee flexor muscles. We also asked them to complete one quasimaximal practice repetition before each MVC. Two quadriceps MVCs then were performed (separated by 1-minute rest periods). Subjects were asked consistently to produce their maximal force rapidly (“as fast and forcefully as possible”) and to maintain the contraction for 4 to 5 seconds. The investigators provided standardized verbal encouragement to all participants throughout the testing protocol. We recorded gravity-corrected force traces with a sampling rate of 100 Hz using the Good Strength software (Metitur). Force traces also were exported, gravity-corrected, and analyzed with Windows-based software (Acqknowledge; BIOPAC Systems Inc, Santa Barbara, CA). We calculated quadriceps isometric MVC strength, ie, the highest force value (Fig. 1A), and peak RFD, ie, the highest positive value from the first derivative of the force signal (ie, the greatest slope of the force-time curve), for the involved and uninvolved sides. Peak RFD generally was obtained within 50 to 100 ms from contraction onset, with very large interindividual variability. Also, quadriceps RFD was derived as the average slope of the force-time curve during intervals of 0 to 50, 0 to 100, and 0 to 200 ms relative to onset of contraction (Fig. 1B) according to the method described by Aagaard et al. [1]. The onset of muscle contraction was defined as the time at which the force curve exceeded baseline force by approximately 15 N [31]. We consistently calculated MVC strength and RFD asymmetry between the uninvolved and involved sides as (uninvolved − involved)/uninvolved × 100.
Fig. 1A–B.
Representative quadriceps strength traces recorded during an isometric MVC trial in a patient who had a TKA, for the involved (dashed line) and uninvolved (solid line) sides are shown. (A) MVC strength is shown (vertical arrows) for respective sides. (B) The first part of the MVC trial is magnified (shaded area in A), and RFDs at different intervals (0–50, 0–100, and 0–200 ms) from contraction onset (black dot) are shown (arrows). MVC strength asymmetry is 18%, whereas RFD asymmetries range from 42% to 55%.
Before performing the statistical analyses on the main dependent variables (ie, MVC strength and RFD asymmetries), we checked the normality assumption using the Shapiro-Wilk’s test, and we verified differences in absolute MVC strength and RFD data between the involved and uninvolved sides using Student’s paired t-test. We determined differences in side-to-side asymmetries between MVC strength and RFD variables (peak RFD, RFD 0–50, 0–100, and 0–200 ms) using a one-way repeated measures ANOVA followed by Tukey’s post hoc testing. We examined the relation between self-reported scores (KOS-ADLS total, symptoms and functional limitations and subjective rating score) and quadriceps asymmetries (MVC strength, peak RFD, RFD 0–50, 0–100, and 0–200 ms) using Pearson’s product-moment correlation coefficients.
Results
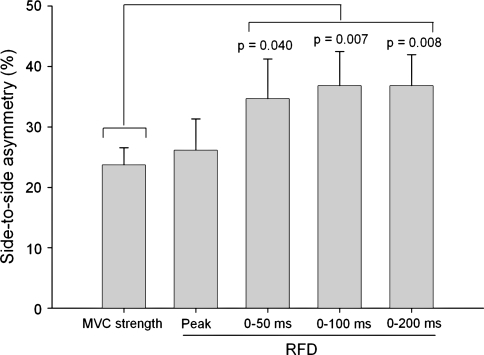
Quadriceps MVC strength and RFD variables were lower (p < 0.001) for the involved compared with the uninvolved side (Table 2). We observed differences in side-to-side asymmetries between the dependent variables (F = 5.3; p < 0.001). Specifically, RFD asymmetries for 0 to 50, 0 to 100, and 0 to 200-ms intervals were larger (p range = 0.04–0.007) than MVC strength asymmetry (Fig. 2). RFD asymmetries for 0 to 100 and 0 to 200-ms intervals also were larger (p = 0.04) than peak RFD asymmetries.
Table 2.
Quadriceps muscle function outcomes by side*
| Outcome | Involved | Uninvolved | p Value |
|---|---|---|---|
| MVC strength (N) | 324.6 ± 133.2 | 422.6 ± 161.2 | p < 0.001 |
| Peak RFD (N/s) | 1787.3 ± 1153.3 | 2442.6 ± 1412.1 | p < 0.001 |
| RFD 0–50 ms (N/s) | 1637.8 ± 1402.1 | 2497.8 ± 1718.1 | p < 0.001 |
| RFD 0–100 ms (N/s) | 1234.2 ± 849.0 | 1927.5 ± 1177.4 | p < 0.001 |
| RFD 0–200 ms (N/s) | 898.1 ± 558.0 | 1411.5 ± 811.0 | p < 0.001 |
* Mean values ± SD. MVC = maximal voluntary contraction; RFD = rate of force development.
Fig. 2.
Side-to-side asymmetries were larger for RFD 0–50, 0–100, and 0–200 ms than for MVC strength.
Correlation coefficients between self-reported scores and quadriceps asymmetries were higher for KOS-ADLS total score (r = −0.45) compared with KOS-ADLS subscores (r = −0.41), and subjective rating score (r = −0.32). Total KOS-ADLS score correlated (p range = 0.02–0.001) with all RFD asymmetry variables but did not correlate (p = 0.08) with MVC strength asymmetry (Fig. 3). As an observation, all correlation coefficients were greater for women (r = −0.69) than for men (r = −0.06). Similar findings were obtained when using KOS-ADLS subscores instead of total score.
Fig. 3A–E.
KOS-ADLS total score was not related to (A) MVC strength asymmetry, but was related to (B) quadriceps peak RFD symmetry, (C) quadriceps RFD 0–50 ms asymmetry, (D) quadriceps RFD 0–100 ms asymmetry, and (E) quadriceps RFD 0–200 ms asymmetry. Correlation coefficients were greater for women (white circles) than for men (black circles).
Discussion
Quadriceps muscle strength is an important predictor of functional abilities in patients having TKAs. However, because several activities of daily life are characterized by a limited time to exert force, quadriceps RFD could better predict functional difficulties than maximal strength [1, 6, 13, 30, 31]. We presumed quadriceps RFD asymmetry is an important functional outcome measure for patients having TKAs. Therefore, we hypothesized that (1) RFD would show larger side-to-side differences compared with MVC strength (construct validity), and (2) RFD asymmetry would correlate with subjective symptoms and/or functional limitations during activities of daily living.
One limitation of this study is related to the use of single-joint isometric contractions, which are rare in daily life. However, measurement of isometric strength in these conditions bears a strong predictive relation with functional capacity [28], and offers the possibility to assess RFD with valid procedures [1]. It is possible the relation between quadriceps RFD asymmetry and subjective knee function could be improved substantially by using short and rapid concentric and/or eccentric actions of the lower limb muscles performed, for example, on a seated leg press machine [2]. Another second limitation is that we made only side-to-side comparisons and no attempt to compare the outcomes of the subjects undergoing TKA with those of healthy matched control subjects. Although this approach might be criticized [19], because approximately 40% of patients with unilateral TKA progress to a TKA in the nonoperated knee by 10 years [18], we believe the approach is valid [28] for several reasons. First, in addition to involved muscle function assessment, strength of the noninvolved quadriceps deserves consideration because it plays an important role in patients’ functional ability [20, 34]. Second, side-to-side comparisons (ie, asymmetries) do not require data normalization by body weight [28] or BMI [20–22], which enormously simplifies between-study comparisons (Table 3). Third, we quantified the osteoarthritis level of the noninvolved knee, which provided validity to our asymmetry index.
Table 3.
Quadriceps isometric MVC strength and RFD asymmetries 6 months after TKA*
| Study | Subject characteristics | MVC | RFD |
|---|---|---|---|
| Gapeyeva et al. [10] | n = 10 (10 ♀); age: 64 years; BMI: 30 kg/m2 | 32% | 40% |
| Lorentzen et al. [16] | n = 30 (25 ♀); age: 74 years; BMI: 30 kg/m2 | 29% | – |
| Mizner et al. [20] | n = 40 (18 ♀); age: 64 years; BMI: 31 kg/m2 | 21% | – |
| Current study | n = 31 (17 ♀); age: 66 years; BMI: 27 kg/m2 | 24% | 36% |
* Asymmetries calculated as: (uninvolved – involved)/uninvolved × 100. MVC = maximal voluntary contraction; RFD = rate of force development; BMI = body mass index.
We found side-to-side differences were larger for RFD in the 0 to 200-ms interval (approximately 36%) than for MVC strength (approximately 24%), which confirms the construct validity of quadriceps RFD asymmetry in patients who have TKAs. According to the benchmarks proposed by Sapega [28], the extent of these side-to-side differences must be considered as “almost certainly abnormal”, more particularly for RFD. Our asymmetries in MVC strength are consistent with those at the same postoperative time reported in several studies [10, 16, 19] (Table 3). Although there have been few studies attempting to quantify neural [3, 10] and muscular [21, 25] impairments after TKA, such quadriceps MVC strength asymmetries are likely associated with reduced neuromuscular activation, reduced muscle size, and probably altered fiber-type composition of the involved quadriceps. According to Mizner et al. [21], diminished neuromuscular activation is by far the largest impairment after TKA. It has been suggested that the most important factor underlying RFD is fast neuromuscular activation [8]. The observation that RFD asymmetries (0–200 ms) were larger than MVC strength asymmetries in our cohort suggests the occurrence of neural impairment following TKA. We believe it is likely that discharge rate of the activated motor units, particularly at the beginning of maximal actions [33], was suboptimal for the involved quadriceps, and this inevitably resulted in lower RFD compared with the uninvolved side. The influence of muscle atrophy [21] and stiffness of the tendon-aponeurosis complex [5] on RFD impairments, however, cannot be ruled out.
Many daily actions for elderly and weakened individuals such as balance recovery after tripping [26], fast walking [31] or stair descending [24] are characterized by a limited time to develop force (50 to 200 ms, depending on the action), which is considerably less time than it takes to achieve maximal strength (approximately 400–600 ms) [31, 32]. Despite that, quadriceps MVC strength assessment is used as the gold standard to identify muscle weakness in patients having TKAs [10, 16, 19–22, 34]. Our asymmetry values were similar to those previously reported (Table 3). Gapeyeva et al. [10] observed large side-to-side differences in quadriceps RFD before and 6 months after TKA. However, they did not investigate the possible link between RFD and daily function. Additionally, because RFD was quantified exclusively at 50% of the MVC strength (ie, 200–300 ms after the onset of the contraction), they probably overlooked the most crucial phase for rapid RFD.
We found the subjective knee function of patients who had TKAs was negatively related to all quadriceps RFD asymmetry variables but not to MVC strength asymmetry, and this was particularly true for women. This finding is similar to the positive correlation reported by Suetta et al. [31] between maximal walking speed and quadriceps RFD in patients who had THAs, while walking speed was not related to MVC strength. More importantly, they observed the postoperative increase in maximal walking speed was correlated to RFD increase, but not to MVC strength improvement [31]. Although we acknowledge correlation does not imply causality, these findings, taken as a whole, suggest quadriceps RFD could be considered as a valid functional outcome measure complementary to MVC strength.
We suggest quadriceps RFD provides an alternative functional outcome measure for individuals undergoing TKA. The ability of rapid force production, which relies on the capacity of fast neuromuscular activation at contraction onset, needs more attention (eg, in TKA rehabilitation programs) because of its relation to symptoms and functional limitation during daily tasks. Specifically, we believe more emphasis is needed on rapid (0–200 ms) rather than on maximal force development following TKA. For example, exercise training programs involving a rapid production of force (ie, ballistic contractions) [33], particularly during the concentric movement phase [6], or visual feedback of muscle activation and strength [29] could be recommended for patients undergoing TKA to promote RFD improvements and minimize side-to-side asymmetries. In addition to maximal quadriceps strength, a rapid RFD is required for good quadriceps function after TKA.
Acknowledgments
We thank the subjects for participation in the study; Kristina Gabel and Sandra Schatt-Spiss for technical assistance; and the following orthopaedic surgeons who performed total knee replacements: Drs. Tomas Drobny and Stefan Preiss.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol. 2002;93:1318–1326. doi: 10.1152/japplphysiol.00283.2002. [DOI] [PubMed] [Google Scholar]
- 2.Bassey EJ. Measurement of muscle strength and power. Muscle Nerve Suppl. 1997;5:S44–S46. doi: 10.1002/(SICI)1097-4598(1997)5+<44::AID-MUS11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 3.Berth A, Urbach D, Neumann W, Awiszus F. Strength and voluntary activation of quadriceps femoris muscle in total knee arthroplasty with midvastus and subvastus approaches. J Arthroplasty. 2007;22:83–88. doi: 10.1016/j.arth.2006.02.161. [DOI] [PubMed] [Google Scholar]
- 4.Bizzini M, Gorelick M. Development of a German version of the knee outcome survey for daily activities. Arch Orthop Trauma Surg. 2007;127:781–789. doi: 10.1007/s00402-006-0200-z. [DOI] [PubMed] [Google Scholar]
- 5.Bojsen-Moller J, Magnusson SP, Rasmussen LR, Kjaer M, Aagaard P. Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of tendinous structures. J Appl Physiol. 2005;99:986–994. doi: 10.1152/japplphysiol.01305.2004. [DOI] [PubMed] [Google Scholar]
- 6.Caserotti P, Aagaard P, Larsen JB, Puggaard L. Explosive heavy-resistance training in old and very old adults: changes in rapid muscle force, strength and power. Scand J Med Sci Sports. 2008;18:773–782. doi: 10.1111/j.1600-0838.2007.00732.x. [DOI] [PubMed] [Google Scholar]
- 7.Codine P, Dellemme Y, Denis-Laroque F, Hérisson C. The use of low velocity submaximal eccentric contractions of the hamstring for recovery of full extension after total knee replacement: a randomized controlled study. Isokinet Exerc Sci. 2004;12:215–218. [Google Scholar]
- 8.Ruiter CJ, Kooistra RD, Paalman MI, Haan A. Initial phase of maximal voluntary and electrically stimulated knee extension torque development at different knee angles. J Appl Physiol. 2004;97:1693–1701. doi: 10.1152/japplphysiol.00230.2004. [DOI] [PubMed] [Google Scholar]
- 9.Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty: a qualitative and systematic review of literature. J Bone J Surg Am. 2004;86:963–974. doi: 10.2106/00004623-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Gapeyeva H, Buht N, Peterson K, Ereline J, Haviko T, Paasuke M. Quadriceps femoris muscle voluntary isometric force production and relaxation characteristics before and 6 months after unilateral total knee arthroplasty in women. Knee Surg Sports Traumatol Arthrosc. 2007;15:202–211. doi: 10.1007/s00167-006-0166-y. [DOI] [PubMed] [Google Scholar]
- 11.Huang CH, Cheng CK, Lee YT, Lee KS. Muscle strength after successful total knee replacement: a 6- to 13-year followup. Clin Orthop Relat Res. 1996;328:147–154. doi: 10.1097/00003086-199607000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80:1132–1145. doi: 10.2106/00004623-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Izquierdo M, Aguado X, Gonzalez R, Lopez JL, Häkkinen K. Maximal and explosive force production capacity and balance performance in men of different ages. Eur J Appl Physiol Occup Physiol. 1999;79:260–267. doi: 10.1007/s004210050504. [DOI] [PubMed] [Google Scholar]
- 14.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee TH, Tsuchida T, Kitahara H, Moriya H. Gait analysis before and after unilateral total knee arthroplasty: study using linear regression model of normal controls–women without arthroplasty. J Orthop Sci. 1999;4:13–21. doi: 10.1007/s007760050068. [DOI] [PubMed] [Google Scholar]
- 16.Lorentzen JS, Petersen MM, Brot C, Madsen OR. Early changes in muscle strength after total knee arthroplasty: a 6-month follow-up of 30 knees. Acta Orthop Scand. 1999;70:176–179. doi: 10.3109/17453679909011258. [DOI] [PubMed] [Google Scholar]
- 17.Marks R, Kumar S, Percy J, Semple J. Force-time measurements of the quadriceps femoris muscles of healthy women and women with osteoarthritis of the knee. Eur J Phys Med Rehabil. 1995;5:88–92. [Google Scholar]
- 18.McMahon M, Block JA. The risk of contralateral total knee arthroplasty after knee replacement for osteoarthritis. J Rheumatol. 2003;30:1822–1824. [PubMed] [Google Scholar]
- 19.Meier W, Mizner RL, Marcus RL, Dibble LE, Peters C, Lastayo PC. Total knee arthroplasty: muscle impairments, functional limitations, and recommended rehabilitation approaches. J Orthop Sports Phys Ther. 2008;38:246–256. doi: 10.2519/jospt.2008.2715. [DOI] [PubMed] [Google Scholar]
- 20.Mizner RL, Petterson SC, Snyder-Mackler L. Quadriceps strength and the time course of functional recovery after total knee arthroplasty. J Orthop Sports Phys Ther. 2005;35:424–436. doi: 10.2519/jospt.2005.35.7.424. [DOI] [PubMed] [Google Scholar]
- 21.Mizner RL, Petterson SC, Stevens JE, Vandenborne K, Snyder-Mackler L. Early quadriceps strength loss after total knee arthroplasty: the contributions of muscle atrophy and failure of voluntary muscle activation. J Bone Joint Surg Am. 2005;87:1047–1053. doi: 10.2106/JBJS.D.01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizner RL, Snyder-Mackler L. Altered loading during walking and sit-to-stand is affected by quadriceps weakness after total knee arthroplasty. J Orthop Res. 2005;23:1083–1090. doi: 10.1016/j.orthres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Moffet H, Collet JP, Shapiro SH, Paradis G, Marquis F, Roy L. Effectiveness of intensive rehabilitation on functional ability and quality of life after first total knee arthroplasty: a single-blind randomized controlled trial. Arch Phys Med Rehabil. 2004;85:546–556. doi: 10.1016/j.apmr.2003.08.080. [DOI] [PubMed] [Google Scholar]
- 24.Nyland J, Frost K, Quesada P, Angeli C, Swank A, Topp R, Malkani AL. Self-reported chair-rise ability relates to stair-climbing readiness of total knee arthroplasty patients: a pilot study. J Rehabil Res Dev. 2007;44:751–759. doi: 10.1682/JRRD.2006.11.0146. [DOI] [PubMed] [Google Scholar]
- 25.Perhonen M, Komi P, Hakkinen H, Bonsdorff H, Partio E. Strength training and neuromuscular function in elderly people with total knee endoprosthesis. Scand J Med Sci Sports. 1992;2:234–243. [Google Scholar]
- 26.Pijnappels M, Bobbert MF, Dieen JH. Control of support limb muscles in recovery after tripping in young and older subjects. Exp Brain Res. 2005;160:326–333. doi: 10.1007/s00221-004-2014-y. [DOI] [PubMed] [Google Scholar]
- 27.Rantanen T, Era P, Heikkinen E. Physical activity and the changes in maximal isometric strength in men and women from the age of 75 to 80 years. J Am Geriatr Soc. 1997;45:1439–1445. doi: 10.1111/j.1532-5415.1997.tb03193.x. [DOI] [PubMed] [Google Scholar]
- 28.Sapega AA. Muscle performance evaluation in orthopaedic practice. J Bone J Surg Am. 1990;72:1562–1574. [PubMed] [Google Scholar]
- 29.Simon AM, Gillespie RB, Ferris DP. Symmetry-based resistance as a novel means of lower limb rehabilitation. J Biomech. 2007;40:1286–1292. doi: 10.1016/j.jbiomech.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 30.Suetta C, Aagaard P, Magnusson SP, Andersen LL, Sipilä S, Rosted A, Jakobsen AK, Duus B, Kjaer M. Muscle size, neuromuscular activation, and rapid force characteristics in elderly men and women: effects of unilateral long-term disuse due to hip-osteoarthritis. J Appl Physiol. 2007;102:942–948. doi: 10.1152/japplphysiol.00067.2006. [DOI] [PubMed] [Google Scholar]
- 31.Suetta C, Aagaard P, Rosted A, Jakobsen AK, Duus B, Kjaer M, Magnusson SP. Training-induced changes in muscle CSA, muscle strength, EMG, and rate of force development in elderly subjects after long-term unilateral disuse. J Appl Physiol. 2004;97:1954–1961. doi: 10.1152/japplphysiol.01307.2003. [DOI] [PubMed] [Google Scholar]
- 32.Thorstensson A, Grimby G, Karlsson J. Force-velocity relations and fiber composition in human knee extensor muscles. J Appl Physiol. 1976;40:12–16. doi: 10.1152/jappl.1976.40.1.12. [DOI] [PubMed] [Google Scholar]
- 33.Cutsem M, Duchateau J, Hainaut K. Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. J Physiol. 1998;513:295–305. doi: 10.1111/j.1469-7793.1998.295by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida Y, Mizner RL, Ramsey DK, Snyder-Mackler L. Examining outcomes from total knee arthroplasty and the relationship between quadriceps strength and knee function over time. Clin Biomech. 2008;23:320–328. doi: 10.1016/j.clinbiomech.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]