Abstract
Young patients with early osteoarthritis wishing to remain functionally active have limited treatment options. Existing studies examining the use of autologous chondrocyte implantation (ACI) have included patients with early degenerative changes; however, none specifically investigated the outcome of ACI with this challenging problem. We prospectively followed 153 patients (155 knees) for up to 11 years after treatment with ACI for early-stage osteoarthritis. Patient pain and function was assessed using WOMAC, modified Cincinnati, SF-36, Knee Society score, and a satisfaction questionnaire. Mean patient age was 38.3 years. On average, 2.1 defects were treated per knee; the mean defect size was 4.9 cm2 and total area per knee was 10.4 cm2. Eight percent of joints were considered treatment failures that went on to arthroplasty and the remaining patients experienced 50% to 75% improvement in WOMAC subscales. Our data suggest that ACI in patients with early osteoarthritis results in clinically relevant reductions in pain and improvement in function. At 5 years postoperatively, 92% of patients were functioning well and were able to delay the need for joint replacement. Given the limited number of treatment options for this subset of patients, autologous chondrocyte implantation may offer improved quality of life for young osteoarthritic patients.
Level of Evidence: Level IV, case series. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Osteoarthritis is a prevalent [4, 12–14] disease that is expected to become symptomatic in almost half the U.S. population within their lifetime [23, 39, 47]. It causes considerable pain, functional limitation, deterioration of health-related quality of life and, in some cases, symptoms of depression [10, 18, 20, 22, 38, 42, 69]. This is especially worrisome for young patients with early degenerative changes because of their high functional demands and long active lifespan. The presence of even asymptomatic chondral defects doubles the rate at which cartilage is lost when compared to intact knees [15], a finding that was confirmed in another study demonstrating disease progression in 81% chondral defects over only 2 years [17].
Surgeons who treat young osteoarthritic patients face the challenge of alleviating debilitating symptoms and satisfying patient expectations of returned function, a task that is further complicated by the limited number of available treatments. Pharmaceutical interventions, such as nonsteroidal antiinflammatory, steroid, and hyaluronan injections, provide symptomatic relief but do not stop or delay disease progression. Arthroscopic débridement or chondroplasty has demonstrated disappointing results for the treatment of degenerative changes in the knee [46]. Knee arthroplasty in young patients remains controversial: while the outcomes of primary total knee arthroplasty are among the best of any orthopaedic procedure, revision arthroplasty is associated with substantially worse outcomes [57] and, presuming a normal lifespan, at least one revision would be a near-certainty in patients having arthroplasty in their late 30s and early 40s or earlier. We therefore routinely attempt to delay arthroplasty as long as possible, oftentimes at great cost to the patient in terms of persistent pain, limited function, and pain medication use. Alternative treatments for this subset of osteoarthritic patients would therefore be important to delay the need for primary knee arthroplasty to a more advanced age when the arthroplasty might outlast the patient’s lifetime, thus avoiding the need for revision surgery.
Autologous chondrocyte implantation (ACI) produces hyaline-like repair tissue [9, 45] in full-thickness cartilage defects and functional improvement with up to 10 years of followup [53–55]. Limited data available on the use of ACI in early osteoarthritis suggest this intervention can reduce symptoms and increase function [43, 44, 59]; however, results are preliminary.
The purpose of this study was to (1) report on the overall failure rate, as defined by revision with arthroplasty, of ACI in a population of young patients with early osteoarthritic changes; (2) describe the functional outcomes seen with this procedure; (3) investigate potential differences in patients requiring concurrent osteotomies; and (4) describe subsequent surgical procedures that become necessary after ACI.
Patients and Methods
This cohort study utilized patients from our cartilage repair database, which includes all patients treated with ACI (Carticel; Genzyme BioSurgery, Cambridge, MA) by the senior author (TM). Institutional review board approval was obtained at its onset in March 1995. All patients provided informed written consent at the time they entered into the database and were followed with questionnaires at regular intervals to document changes from their preoperative baseline. We utilized the following criteria to determine whether patients were suitable to undergo treatment of cartilage defects with ACI: full-thickness chondral defect(s) Outerbridge Grade 3 or 4 [52] of the knee with consistent history, physical examination, imaging and arthroscopy; no inflammatory joint disease, unresolved septic arthritis, deficient soft tissue coverage, metabolic or crystal disorders; no or correctable ligamentous instability, malalignment, or meniscal deficiency; not more than 50% loss of joint space on weight-bearing radiographs. Specific inclusion criteria for the present study were: (1) minimum followup of 2 years; and (2) evidence of early arthritis, as defined by radiographic and clinical criteria: radiographically, patients were eligible if they had peripheral intra-articular osteophyte formation and/or 0% to 50% joint space narrowing as defined by Ahlback Stage 0 or 1 classification [1]. Clinically, patients were also included if they had normal radiographs but evidence of bipolar (kissing) lesions, or generalized chondromalacia noted at the time of surgery. There were no exclusion criteria. From among the more than 500 patients treated with ACI at our center since March 1995, 328 had completed more than 2 years of followup by the time data collection began for this study, having been treated between March 1995 and November 2004. From among this group, 153 patients with 155 treated knee joints had been classified as having early osteoarthritis by the abovementioned criteria and were therefore included in the study (Table 1).
Table 1.
Demographic data of study patients and defect characteristics
| Baseline characteristics | Value |
|---|---|
| Number of patients (knees) | 153 (155) |
| Followup [%] | 87 |
| Age, mean (range) [years] | 38.3 (17–60) |
| Followup, mean (range) [months] | 64.2 (24–132) |
| Females—Males | 70–83 |
| Worker’s compensation, % of patients | 22/153 (14%) |
| Osteochondritis dissecans, % of knees | 12/155 (8%) |
| Mean number of defects per knee | 2.1 |
| Mean size (range) of primary lesion [cm2] | 6.7 (1–15.1) |
| Mean size (range) across all lesions [cm2] | 4.9 (0.6–15.1) |
| Mean total resurfaced area (range) per knee [cm2] | 10.4 (1.5–31.6) |
| Defect location | |
| Medial femoral condyle | 115 |
| Trochlea | 85 |
| Patella | 60 |
| Lateral femoral condyle | 45 |
| Medial tibial plateau | 7 |
| Lateral tibial plateau | 7 |
| Bipolar (kissing) defects | |
| Patellofemoral | 30 joints |
| Medial compartment | 6 joints |
| Lateral compartment | 6 joints |
The autologous chondrocyte implantation (ACI) procedure was performed as described previously [9, 24, 45, 54, 55]: in summary, after initial arthroscopic cartilage biopsy, chondrocytes were cultured (Genzyme BioSurgery) for 4 to 6 weeks. Patients then returned for reimplantation, which was performed through a standard arthrotomy. Degenerated cartilage, as well as the layer of calcified cartilage, was removed from the defect leaving a stable shoulder of healthy cartilage; defect size was then measured. Periosteum was harvested from the proximal tibia or distal femur and sutured over the defect with multiple, interrupted 6-0 Vicryl sutures. The suture line was water-proofed with fibrin glue (Tisseel; Baxter Biosurgery, Deerfield, IL) sealant and the chondrocytes were injected underneath the patch to fill the defect.
An average of 2.1 defects per knee was treated with an average defect size of 4.9 cm2 and a total treated surface area of 10.4 cm2 per knee joint. Lesions were most commonly located in the following location, by order of incidence: medial femoral condyle, trochlea, patella, and lateral femoral condyle. Bipolar (kissing) lesions were present in 27% (42 of 155) of knee joints. In addition to ACI, many concurrent procedures were performed, most commonly to correct tibiofemoral malalignment in 31% (48 of 155) and patellar maltracking in 28% (44 of 155) of implanted knees (Table 2).
Table 2.
Overview of procedures performed concurrently with ACI
| Procedure | Number |
|---|---|
| High-tibial osteotomy | |
| Opening wedge | 11 |
| Closing wedge | 36 |
| Distal femoral osteotomy | 1 |
| Tibial tubercle osteotomy | 44 |
| Ligament reconstruction | 4 |
| Meniscal allograft transplantation | 7 |
Patients with 2° or more of malalignment were treated by realignment osteotomy and overcorrected by 2° to unload and optimize the mechanical environment of the compromised compartment. We intentionally avoided additional overcorrection by 3° to 5° as recommended for isolated osteotomy [16], due to the risk of overload and accelerated failure of the contralateral compartment, but rather corrected the weight-bearing line to the contralateral tibial spine. Patients with patellofemoral defects had a concurrent anteromedialization tibial tubercle osteotomy, lateral release, and vastus medialis obliquus advancement if there was evidence of patellar subluxation and tilt as noted by physical examination, radiographs, and/or CT scan assessment.
The postoperative rehabilitative protocol focused on graft protection and restoration of motion, muscle tone, and control. Rehabilitation was progressed in stages: Stage I (weeks 1–6 after surgery) included touch-down weight bearing on two crutches in a brace, the use of continuous passive motion (CPM) for 6 to 8 hours per day, range-of-motion (ROM) and isometric muscle exercises; Stage II (7–12 weeks) included active ROM exercises, functional muscle usage, and progression from partial to full weight bearing at 12 weeks after the index surgery. Stage III (12 + weeks) continued to advance functional activities. Patients were restricted from inline impact activities (running) for 12 to 18 months and from cutting sports for at least 18 months. The protocol was adjusted by the senior surgeon for each patient’s specific reconstruction, concurrent procedures, degree of graft maturation, and previous activity level.
The outcome evaluation was performed with five validated, generic- and disease-specific instruments measuring changes in symptoms, function, and sports activity level to avoid ceiling and floor effects. Data were routinely collected preoperatively, at 1 and 2 years postoperatively, and then biennially; only the preoperative, 2-year, and final followup data for each patient were analyzed for this study. Patients completed questionnaires including an overall well-being and quality-of-life survey (Short Form-36) [66], a knee-specific (Knee Society score, KSS) and sports activity-based instrument (modified Cincinnati Rating scale) [50], and a generalized nonspecific arthritis score (WOMAC) [7]. Also, they were asked to answer questions pertaining to their satisfaction with the procedure. Questionnaires were answered independently by the patients without physician interaction, mostly at home, and mailed in. Patients presented for clinical, postoperative followup as dictated by standard clinical routine.
The WOMAC osteoarthritis index is a disease-specific, self-administered instrument that was used to measure patient pain, stiffness, and physical function. This health status instrument has been statically tested for reliability, validity, and responsiveness in a number of osteoarthritic populations [21, 63]. Questions consist of five-point Likert scales (score of 0 = none, 1 = slight, 2 = moderate, 3 = severe, 4 = extreme). The pain, stiffness, and function subscales include five, two, and 17 questions, respectively. We reported WOMAC scores as mean scores; higher scores indicated worse symptom and functional status. Minimal clinically important difference (MCID) was defined as the smallest difference in score that patients perceive as beneficial [3, 30]. According to previously published studies, the MCID of improvement in patients with osteoarthritis of the knee has been defined as a 17% to 22% change from baseline WOMAC subscale scores [3, 26].
The patient perception scale of the Cincinnati knee rating system for overall condition was modified as previously described [42] and used to measure patient function at baseline and followup. Administration of this scale required patients to rate their overall condition on a 10-point scale (Fig. 1). The Cincinnati knee rating system has been used in various patient populations including those with high tibial osteotomies, anterior cruciate ligament deficient knees, and meniscal repairs [6, 49, 51].
Fig. 1.
An overview of the questions used in the modified Cincinnati score is given.
Commonly used by orthopaedic surgeons, the Knee Society score health survey captures a diagnosis-based estimate of functional impairment [29]. Two scores (0 to 100 points) are derived from this survey. The knee score rates only the knee joint itself and the functional score rates the patient’s ability to walk and climb stairs. The highest score (100 points) that can be obtained represents a well-aligned knee with no pain, 125° motion, negligible anteroposterior and mediolateral instability, walking an unlimited distance, and climbing up and down stairs normally [29].
The Patient Satisfaction Questionnaire asked patients (1) to rate the operated joint compared to 1 year ago; (2) to rate the operated joint compared to before surgery; (3) to assess their overall satisfaction with their surgery; (4) to assess if they would have the surgery again; and (5) to rate the results of their surgery. For each item, patients were instructed to select one of five possible responses (Table 3).
Table 3.
Satisfaction with the procedure at final followup for patients with successful ACI surgery (n = 143) versus satisfaction scores of patients who subsequently required revision to partial or total knee arthroplasty (n = 12)
| Success | Failure | |
|---|---|---|
| Compared to 1 year ago, how would you rate your operated joint now? | ||
| Better | 73.4% | 25.0% |
| About the same | 23.1% | 75.0% |
| Worse | 3.5% | 0.0% |
| Compared to before each surgery, how would you rate your operated joint now? | ||
| Better | 90.2% | 33.3% |
| About the same | 7.0% | 33.3% |
| Worse | 2.8% | 33.3% |
| What is your overall satisfaction level with the joint surgery? | ||
| Satisfied | 91.6% | 58.3% |
| Neutral | 6.3% | 25.0% |
| Dissatisfied | 2.1% | 16.7% |
| If you could go back in time and make the decision again, would you choose to have your joint surgery? | ||
| Yes | 92.3% | 91.7% |
| Completely uncertain | 3.6% | 8.3% |
| No | 4.1% | 0.0% |
| How would you rate the results of your joint surgery | ||
| Good or Excellent | 88.8% | 25.0% |
| Fair | 9.8% | 50.0% |
| Poor | 1.4% | 25.0% |
The Short-Form 36 health status survey, a widely used, self-administered, generic health instrument, has been validated in the general population and disease-specific populations [66, 68]. The items of the SF-36 instrument measure eight health domains: physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health. Domain and summary scores range from 0 to 100 points, with higher scores indicating a better health state [67, 68].
Data were collected independent of the surgeon by mailed questionnaires, and statistical analysis was performed by an independent outside statistician (JHK). Treatment success for the purpose of this study was defined as avoidance of partial or total joint replacement. The functional data for successful and failed grafts were analyzed separately. We determined differences in functional scores (WOMAC, KSS, SF-36, and modified Cincinnati) between three time points (preoperatively, 24 months after implantation, and at latest followup) using the Wilcoxon signed rank test. For sub-analysis, patients were grouped based on the type of osteotomy performed concurrently with ACI, if any. Differences in functional scores at baseline and final followup were then determined between these groups using the Student’s t-test. We determined survival using the Kaplan-Meier method and computing 95% confidence intervals.
Results
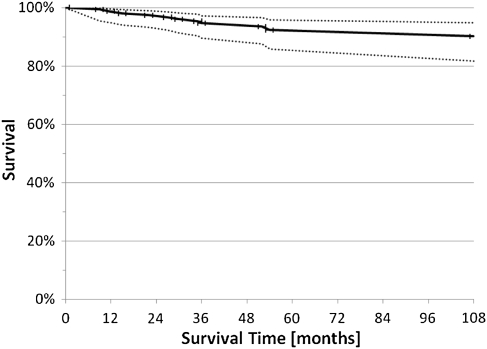
Twelve of the 155 knees (8%) were considered treatment failures and revised to partial (two) or total (10) joint arthroplasty at an average of 38 months (range, 9–118 months) after ACI. The survival was 93% (CI 86% to 96%) at 60 months (Fig. 2). The reasons for revision included complete graft failure in three patients, inadequate pain relief in one patient, and progression of osteoarthritic disease beyond the originally transplanted defect area in eight patients.
Fig. 2.
A graph demonstrating survival of ACI over time utilizing a Kaplan-Meier survival curve with 95% confidence interval is shown. The survival was 93% (CI 86% to 96%) at 60 months.
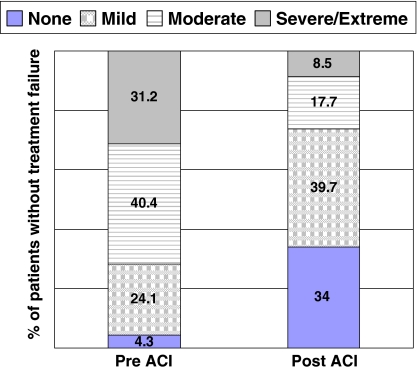
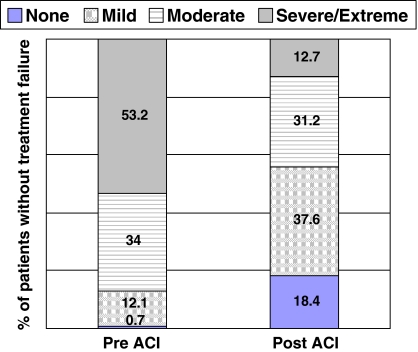
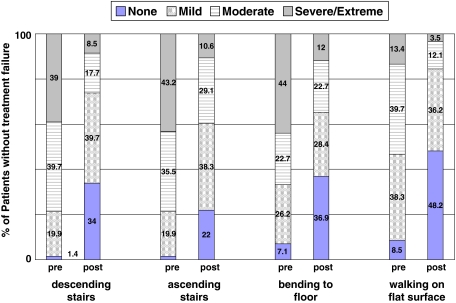
The 147 knees (92%) not considered treatment failures within the followup time experienced improvements (p < 0.001) in all scores from baseline to final followup. Specifically, changes in the WOMAC pain and function scores improved from baseline to followup, exceeding the definition of MCID (Table 4), with mean improvements in the 20-point WOMAC pain score and 68-point WOMAC function scores of 4.9 points (51% improvement) and 15.7 points (53% improvement), respectively. The proportion of patients who experienced severe or extreme pain while walking on a flat surface decreased by 73% (Fig. 3), while the percentage of patients with similar pain walking up and down stairs decreased by 76% (Fig. 4). Similar reductions also were seen in the proportion of patients who had severe or extreme difficulty when descending stairs, ascending stairs, bending to the floor, and walking on a flat surface; the proportion of patients with this level of difficulty decreased by 78%, 75%, 73%, and 74%, respectively (Fig. 5). We also observed improvements (p < 0.001) in mean scores for the modified Cincinnati knee rating system, KSS function scale, KSS pain scale, and all eight SF-36 domain scales (Table 4, Fig. 6). Furthermore, 91.6% of patients were satisfied with their outcome after treatment with autologous chondrocyte implantation, 90.2% rated their knees better than before the surgery, and 91.3% would have the same procedure again (Table 3).
Table 4.
Functional scores preoperatively, at 24 months, and at final followup. Data are provided as mean (range). [n = number of patients at the respective time point]
| Type of test | Score | p Value | ||
|---|---|---|---|---|
| Preoperative (n = 143) | 24 months followup (n = 143) | Latest followup (> 2 years) (n = 132) | ||
| Modified Cincinnati | 3.6 (1–8) | 6.1 (2–10) | 6.7 (2–10) | < 0.001 |
| KSS—knee | 59.0 (24–100) | 82.6 (45–100) | 88.0 (50–100) | < 0.001 |
| KSS—function | 60.6 (15–100) | 74.2 (40–100) | 79.4 (40–100) | < 0.001 |
| WOMAC—pain | 9.6 (0–20) | 5.8 (0–16) | 4.7 (0–17) | < 0.001 |
| WOMAC—stiffness | 3.9 (0–8) | 2.7 (0–7) | 2.4 (0–8) | < 0.001 |
| WOMAC—function | 29.4 (2–62) | 17.3 (0–51) | 13.7 (0–49) | < 0.001 |
| SF-36—PCS | 37.2 (23–60) | 44.0 (25–60) | 45.4 (9–60) | < 0.001 |
| SF-36—MCS | 38.4 (9–55) | 41.3 (9–53) | 43.8 (9–56) | < 0.001 |
Fig. 3.
Patients’ responses to WOMAC Pain Scale-Item 1: “Amount of pain experienced when walking on a flat surface”. Graph depicts the percentage of patients responding within each answer category.
Fig. 4.
Patients’ responses to WOMAC Pain Scale-Item 2: “Amount of pain experienced when going up or down stairs”. Graph depicts the percentage of patients responding within each answer category.
Fig. 5.
Patients’ responses to WOMAC Function Scale questions demonstrating the degree of difficulty with various tasks are shown.
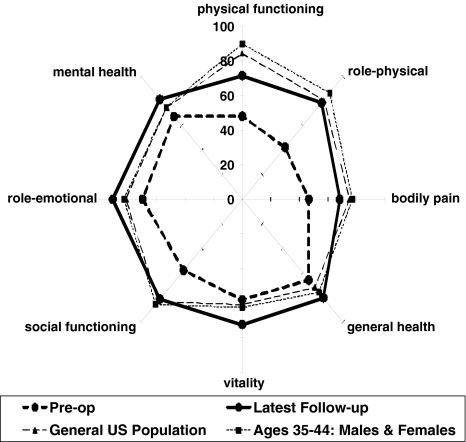
Fig. 6.
Graph visually demonstrating average patient scores for the 8 SF-36 domains. Results are provided for ACI patients pre-operatively and at the time of latest follow-up; as well as reference values for the general US population and those aged 35–44 years.
Patients with and without concurrent osteotomies experienced improvements (p < 0.001) in all functional scores. There were no substantial differences between patients without osteotomies and individual subgroups of patients with isolated tibial tubercle osteotomy (TTO) (p values ranged from 0.16 to 0.23 for the different functional scores), or a combination of TTO and high tibial osteotomy (HTO) (p values, 0.09 to 0.16); differences were small in comparison with a group of patients with isolated HTO (p values, 0.04 to 0.13). Comparing patients with HTO and patients who had undergone TTO demonstrated better outcomes for the HTO group at final followup (Table 5) when considering absolute levels; however, TTO patients started with worse scores.
Table 5.
Functional scores for the osteotomy groups preoperatively and at final followup. Data are provided as mean; p values compare the two groups at each time-point [n = number of patients at the respective time point]
| Type of test | Preoperative | Latest Followup (> 2 years) | ||||
|---|---|---|---|---|---|---|
| HTO (n = 29) | TTO (n = 23) | p Value | HTO (n = 27) | TTO (n = 23) | p Value | |
| Modified Cincinnati | 3.4 | 3.3 | 0.9 | 7.3 | 6.1 | 0.002 |
| KSS—knee | 61.7 | 52.9 | 0.03 | 92.0 | 84.1 | 0.02 |
| KSS—function | 64.5 | 59.1 | 0.2 | 82.6 | 74.3 | 0.04 |
| WOMAC—pain | 9.5 | 11.5 | 0.08 | 3.3 | 6.2 | 0.01 |
| WOMAC—stiffness | 4.1 | 4.9 | 0.1 | 1.9 | 3.2 | 0.01 |
| WOMAC—function | 30.0 | 34.9 | 0.2 | 10.8 | 19.7 | 0.007 |
| SF-36—PCS | 36.7 | 35.6 | 0.6 | 48.6 | 43.3 | 0.03 |
| SF-36—MCS | 39.1 | 38.4 | 0.7 | 43.7 | 43.7 | 0.99 |
HTO = high tibial osteotomy.
TTO = tibial tubercle osteotomy.
Subsequent surgical procedures (SSP) after the index implantation were performed in 95 of the 155 knees (61%), the majority (52 of 95) for periosteal hypertrophy. Other indications included arthrofibrosis (32 of 95), graft complications (23 of 95), and periosteal delamination (11 of 95), with several patients having more than one diagnosis treated during a SSP. Graft complications were partial graft delamination, affecting less than 20% of the defect area, in 21 knees, treated with arthroscopic interventions, either microfracture (eight), abrasion arthroplasty (five) or OATS (eight). None of the patients undergoing a subsequent surgical procedure went on to complete failure requiring joint replacement.
Discussion
Large, multiple, and bipolar cartilage defects represent an early stage in the wide spectrum of disease termed osteoarthritis. Treatment options for these patients are controversial since many are too young for joint replacement, yet cartilage repair such as ACI has traditionally been contraindicated for these degenerative lesions. The purpose of this study was to (1) report on the overall failure rate, as defined by revision with arthroplasty, of ACI in a population of young patients with early osteoarthritic changes; (2) describe the functional outcomes seen with this procedure; (3) investigate potential differences in patients requiring concurrent osteotomies; and (4) provide an overview of subsequent surgical procedures that become necessary after ACI.
Our study has several limitations. First, observational case series without a control group are subject to the effects of potential confounding factors that may either obscure a relationship or suggest an association where none actually exists. Second, most of our patients presented to us specifically to avoid or postpone knee arthroplasty; a randomized controlled study comparing cartilage repair with arthroplasty, while important, was therefore not feasible in our patient population. Our patients, on average, had previously undergone between two and three unsuccessful procedures directed at cartilage repair, such as débridement, chondroplasty, or marrow stimulation. They were therefore not agreeable to randomization for a study of ACI versus such techniques, which they had already failed in the past. Third, the indications of ACI and other cartilage repair procedures, especially microfracture, do not overlap, complicating the design of a randomized study: due to its invasiveness, ACI should be reserved for larger lesions above 4 cm2, whereas microfracture is indicated for smaller lesions. We believe, however, that in the absence of randomized data, observational studies can provide important information on outcomes of specific procedures. Further, our study was prospective and the followup data were collected by observers other than the surgeon, we used numerous validated instruments to measure clinical outcomes, the followup response rate was high, and there was minimal variability in treatment technique and rehabilitation protocols due to the single-surgeon experience. Finally, the term osteoarthritis is used to describe a wide spectrum of disease, ranging from localized chondral damage to complete loss of joint space. There is also controversy whether osteoarthritis is a disease affecting the cartilage, or a disease of the entire joint, including the subchondral bone and synovial lining. We chose our definition of early OA based on the radiographic criteria.
Young patients with early arthritic changes present a challenging problem and treatment with joint arthroplasty is frequently recommended. Primary total (TKR) or unicompartmental (UKR) knee arthroplasty provides excellent pain relief with patient satisfaction ranging between 73% and 85% in older patient age groups [5, 11, 28, 48]. Large implant survival studies drawn from joint registry programs have demonstrated 10-year survival rates of 60% to 70% for partial knee arthroplasties and 80% to 90% for total knee arthroplasties [25, 33]. Younger patients seem less satisfied with the outcome and also demonstrate higher implant failure rates [65]. In a report on knee arthroplasty in patients younger than 40 years, a group comparable to the one presented in our study, the authors noticed good and excellent Knee Society function scores in only 50% of patients and a revision rate of 12.5% at 8 years [35]. The subsequent revision surgeries, all but guaranteed within the lifetime of this young patient group, have an even more guarded prognosis: increasingly complex, these procedures result in progressively compromised outcomes [19, 56], and patient satisfaction after revision knee arthroplasty has been reported as low as 59% [57]. Furthermore, prosthesis survival time is lessened: in younger patients (< 65 years), 5-year survival as low as 82% has been reported in a large registry study focused on revision knee arthroplasty [60]. Lastly, revision surgery is extremely costly, with average charges of $73,000 in a recent study [34]. It therefore seems reasonable to attempt delaying arthroplasty as long as possible in young patients, utilizing nonoperative measures such as physical therapy, antiinflammatories and injections, followed by limited surgical measures such as arthroscopic chondroplasty. A subset of patients, however, fails to experience pain relief with these measures and require more invasive interventions, such as cartilage repair with ACI for large, degenerative lesions, as presented here.
Eight percent (12 of 155) of knees treated with ACI met the criteria of treatment failure at an average of 64 months as defined by revision with partial or total knee arthroplasty; six of these patients received payments through worker’s compensation (WC), resulting in a failure rate of 27.3% (six of 22) in that subgroup. ACI in non-WC patients failed at a rate of 4.5% (six of 133). The definition of failure for this study was limited to revision by arthroplasty: patients in this group usually presented to our office to avoid arthroplasty, which the majority had previously been recommended; we therefore wanted to measure our success in avoiding this outcome. We have used a more stringent definition of failure in the past, which includes any open revision surgery, including repeat cartilage repair with ACI or osteochondral allograft. Based on this more stringent definition, our failures would include two additional joints, which were successfully revised with repeat ACI, for a total failure rate of 9% (14 of 155). Bipolar (kissing) lesions have long been considered particularly challenging and are considered a contraindication to ACI by some. Of the 42 knees with bipolar lesions, five failed (12%): two of 30 patellofemoral bipolar lesions, two of six in the medial compartment, and one of six in the lateral compartment.
The large number of patients who had concurrent procedures performed to correct malalignment and maltracking illustrate the prevalence of these comorbidities in this patient population. As indicated in this and other studies, treatment success of ACI is dependent upon the careful detection and treatment of these comorbidities at the time of implantation; for example, we always perform unloading osteotomies in the setting of bipolar lesions. One concern of osteotomies is the potential to compromise the results of subsequent knee arthroplasty. Especially in young patients, the overall burden of surgery should be taken into consideration: it would not be useful to perform an osteotomy with cartilage repair, delaying knee arthroplasty by several years, if by doing so one would decrease the longevity and functional outcome of the arthroplasty, as suggested by early studies [2, 37]. More recently, however, multiple studies have demonstrated clinical outcomes of arthroplasty after tibial osteotomy that are not different from primary arthroplasty, even though the procedure is technically more challenging [31, 32, 40, 41, 58, 64]. The role of ACI in conjunction with realignment osteotomy has been viewed critically by many, who raise the valid question of how much additional benefit the cartilage transplant confers. If the majority of pain relief were derived from osteotomy, rather than ACI, one would expect revision rates of isolated osteotomy to be similar to those of osteotomy with ACI. However, isolated realignment osteotomy has demonstrated conversion rates to arthroplasty that approached 10% to 20% at 5 years and 20% to 50% at 10 years [8, 16, 61, 62], much higher than the reported rates for patients treated with ACI [55]. We therefore believe that while the role of osteotomy is important to normalize joint biomechanics, ACI provides an important clinical benefit above and beyond that of the osteotomy.
At baseline, study patients had considerably lower physical functioning, greater bodily pain, and greater role limitations due to physical problems than a cohort of similarly aged people from the general U.S. population (Fig. 6). Assessments measured with the WOMAC pain, WOMAC function, modified Cincinnati knee rating system, KSS knee, and KSS function scales indicated that patients rated their baseline symptoms and function as poor to fair [6, 21, 36]. With limited treatment options, young osteoarthritic patients are a clinical challenge to treating surgeons. These patients have debilitating symptoms, a long active lifespan, and a strong desire to return to an age-appropriate level of function. We found that patients who were treated with autologous chondrocyte implantation for early osteoarthritis had clinically relevant reductions in pain at minimum of 2 years after treatment. On average, the percentage of patients experiencing severe and extreme pain and difficulty with various tasks including walking, bending and stairs fell by ¾. The findings from this study are consistent with the results reported previously in nonarthritic patient populations [42–44]. Furthermore, results from additional analyses showed that having a high tibial osteotomy or tibial tubercle osteotomy in combination with autologous chondrocyte implantation did not result in different outcomes when compared to treatment with autologous chondrocyte implantation alone.
Sixty-one percent of patients underwent repeat surgery, the majority for periosteum-related complications such as periosteal hypertrophy. This very high reoperation rate will decrease as collagen membranes replace periosteum for defect coverage; randomized trials in Europe have demonstrated reoperation rates of less than 5% with this technique [27]. Importantly, all of these subsequent surgical procedures were arthroscopic, rather than open procedures, and none of the patients undergoing subsequent surgical procedures went on to requiring joint arthroplasty due to failure of ACI.
Our data demonstrate that autologous chondrocyte implantation results in clinically relevant reductions in pain and improvement in function, while apparently delaying the need for knee arthroplasty for over 5 years in 92% of patients. Careful and thorough discussion of the invasive surgical procedure, complex rehabilitation and long recovery, as well as high likelihood of repeat surgery, is paramount to ensure a reasonable level of patient expectations and satisfaction with the outcome. Given the limited treatment options for this subset of patients, ACI may be a plausible treatment for young osteoarthritic patients to delay the need for joint arthroplasty in the hope of obviating subsequent revision surgery, which is associated with much less satisfactory outcomes than primary procedures.
Acknowledgments
We thank Jae Hee Kang, ScD, Harvard School of Public Health, for her assistance with the statistical analyses.
Footnotes
Drs. Minas and Gomoll have received personal and institutional support from Genzyme BioSurgery Inc. (Cambridge, MA).
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Cartilage Repair Center, Brigham and Women’s Hospital and Harvard Medical School, Boston, Mass.
References
- 1.Ahlback S. Osteoarthrosis of the knee: a radiographic investigation. Acta Radiol Diagn (Stockh). 1968;Suppl 277:7–72. [PubMed]
- 2.Amendola A, Rorabeck CH, Bourne RB, Apyan PM. Total knee arthroplasty following high tibial osteotomy for osteoarthritis. J Arthroplasty. 1989;4(Suppl):S11–17. doi: 10.1016/S0883-5403(89)80002-6. [DOI] [PubMed] [Google Scholar]
- 3.Angst F, Aeschlimann A, Michel BA, Stucki G. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. J Rheumatol. 2002;29:131–138. [PubMed] [Google Scholar]
- 4.Aroen A, Loken S, Heir S, Alvik E, Ekeland A, Granlund OG, Engebretsen L. Articular Cartilage Lesions in 993 Consecutive Knee Arthroscopies. Am J Sports Med. 2004;32:211–215. doi: 10.1177/0363546503259345. [DOI] [PubMed] [Google Scholar]
- 5.Baker PN, Meulen JH, Lewsey J, Gregg PJ. The role of pain and function in determining patient satisfaction after total knee replacement. Data from the National Joint Registry for England and Wales. J Bone Joint Surg Br. 2007;89:893–900. doi: 10.1302/0301-620X.89B7.19091. [DOI] [PubMed] [Google Scholar]
- 6.Barber-Westin SD, Noyes FR, McCloskey JW. Rigorous statistical reliability, validity, and responsiveness testing of the Cincinnati Knee Rating System in 350 subjects with uninjured, injured, or anterior cruciate ligament-reconstructed knees. Am J Sports Med. 1999;27:402–416. doi: 10.1177/03635465990270040201. [DOI] [PubMed] [Google Scholar]
- 7.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 8.Billings A, Scott DF, Camargo MP, Hofmann AA. High tibial osteotomy with a calibrated osteotomy guide, rigid internal fixation, and early motion. Long-term follow-up. J Bone Joint Surg Am. 2000;82:70–79. doi: 10.2106/00004623-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 10.Buckwalter JA. Articular cartilage: composition, structure, response to injury, and methods of facilitating repair. In: Ewing J, editor. Articular Cartilage and Knee Joint Function: Basic Science and Arthroscopy. New York, NY: Raven Press; 1990. pp. 19–56. [Google Scholar]
- 11.Bullens PH, Loon CJ, Waal Malefijt MC, Laan RF, Veth RP. Patient satisfaction after total knee arthroplasty: a comparison between subjective and objective outcome assessments. J Arthroplasty. 2001;16:740–747. doi: 10.1054/arth.2001.23922. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention Prevalence of disabilities and associated health conditions among adults-United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50:120–125. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Prevalence of self-reported arthritis or chronic joint symptoms among adults-United States, 2001. MMWR Morb Mortal Wkly Rep. 2002;51:948–950. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention Prevalence of doctor-diagnosed arthritis and possible arthritis-30 states, 2002. MMWR Morb Mortal Wkly Rep. 2004;53:383–386. [PubMed] [Google Scholar]
- 15.Cicuttini F, Ding C, Wluka A, Davis S, Ebeling PR, Jones G. Association of cartilage defects with loss of knee cartilage in healthy, middle-age adults: a prospective study. Arthritis Rheum. 2005;52:2033–2039. doi: 10.1002/art.21148. [DOI] [PubMed] [Google Scholar]
- 16.Coventry MB, Ilstrup DM, Wallrichs SL. Proximal tibial osteotomy. A critical long-term study of eighty-seven cases. J Bone Joint Surg Am. 1993;75:196–201. doi: 10.2106/00004623-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Davies-Tuck ML, Wluka AE, Wang Y, Teichtahl AJ, Jones G, Ding C, Cicuttini FM. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16:337–342. doi: 10.1016/j.joca.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 18.DeBock GH, Kaptein AA, Touw-Otten F, Mulder J. Health-related quality of life in patinets with osteoarthritis in a family practice setting. Arthritis Care Res. 1995;8:88–93. doi: 10.1002/art.1790080206. [DOI] [PubMed] [Google Scholar]
- 19.Deehan DJ, Murray JD, Birdsall PD, Pinder IM. Quality of life after knee revision arthroplasty. Acta Orthop. 2006;77:761–766. doi: 10.1080/17453670610012953. [DOI] [PubMed] [Google Scholar]
- 20.Dexter P, Brandt K. Distribution and predictors of depressive symptoms in osteoarthritis. J Rheumatol. 1994;21:279–286. [PubMed] [Google Scholar]
- 21.Ehrich EW, Davies GM, Watson DJ, Bolognese JA, Seidenberg BC, Bellamy N. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities Osteoarthritis Index Questionnaire and Global Assessments in patients with osteoarthritis. J Rheumatol. 2000;27:2635–2641. [PubMed] [Google Scholar]
- 22.Ethgen O, Vanparijs P, Delhalle S, Rosant S, Bruyere O, Reginster J-Y. Social support and health-related quality of life in hip and knee osteoarthritis. Qual Life Res. 2004;13:321–330. doi: 10.1023/B:QURE.0000018492.40262.d1. [DOI] [PubMed] [Google Scholar]
- 23.Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am. 2004;42:1–9. doi: 10.1016/S0033-8389(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 24.Gillogly SD, Voight M, Blackburn T. Treatment of articular cartilage defects of the knee with autologous chondrocyte implantation. J Orthop Sports Phys Ther. 1998;28:241–251. doi: 10.2519/jospt.1998.28.4.241. [DOI] [PubMed] [Google Scholar]
- 25.Gioe TJ, Novak C, Sinner P, Ma W, Mehle S. Knee arthroplasty in the young patient: survival in a community registry. Clin Orthop Relat Res. 2007;464:83–87. doi: 10.1097/BLO.0b013e31812f79a9. [DOI] [PubMed] [Google Scholar]
- 26.Goldsmith CH, Boers M, Bombardier C, Tugwell P. Criteria for clinically important changes in outcomes: development, scoring and evaluation of rheumatoid arthritis patient and trial profiles. OMERACT committee. J Rheumatol. 1993;20:561–565. [PubMed] [Google Scholar]
- 27.Gooding CR, Bartlett W, Bentley G, Skinner JA, Carrington R, Flanagan A. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: Periosteum covered versus type I/III collagen covered. Knee. 2006;13:203–210. doi: 10.1016/j.knee.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Hawker G, Wright J, Coyte P, Paul J, Dittus R, Croxford R, Katz B, Bombardier C, Heck D, Freund D. Health-related quality of life after knee replacement. J Bone Joint Surg Am. 1998;80:163–173. doi: 10.2106/00004623-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Install JN, Dorr LD, Scott RD, Scott WN. Rationale of the knee society clincial rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 30.Jaeschke J, Singer J, Guyatt G. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 31.Karabatsos B, Mahomed NN, Maistrelli GL. Functional outcome of total knee arthroplasty after high tibial osteotomy. Can J Surg. 2002;45:116–119. [PMC free article] [PubMed] [Google Scholar]
- 32.Kazakos KJ, Chatzipapas C, Verettas D, Galanis V, Xarchas KC, Psillakis I. Mid-term results of total knee arthroplasty after high tibial osteotomy. Arch Orthop Trauma Surg. 2008;128:167–173. doi: 10.1007/s00402-007-0488-3. [DOI] [PubMed] [Google Scholar]
- 33.Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50, 493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79:499–507. doi: 10.1080/17453670710015490. [DOI] [PubMed] [Google Scholar]
- 34.Lavernia C, Lee DJ, Hernandez VH. The increasing financial burden of knee revision surgery in the United States. Clin Orthop Relat Res. 2006;446:221–226. doi: 10.1097/01.blo.0000214424.67453.9a. [DOI] [PubMed] [Google Scholar]
- 35.Lonner JH, Hershman S, Mont M, Lotke PA. Total knee arthroplasty in patients 40 years of age and younger with osteoarthritis. Clin Orthop Relat Res. 2000;380:85–90. doi: 10.1097/00003086-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Luck JV. The lower extremities. In: Cocchiarella L, Anderson GBJ, editors. Guides to the Evaluation of Permanent Impairment. 5. Chicago, IL: American Medical Association; 2001. pp. 546–548. [Google Scholar]
- 37.Madan S, Ranjith RK, Fiddian NJ. Total knee replacement following high tibial osteotomy. Bull Hosp Jt Dis. 2002;61:5–10. [PubMed] [Google Scholar]
- 38.Mankin HJ. Current concepts review. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460–466. [PubMed] [Google Scholar]
- 39.Mankin HJ, Mow VC, Buckwalter JA. Articular cartilage repair and ostearthritis. In: Buckwalter JA, Einhorn TA, Simon SR, editors. Orthopaedic Basic Science: Biology and Biomechanics of the Musculoskeletal System. 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2000. [Google Scholar]
- 40.Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. Clin Orthop Relat Res. 2000;375:175–184. doi: 10.1097/00003086-200006000-00021. [DOI] [PubMed] [Google Scholar]
- 41.Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82:1252–1259. doi: 10.2106/00004623-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Minas T. Autologous chondrocyte implantation for focal chondral defects of the knee. Clin Orthop Relat Res. 2001;391S:S349–361. doi: 10.1097/00003086-200110001-00032. [DOI] [PubMed] [Google Scholar]
- 43.Minas T. Autologous Chondrocyte Implantation in the Arthritic Knee. Orthopedics. 2003;26:945–947. doi: 10.3928/0147-7447-20030901-28. [DOI] [PubMed] [Google Scholar]
- 44.Minas T. Autologous Chondrocyte Implantation in the Osteoarthritic Knee. In: Cole BJ, Malek MM, editors. Articular cartilage lesions: A practical guide to assessment and treatment. New York: Springer; 2004. pp. 105–118. [Google Scholar]
- 45.Minas T, Peterson L. Advanced techniques in autologous chondrocyte transplantation. Clin Sports Med. 1999;18:13–44. doi: 10.1016/S0278-5919(05)70128-9. [DOI] [PubMed] [Google Scholar]
- 46.Moseley JB, O’Malley K, Petersen NJ, Menke TJ, Brody BA, Kuykendall DH, Hollingsworth JC, Ashton CM, Wray NP. A Controlled Trial of Arthroscopic Surgery For Osteoarthritis of the Knee. N Engl J Med. 2002;347:81–88. doi: 10.1056/NEJMoa013259. [DOI] [PubMed] [Google Scholar]
- 47.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, Dragomir A, Kalsbeek WD, Luta G, Jordan JM. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.NIH NIH Consensus Statement on total knee replacement December 8–10, 2003. J Bone Joint Surg Am. 2004;86-A:1328–1335. doi: 10.2106/00004623-200406000-00031. [DOI] [PubMed] [Google Scholar]
- 49.Noyes FR, Barber-Westin SD. Arthroscopic-assisted allograft anterior cruciate ligament reconstruction in patients with symptomatic arthrosis. Arthroscopy. 1997;13:24–32. doi: 10.1016/s0749-8063(97)90206-1. [DOI] [PubMed] [Google Scholar]
- 50.Noyes FR, Barber SD, Mooar LA. A rationale for assessing sports activity levels and limitations in knee disorders. Clin Orthop Relat Res. 1989;246:238–249. [PubMed] [Google Scholar]
- 51.Noyes FR, Barber SD, Simon R. High tibial osteotomy and ligament reconstruction in varus angulated, anterior cruciate ligament-deficient knees: A two to seven year follow-up study. Am J Sports Med. 1993;21:2–12. doi: 10.1177/036354659302100102. [DOI] [PubMed] [Google Scholar]
- 52.Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43:752–757. doi: 10.1302/0301-620X.43B4.752. [DOI] [PubMed] [Google Scholar]
- 53.Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30:2–12. doi: 10.1177/03635465020300011601. [DOI] [PubMed] [Google Scholar]
- 54.Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation - Results at two to ten years. J Bone Joint Surg Am. 2003;85-A:17–24. doi: 10.2106/00004623-200300002-00003. [DOI] [PubMed] [Google Scholar]
- 55.Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212–234. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 56.Rand JA, Trousdale RT, Ilstrup DM, Harmsen WS. Factors affecting the durability of primary total knee prostheses. J Bone Joint Surg. 2003;85:259–265. doi: 10.2106/00004623-200302000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Robertsson O, Dunbar M, Pehrsson T, Knutson K, Lidgren L. Patient satisfaction after knee arthroplasty: a report on 27, 372 knees operated on between 1981 and 1995 in Sweden. Acta Orthop Scand. 2000;71:262–267. doi: 10.1080/000164700317411852. [DOI] [PubMed] [Google Scholar]
- 58.Rozkydal Z, Pink T. Total knee replacement following high tibial osteotomy. Acta Chir Orthop Traumatol Cech. 2003;70:158–163. [PubMed] [Google Scholar]
- 59.Saleh KJ, Arendt EA, Eldridge J, Fulkerson JP, Minas T, Muhall KJ. Symposium. Operative treatment of patellofemoral arthritis. J Bone Joint Surg. 2005;87-A:659–670. [DOI] [PubMed]
- 60.Sheng PY, Konttinen L, Lehto M, Ogino D, Jamsen E, Nevalainen J, Pajamaki J, Halonen P, Konttinen YT. Revision total knee arthroplasty: 1990 through 2002. A review of the Finnish arthroplasty registry. J Bone Joint Surg Am. 2006;88:1425–1430. doi: 10.2106/JBJS.E.00737. [DOI] [PubMed] [Google Scholar]
- 61.Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Am. 2003;85-A:469–474. [PubMed] [Google Scholar]
- 62.Stukenborg-Colsman C, Wirth CJ, Lazovic D, Wefer A. High tibial osteotomy versus unicompartmental joint replacement in unicompartmental knee joint osteoarthritis: 7–10-year follow-up prospective randomised study. Knee. 2001;8:187–194. doi: 10.1016/S0968-0160(01)00097-7. [DOI] [PubMed] [Google Scholar]
- 63.Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, Bombardier C, Felson D, Hochberg M, Heijde Dvd, Dougados M. Evaluation of clinically relevant states in patient reported outcomes in knee and hip osteoarthritis: the patient acceptable symptom state. Ann Rheum Dis. 2005;64:34–37. doi: 10.1136/ard.2004.023028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raaij TM, Bakker W, Reijman M, Verhaar JA. The effect of high tibial osteotomy on the results of total knee arthroplasty: a matched case control study. BMC Musculoskelet Disord. 2007;8:74. doi: 10.1186/1471-2474-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vazquez-Vela Johnson G, Worland RL, Keenan J, Norambuena N. Patient demographics as a predictor of the ten-year survival rate in primary total knee replacement. J Bone Joint Surg Br. 2003;85:52–56. doi: 10.1302/0301-620X.85B1.12992. [DOI] [PubMed] [Google Scholar]
- 66.Ware J, Sherbourne C. The MOS 36-item short form health survey (SF-36): conceptual framework and item selection. Med Care. 1992;30:473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 67.Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. 5. Boston, MA: Health Assessment Lab; 1994. [Google Scholar]
- 68.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual & Interpretation Guide. Boston, MA: The Health Institute; 1997. [Google Scholar]
- 69.Yelin E, Lubeck D, Holman H, Epstein W. The impact of rheumatoid arthritis and osteoarthritis: the activities of patients with rheumatoid arthritis and osteoarthritis compared to controls. J Rheumatol. 1987;14:710–717. [PubMed] [Google Scholar]