Abstract
We have developed a mouse in which the Cre recombinase gene has been targeted to exon 1 of the matrilin-1 gene (Matn1) to investigate the origins of articular chondrocytes and the development of the knee joint. Analysis of joints from offspring of Matn1-Cre/R26R crosses demonstrates that articular chondrocytes are derived from cells that have never expressed matrilin-1, whereas the remainder of the chondrocytes in the cartilage anlagen express matrilin-1. A band of chondrocytes adjacent to the developing interzone in the E13.5 day knee joint becomes apparent because these chondrocytes do not turn on expression of matrilin-1 along with all the other chondrocytes of the anlagen. Articular chondrocytes therefore appear to arise directly from a subpopulation of early chondrocytes that do not activate matrilin-1 expression rather than by redifferentiation from the flattened cells of the interzone. In addition, lineage tracing using both Matn1-Cre/R26R and Col2a1-Cre/R26R lines indicate that non-cartilaginous structures in the knee such as cruciate ligament, synovium and some blood vessels are formed by cells derived from the early chondrocytes of the anlagen.
Keywords: articular chondrocytes, matrilin-1, collagen II, linage tracing, cre recombinase, joint development, knee development
INTRODUCTION
Synovial joints are complex structures comprised of several tissues including articular cartilage, bone, ligament and synovium. During early development mesenchymal cells undergo a condensation process, switch on collagen II expression and form a cartilaginous model of the future skeletal elements. These intermediate structures are later largely replaced by mineralised bone in a process known as endochondral ossification. In the hind limb the early condensation appears as a continuous Y-shaped structure, in which the ‘arms’ of the ‘Y’ give rise to the tibia and fibula and the ‘shaft’ gives rise to the femur (Hinchcliffe and Johnson 1980). The knee joint forms at the junction of the “arms” and the “shaft” dividing the condensation into 3 separate skeletal elements. Currently it is proposed that a secondary remodelling event occurs in the presumptive joint region (Archer et al. 2003), where the early chondrocytes change shape becoming thin, elongated, and closely associated; the change in cell morphology coincides with a switch from collagen II to collagen I expression (Craig et al. 1987, Nalin et al. 1995). This area of remodelling is known as the interzone; it is the first histological sign of joint development and provides a clear delineation between the cartilaginous elements of the presumptive tibia and femur. As well as being apparent histologically, the interzone has been defined by the expression of several markers including Wnt9a (Hartmann and Tabin, 2001) and growth and differentiation factor 5 (Gdf5) mutations in which were shown to cause brachypodism in the mouse (Storm et al. 1994).
The exact role that the interzone plays in joint development is not entirely clear but it is believed to give rise to some joint structures. Ito and Kida (2000) carried out a detailed ultrastructural study of joint formation in the rat knee and concluded that articular chondrocytes are derived from the elongated cells of the interzone, which disperse, recover from their flattened state, and form the articular cartilage. More recently, lineage tracing has revealed that articular chondrocytes of the interphalangeal joints in mice are derived from cells that have expressed Gdf5 (Rountree et al. 2004) further supporting the hypothesis that articular chondrocytes arise by redifferentiation from the interzone cells. Others however, have suggested that articular chondrocytes develop from the outer chondrogenous layers of the interzone based on the presence of collagen V around both these cells and mature articular chondrocytes (Bland and Ashhurst 1996).
Articular chondrocytes persist throughout life and maintain the articular cartilage whereas ‘epiphyseal’ chondrocytes lay down the cartilaginous anlagen but are subsequently consumed by the endochondral ossification process. A marker that clearly distinguishes these two cell types is matrilin-1 which is expressed by epiphyseal chondrocytes but not by articular chondrocytes (Aszodi et al. 1994, Murphy et al 1999). To address the origin of articular chondrocytes we have generated a matrilin-1-Cre (Matn1-Cre) knock-in mouse, where the Cre recombinase gene is expressed under the control of the matrilin-1 promoter. The Matn1-Cre mouse was then crossed with mice carrying the floxed ROSA26 reporter transgene (R26R) (Mao et al. 1999). This mouse has a lacZ gene inserted downstream of the ROSA26 house-keeping gene that is flanked by loxP sites so that when Cre recombinase is expressed the ROSA26 gene is excised and lacZ is expressed under the control of the ROSA26 promoter (Mao et al. 1999). The generation of this mouse model has allowed us to lineage trace cells that during their history have expressed matrilin-1 and to compare these findings with those obtained with a previously generated Col2a1-Cre mouse model (Sakai et al. 2001). The findings of this analysis are reported here and provide new insights into the origin of articular chondrocytes and associated tissues within the synovial joint.
MATERIALS AND METHODS
Gene targeting
To prepare the targeting construct, the Cre recombinase gene was inserted into exon 1 of the matrilin-1 gene (Fig 1A) in the process destroying the endogenous ATG start codon.
Figure 1.
(A) Generation of Matn1-Cre DNA targeting construct. The Cre gene and floxed neotk cassette were inserted into the first exon of the matrilin-1 gene by homologous recombination in ES cells. (B) Southern analysis of DNA isolated from G418 resistant ES clones probed with the external probe. The middle track is from a homologously recombined ES clone the exhibits both the wt 12 kb and homologously recombined 3 kb alleles. (C) In situ hybridisation of E15.5 knee sections for Cre (a), matrilin-1 (b) and α1(II) collagen (c) all developed for 48 hours.
The linearised construct was electroporated into mouse R1 ES cells, homologously recombined clones identified by Southern blotting, microinjected into C57Bl6 blastocysts and transferred to psuedopregnant foster mothers. Chimeric male offspring were then bred with C57Bl6 females and agouti offspring genotyped by Southern blot (Fig 1B) (Talts et al. 1999). Transgenic lines were then established by breeding to homozygosity. Male mice homozygous for the Matn1-Cre locus were bred with females homozygous for the ROSA26 reporter locus (R26R) producing offspring heterozygous for both loci. These offspring were sacrificed at a range of developmental stages and the knee joints analysed. A Collagen II-Cre mouse (Col2a1-Cre, Sakai et al. 2001) was also used as described above.
Detection of β-galactosidase (lacZ) activity
lacZ activity was detected as described previously (Sakai et al. 2001). In brief, mouse limbs and embryos were fixed for 1 h with 0.2% (v/v) gluteraldehyde, 5 mM EGTA, 2mM MgCl2 in 0.1 M sodium phosphate buffer pH 7.3. and washed three times for 30 minutes in a detergent solution containing 2 mM MgCl2, 0.2% (v/v) NP40 and 0.1% (w/v) sodium deoxycholate in 0.1 M sodium phosphate buffer. Staining was performed at room temperature for 24 hours in the detergent solution supplemented with 1 mg/ml X-gal, 5 mM potassium ferrocyanide and 5 mM potassium ferricyanide. After staining the embryos were washed with PBS and stored in 4% (v/v) formaldehyde at 4°C. For histological examination, embryos/limbs were dehydrated, embedded in paraffin and used to generate 7 μm thick sections, which were counterstained with eosin.
Probes and in situ hybridization
The Gdf5 probe was a kind gift from C. Hartmann (IMP, Vienna). The α1(II) Collagen probe (Col2a1) was a 600 bp insert encoding 3′ UTR from I.M.A.G.E clone #735113 subcloned into the pT7T3 vector. The Matrilin-1 probe (Matn1) was a 500 bp fragment of the 3′ UTR from I.M.A.G.E clone #660961 in the pT7T3 vector. The Cre probe was a 700bp fragment of the gene also in the pT7T3 vector. Mouse embryos were harvested at E13.5 - E15.5 days and fixed overnight in 4% (w/v) paraformaldehyde (PFA) in PBS at 4°C, washed and dehydrated through an ethanol series into 100% ethanol and then embedded in 100% paraffin. 7 μm sections were cut from the blocks and dried onto glass slides overnight at 37°C.
Probes were synthesised from linearised DNA using DIG RNA labelling mix (Roche) and RNA precipitated using 0.1M LiCl, 5mM EDTA, 75% ethanol (v/v) in DEPC-treated water. Tissue sections were prepared for hybridization by dewaxing in xylene and then rehydrating through an ethanol series into PBS prepared in DEPC-treated water.
Sections were fixed in 4% (w/v) PFA/PBS for 10 minutes then treated with 1 μg/ml proteinase K for 10 minutes prior to being re-fixed in 4% (w/v) PFA/PBS. Sections were given three 5 minute washes in PBS, 0.1% (v/v) Tween DEPC treated (PBT), between each step. Finally the sections were treated with 0.0025% (v/v) acetic anhydride in 0.1M triethanolaminehydrochloride for 15 minutes, washed in PBT and allowed to dry at room temperature.
Hybridization was carried out in a hybridization buffer (10 mM Tris pH7.5, 600 mM NaCl, 1 mM EDTA, 0.25% (w/v) SDS, 10% (w/v) dextran sulphate, 1 × Denhardt’s, 50% (v/v) formamide and 200 μg/ml yeast tRNA). 1 μl of DIG-labelled probe was added per 100 μl of hybridisation solution and heated to 85°C for 3 minutes before adding to the tissue sections. Sections were hybridised overnight at 65°C. The following post-hybridization washes were carried out before antibody detection: 1× SSC (20× SSC: 3M NaCl, 0.6M tri sodium citrate, pH7.0) /50% formamide (v/v) in water for 30 minutes at 65°C, TNE (10mM Tris/HCl, 0.5M NaCl, 1mM EDTA, pH 7.5) for 10 minutes at 37°C, RNase A (20μg/ml) in TNE for 30 minutes at 37°C, TNE for 10 minutes at 37°C, 2× SSC for 20 minutes at 65°C and finally, 2 washes in 0.2× SSC for 20 minutes at 65°C. Sections were then given two 5 minute washes in MABT (100mM maleic acid, 150mM NaCl, 0.2% (v/v) Tween, pH7.5) prior to been blocked for 1 hour at room temperature in 20% (v/v) heat inactivated sheep serum (HISS) in MABT. Antibody detection was carried out using α-DIG-AP (Roche) diluted 1:2000 in 2% (v/v) HISS in PBS overnight at 4°C. The antibody was visualized using BCIP/NBT (Sigma) in the dark at room temperature for 1-3 days.
RESULTS
Generation of Matrilin-1-Cre transgenic mice
360 G418-resistant ES cell clones were isolated after electroporation of the construct (Fig. 1A) of which in excess of 20% were found to be homologously recombined by Southern analysis due to the presence of the 3 kb recombinant as well as 12 kb wild type band (Fig. 1B). Two independent homologously recombined ES cell clones were used to generate germ-line chimeras. Offspring from both lines were crossed with a deletor-cre mouse line to delete the floxed neo selection cassette.
Analysis of Cre activity in Matn1-Cre /R26R mice
To test for accurate spatial expression of Cre recombinase from the targeted Matrilin-1 allele In situ localisation of Cre, Matn1 and Col2a1 was carried out on serial sections of an E15.5 Matn1-Cre heterozygous murine knee. The spatial expression of Cre and endogenous Matn1 matches, as can be seen in Fig. 1C. To further investigate Cre recombinase expression from the Matrillin-1 allele, Matn1-Cre transgenic mice were crossed with ROSA26 reporter mice (R26R) and the expression of lacZ examined in offspring. The two independently targeted lines of Matn1-Cre mice gave indistinguishable results (data not shown). Expression of Matn1-Cre was not detectable at E12.5 days but was apparent by E13.5 (Fig. 2A). LacZ expression as a result of the Matn1-Cre allele activity was limited to the cartilaginous anlagen of the developing skeleton. At E13.5, the upper limb stained along its complete length whereas in the lower limb, staining distal to the fibula and tibia was not apparent. The skeletal pattern of expression for Matn1-Cre was similar to that of Col2a1-Cre (Fig 2B) but less intense and did not extend into the cartilaginous elements of the tail or, as described above, the foot. Furthermore, whereas the staining for lacZ activity appeared continuous through the long-bone anlagen of the developing limbs in the Col2a1-Cre mice, the lacZ expression in Matn1-Cre mice was interrupted at sites of presumptive joint formation such as the developing digits and knee (see Fig. 2 inset).
Figure 2.
β-galactosidase staining indicating Cre activity in whole-mount E13.5 embryos. Cre gene expression driven by either the endogenous matrilin-1 (Matn1-Cre/R26R) or transgenic collagen II (Col2a1-Cre/R26R) promoter.
Articular chondrocytes have never expressed matrilin-1
Examination of knee joints from new born, 1 and 3 week old Matn1-Cre/R26R animals revealed that in contrast to epiphyseal chondrocytes the articular chondrocytes did not stain for β-galactosidase activity (Fig. 3) whereas all of the chondrocytes including the articular chondrocytes in the Col2a1-Cre/R26R knee joint were positive (Fig. 3). Furthermore, upon formation of the secondary centre of ossification between 1 and 3 weeks of age, the lacZ-expressing Matn1-Cre positive epiphyseal chondrocytes were replaced by bone whereas the Matn1-Cre negative chondrocytes remained as the articular chondrocytes (Fig. 3F). The few Matn1-Cre positive chondrocytes which are present at 3 weeks of age are located at the ossification front or at the very periphery of the joint. Articular chondrocytes can therefore be distinguished from ‘epiphyseal’ chondrocytes, which are destined to be replaced by bone during endochondral ossification, on the basis that the former have a collagen II positive but matrilin-1 negative expression history whereas the latter are positive for both.
Figure 3.
β-galactosidase staining indicating cells that have expressed Cre activity from either the matrilin-1 or collagen II promoter in mouse knee joints: (A) New born Col2a1-Cre/R26R (B) New born Matn1-Cre/R26R (C) 1 wk Col2a1-Cre/R26R (D) 1 wk Matn1-Cre/R26R (E) 3 wk Col2a1-Cre/R26R (F) 3 wk Matn1-Cre/ R26R. F = Femur, AC = Articular Cartilage.
Articular chondrocytes are apparent at E13.5 days as the interzone forms
We examined how early in development we could detect collagen II-positive matrilin-1 negative chondrocytes in regions destined to be articular cartilage. Since E13.5 was the earliest time at which Matn-1 expression could be detected by way of Cre recombinase activity (see Fig 2) we sectioned β-galactosidase stained knee joints from E13.5 day Matn1-Cre/R26R embryos. At E13.5 days, the interzone first becomes apparent as a flattened zone of cells intervening between two populations of round chondrocytes (see Fig. 4B). β-galactosidase staining around the Matn1- Cre/R26R presumptive knee joint was never detected in chondrocytes immediately adjacent to the forming interzone but was restricted to chondrocytes several cell diameters removed from the interzone. In contrast, β-galactosidase staining in the equivalent Col2a1-Cre/R26R sections revealed that all the cells stained strongly. (Fig. 4A). In situ localisation of Matn1 and Col2a1 (Fig. 4C & D, respectively) provided corroborating evidence for the presence of matrilin-1 negative, collagen II positive chondrocytes adjacent to the forming interzone in the E13.5 day knee joint. Comparison of β-galactosidase staining in the Col2a1-Cre/R26R sections (Fig. 4A) with Col2a1 In situ localisation (Fig. 4D) confirms that the interzone cells are no longer expressing Col2a1, however they stain positive for β-galactosidase due to their expression of the gene prior to their dedifferentiation.
Figure 4.
β-galactosidase staining indicating Cre activity in E13.5 mouse knee sections from Col2a1-Cre/R26flox (A) and Matn1-Cre/R26R (B) mice. In situ localisation of matrilin-1 (C) and collagen II (D) in E13.5 mouse knee sections. AC = articular chondrocytes, EC = epiphyseal chondrocytes., IZ = interzone.
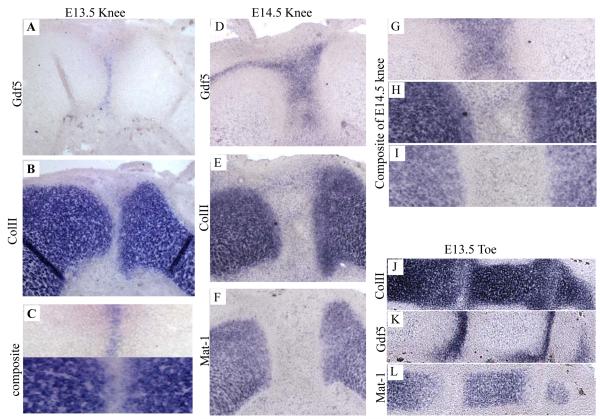
In situ localisation of Gdf5 and Col2a1 was undertaken to verify the exact location of the boundary between the interzone and cartilage. In the E13.5 knee joints, Gdf5 expression was limited to the region of flattened cells intervening between the presumptive articular surfaces as defined by the Col2a1-expressing chondrocytes (Fig. 5 A-C). This was also the case in E14.5 knee joints where a clear band of Matn1 negative, Col2a1 positive cells can be seen adjacent to the Gdf5 expressing interzone cells (Fig. 4D-G).
Figure 5.
Expression of Gdf5, α1(II) Collagen and Matrilin-1 in the developing knee joint. In Situ hybridisation of E13.5 knee sections for Gdf5 (A) and Col2a1 (B), high power comparison (C). In Situ hybridisation of E14.5 knee sections for Gdf5 (D) and Col2a1 (E) and matrilin-1 (F) high power comparison (G′-G′′′). In Situ hybridisation of E13.5 toe sections for Col2a1 (H) and Gdf5 (I) Matrilin-1 (J).
Joint development occurs in a proximal to distal order with the joints of the hind limbs forming later than those of the forelimbs. Therefore, to investigate the earliest stage of joint development when matrilin-1 is expressed we analysed the interphalangeal joints of the E13.5 day murine foot. Even at this early stage of joint development it is clear that the Matrilin-1 negative band of cells is much wider than the Gdf5 positive band, again confirming the presence of Matrilin-1 negative, col2a1 positive cells flanking the interzone (Fig. 4H-J).
Non-cartilaginous tissues of the mature knee joint are derived from the chondrocytes that formed the original anlagen
Examination of developed knee joints from 1 week old Col2a1-Cre/R26R mice revealed that virtually all of the cells within the tissues of the synovial joint stained for β-galactosidase (Fig. 6). This is particularly noteworthy for the cells of non-cartilaginous tissues such as cruciate ligament and synovium and for the smooth muscle and endothelial cells of arterioles perfusing the extremities of the meniscal cartilage. These cells did not stain for β-galactosidase activity in the age-matched Matn1-Cre/R26R mouse (Fig. 6). It should also be noted that Col2a1 expression is not a common feature of blood vessel development, and that no β-galactosidase positive cells were found in the blood vessels of the spleen, heart, kidney or muscle of 3 week old Col2a1-Cre/R26R mice (data not shown). This suggests that tissues such as ligament, synovium and even blood vessels within the joint have been derived from the early chondrocytes of the skeletal anlagen.
Figure 6.
β-galactosidase staining indicating Cre activity in a section of whole-mount stained 1 week Col2a1-Cre/R26R embryo knee (A). High power image of cruciate ligament seen in A (B). Blood vessels (arrow) profusing peripheral region of meniscal cartilage (C). High power image of β-galactosidase stained blood vessel in periphery of meniscal cartilage (D). High power image of β-galactosidase-negative blood vessel from surrounding muscle (E). Image of β-galactosidase stained blood vessel (arrow) perfusing muscle adjacent to the perichondrium (F). Equivalent section to (A) from Matn1-Cre/R26R embryo knee, β-galactosidase showing staining limited to the epiphyseal chondrocytes in this case (G).
DISCUSSION
Although the destruction of articular cartilage in diseases like osteoarthritis affect a large percentage of the population, relatively little is known about how articular cartilage develops and is maintained. Here we have generated a Matrilin-1-Cre transgenic mouse line, which was crossed with the R26R indicator mouse strain to trace the lineage of matrilin-1 expressing cells. Matrilin-1 is an extracellular matrix protein which has been shown by immunohistochemical and in situ localisation to be expressed in the cartilage of the developing skeletal anlagen with the notable exception of the articular cartilage (Aszodi et al. 1994, Murphy et al 1999). The chondrocyte-based expression of Cre (Fig. 1C) correlates with In situ localisation of endogenous matrilin-1 on serial sections, indicting that the Matn1-Cre allele is regulated in a similar manner to the wild-type matrillin-1 gene. We demonstrate here that articular chondrocytes are distinguishable from ‘epiphyseal’ chondrocytes of the developing joint by the fact that the articular chondrocytes have never expressed matrilin-1 (as shown by their lack of β-galactosidase activity in the Matn1-Cre/R26R mouse) whereas the epiphyseal chondrocytes have expressed matrilin-1 (Fig. 3). In contrast, all chondrocytes within the developing joint stain for β-galactosidase activity in the Col2a1-Cre/R26R mouse (Fig. 3). By 3 weeks of age, virtually all of the matrilin-1 positive chondrocytes of the femur and tibia, apart from those in the growth plates and at the ossification front, have been ablated and replaced by the bone and marrow leaving only the matrilin-1 negative, collagen II positive chondrocytes of the articular cartilage (Fig. 3).
Not all chondrocytes within the epiphyseal region of the joint stain positive for β-galactosidase activity in the Matn1-Cre/R26R mice (Figs 2 and 3). We have shown that pattern of expression of the Cre transcript from the matrilin-1 allele matches matrillin-1 (Fig. 1C). However, it was consistently more difficult to visualise Cre compared to matrilin-1 expression by In situ hybridisation (Fig. 1C, compare a and b) despite the fact that the Cre probe was 28% longer than the matrilin-1 probe and that the sections were developed for the same length of time. We therefore believe that the steady-state level of Cre mRNA (and by implication Cre protein) is considerably reduced compared to matrilin-1 and that this accounts for not all cells ablating their floxed allele and expressing β-galactosidase. In addition, it should be noted that whilst matrilin-1 expression distinguishes articular from ‘epiphyseal’ chondrocytes, matrilin-1 itself is not essential for either the formation of joints or the articular cartilage since these tissues form normally in the matrilin-1 knockout mouse (Aszodi et al. 1999; Huang et al. 1999).
The molecular distinction between the permanent chondrocytes of the articular cartilage and the transient chondrocytes of the remainder of the cartilaginous skeletal anlagen led us to examine how early in development articular chondrocytes could first be identified based on their distinctive matrilin-1 and collagen II expression patterns. Articulating joints develop across a continuous anlagen and eventually segment the cartilage and lead to the development of the different tissues of the joint such as the synovium, ligaments and, in the case of the knee, the meniscus (Archer et al. 2003, Pacifici et al. 2005). The first histological evidence of joint formation, where chondrocytes of the anlagen flatten out and form the spindle-shaped cell layer known as the interzone, is apparent at E13.5 dpc in the developing mouse knee (see Fig. 4). This coincides with the appearance of matrilin-1 expression, based on Cre-mediated induction of β-galactosidase from the targeted ROSA26 reporter locus in the Matn1-Cre/R26R mouse (Fig. 2). As matrilin-1 expression becomes up-regulated, a zone of chondrocytes adjacent to the interzone become apparent that are entirely negative for matrilin-1 expression yet, positive for collagen II expression in equivalent sections from the Col2a1-Cre/R26R mouse (Fig. 4). This zone of matrilin-1-negative, collagen II-positive chondrocytes is apparent at all subsequent stages of knee joint development, at first adjacent to the differentiating interzone (Figs 4 and 5), during cavitation at E15.5 (Fig.1C), and after cavitation, within and defining the articular cartilage (e.g. Fig. 3 and Aszodi et al. 1994; 1999).
As Gdf5 has been shown by lineage tracing to have been expressed by articular chondrocytes of the interphalangeal joints (Rountree et al. 2004) we performed in situ hybridisation to ascertain in the developing knee precisely how Gdf5 expression relates to the interzone and the adjacent chondrocytes that express collagen II but not matrilin-1 (Fig. 5). Gdf5 expression in both the E13.5 and E14.5 day developing knee joints was restricted to the non-collagen II expressing cells of the interzone. Furthermore, in the interphalangeal joints of the E13.5 day murine foot (the earliest stage of joint development when matrilin-1 is expressed) a band of matrilin-1 negative chondrocytes can be seen adjacent to the Gdf5 expressing cells. This demonstrates that even at this early stage of joint development articular chondrocytes can be distinguished, but do not express Gdf5. However, it has been previously reported (Storm et al. 1999, Francis-west et al. 1999) that at earlier stages of joint development Gdf5 expression is broader and more diffuse. Lineage tracing using Gdf5 is therefore likely to be following the fate of chondrocytes that are in the vicinity of the developing joint rather than just cells that are derived solely from the interzone. This would explain the large pool of β-galactosidase expression evident in the region of the E14.5 day knee joint of the previously reported Gdf5-Cre/R26R mouse (Rountree et al. 2004).
The results presented here demonstrate that as the interzone becomes apparent, a population of articular chondrocytes is distinguishable from ‘epiphyseal’ chondrocytes based on matrilin-1 expression. Therefore, articular chondrocytes do not develop from the dispersal of the flattened interzone cells (Ito & Kido, 2000) (although some of these cells may be subsequently recruited into the developing articular cartilage) but arise directly from a sub-population of the early chondrocytes of the anlagen, as suggested by Bland & Ashurst (1996). This does not prove that articular chondrocytes have an independent precursor population to the rest of the chondrogenic anlagen. However, it does demonstrate that the articular chondrocytes have become distinct from the rest of the chondrogenic anlagen, at the latest by E13.5 days, when the joint is in the initial stages of formation.
Examination of the non-cartilaginous tissues such as cruciate ligament and synovium of the fully formed knee joints in the Col2a1-Cre/R26R and Matn1-Cre/R26R mice revealed that all of these tissues had an expression history that was collagen II positive and matrilin-1 negative. We therefore conclude in agreement with other lineage tracing experiments (Rountree et al. 2004) that these non-cartilaginous tissues have arisen from early chondrocytes of the anlagen that formed the interzone and articular cartilage prior to the expression of matrilin-1. In addition, it is noteworthy that arterioles perfusing the peripheral regions of the meniscal cartilage and some of the vessels perfusing muscle adjacent to the perichondrium also stained heavily for β-galactosidase in the Col2a1-Cre/R26R mice (Fig. 6). This staining was not a generalised feature of blood vessels (Fig. 6E and G) and was not detectable in the blood vessels of the spleen, heart, kidney or muscle of 3 week old Col2a1-Cre/R26R mice (data not shown). While it is possible that these vascular cells expressed Col2a1 independently of the chondrogenic anlagen, the fact that β-galactosidase staining is not a common feature of blood vessels in the Col2a1-Cre/R26R mice, and that it is only seen in vessels in close proximity to the chondrogenic anlagen, suggests that early chondrocytes are multipotent and are capable of subsequently transforming not only into ligament fibroblasts and synoviocytes but also vascular cells.
In conclusion, the demonstration that permanent articular chondrocytes can be distinguished from the transient ‘epiphyseal’ chondrocytes of the anlagen based on matrilin-1 expression is of significance. In osteoarthritis, matrilin-1 expression by articular chondrocytes has been described previously (Okimura et al. 1997). In our own studies of idiopathic OA in the guinea pig, the expression of matrilin-1 by articular chondrocytes prior to any histological evidence of damage is a prominent finding suggesting that a phenotypic switch from an articular to an epiphyseal chondrocyte may be a significant step in disease pathogenesis (Meziane, Boot-Handford and Wallis, manuscript submitted)
ACKNOWLEDGEMENTS
R Fässler is thanked for hosting RBH on research leave and providing training and expertise in gene targeting techniques. This work was supported by the Wellcome Trust and Arthritis Research Campaign.
REFERENCES
- Archer CD, Dowthwaite GP, Francis-West PH. Development of synovial joints. Birth Defects Research. (Part C) 2003;69:144–155. doi: 10.1002/bdrc.10015. [DOI] [PubMed] [Google Scholar]
- Aszodi A, Modis L, Paldi A, Rencendorj A, Kiss I, Bosze Z. The Zonal expression of chicken cartilage matrix protein gene in the developing skeleton of transgenic mice. Mat. Biol. 1994;14:181–190. doi: 10.1016/0945-053x(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Aszodi A, Beier DR, Hiripi L, Bosze Z, Fassler R. Sequence, structure and chromosomal localization of crtm gene encoding mouse cartilage matrix protein and its exclusion as a candidate for murine achondroplasia. Mat. Biol. 1997;16:563–573. doi: 10.1016/s0945-053x(98)90067-1. [DOI] [PubMed] [Google Scholar]
- Aszodi A, Bateman JF, Hirsch E, Baranyi M, Hunziker EB, Hauser N, Bosze Z, Fassler R. Normal skeletal development of mice lacking matrilin 1: redundant function of matrilins in cartilage? Mol Cell Biol. 1999;19:7841–5. doi: 10.1128/mcb.19.11.7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland YA, Ashhurst DE. Development and ageing of the articular cartilage of the rabbit knee joint: distribution of fibrillar collagens. Anat. Embryol. 1996;194:607–619. doi: 10.1007/BF00187473. [DOI] [PubMed] [Google Scholar]
- Craig FM, Bentley G, Archer CW. The spatial and temporal pattern of collagens I and II and keratin sulphate in the developing chick metatarsophalangeal joint. Dev. 1987;99:383–391. doi: 10.1242/dev.99.3.383. [DOI] [PubMed] [Google Scholar]
- Francis-West PH, Abdelfattah A, Chen P, Allen C, Parish J, Ladher R, Allen S, MacPherson S, Luyten FP, Archer CW. Mechanisms of Gdf5 action during skeletal development. Dev. 1999;126:1305–1315. doi: 10.1242/dev.126.6.1305. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001;104:341–351. doi: 10.1016/s0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- Hinchliffe JR, Johnson DR. The development of the vertebrate limb. Oxford university press; Oxford: 1980. [Google Scholar]
- Huang X, Birk DE, Goetinck PF. Mice lacking matrilin-1 (cartilage matrix protein) have alterations in type II collagen fibrillogenesis and fibril organization. Dev. Dyn. 1999;216:434–41. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<434::AID-DVDY11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Ito MM, Kido MY. Morphological and biochemical re-evaluation of the process of cavitation in the rat knee joint: cellular and cell strata alterations in the interzone. J. Anat. 2000;197:659–679. doi: 10.1046/j.1469-7580.2000.19740659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc. Natl. Acad. Sci. USA. 1999;96:5037–5042. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Heinegard D, McIntosh A, Sterchi D, Barry FP. Distribution of cartilage molecules in the developing mouse joint. Mat. Biol. 1999;18:487–497. doi: 10.1016/s0945-053x(99)00042-6. [DOI] [PubMed] [Google Scholar]
- Nalin AM, Greenlee TK, Jr, Sandell LJ. Collagen gene expression during development of avian synovial joints: Transient expression of types II and XI collagen genes in the joint capsule. Dev. Dyn. 1995;203:352–362. doi: 10.1002/aja.1002030307. [DOI] [PubMed] [Google Scholar]
- Okimura A, Okada Y, Makihira S, Pan H, Yu L, Tanne K, Imai K, Yamada H, Kawamoto T, Noshiro M, Yan W, Kato Y. Enhancement of cartilage matrix protein synthesis in arthritic cartilage. Arthritis Rheum. 1997;40:1029–1036. doi: 10.1002/art.1780400606. [DOI] [PubMed] [Google Scholar]
- Pacifici M, Koyama E, Iwamoto M. Mechanisms of synovial joint and articular cartilage formation: Recent advances, but many lingering mysteries. Birth Defects Research (Part C) 2005;75:237–248. doi: 10.1002/bdrc.20050. [DOI] [PubMed] [Google Scholar]
- Rountree RB, Schoor M, Chen H, Marks ME, Harley V, Mishina Y, Kingsley DM. BMP receptor signalling is required for postnatal maintenance of articular cartilage. PLoS Biology. 2004;2:1815–1827. doi: 10.1371/journal.pbio.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Hiripi L, Glumoff V, Brandau O, Eerola R, Vuorio E, Bosze Z, Fassler R, Aszodi A. Stage - and tissue-specific expression of a Col2a1-Cre fusion gene in transgenic mice. Mat. Biol. 2001;19:731–767. doi: 10.1016/s0945-053x(00)00122-0. [DOI] [PubMed] [Google Scholar]
- Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley DM, Lee S. Limb alterations in brachypodism mice due to mutations in a new member of the TGFβ-superfamily. Nature. 1994;368:639–643. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- Storm EE, Kingsley DM. GDF5 coordinates bone and joint formation during digit development. Dev. Biol. 1999;209:11–27. doi: 10.1006/dbio.1999.9241. [DOI] [PubMed] [Google Scholar]
- Talts JF, Brakebusch C, Fässler R. Integrin gene targeting. Meth. Mol. Biol. 1999;129:153–187. doi: 10.1385/1-59259-249-X:153. [DOI] [PubMed] [Google Scholar]