Abstract
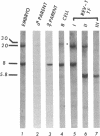
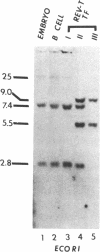
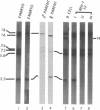
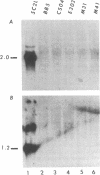
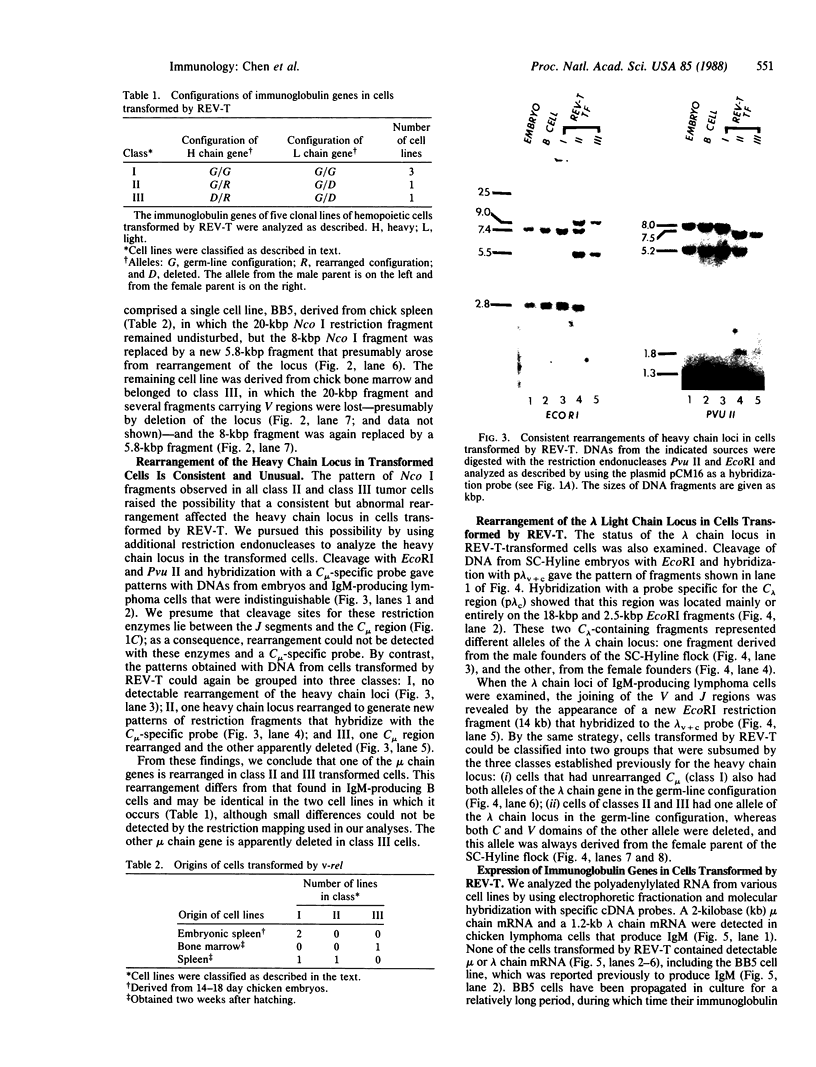
The retroviral oncogene v-rel transforms poorly characterized lymphoid cells. We have explored the nature of these cells by analyzing the configuration and expression of immunoglobulin genes in chicken hemopoietic cells transformed by v-rel. None of the transformed cells expressed their immunoglobulin genes. The cells fell into three classes: class I cells have their immunoglobulin genes potentially in an embryonic configuration; class II and class III cells have lost one copy of the lambda light chain locus and have one copy of the heavy chain locus rearranged into a configuration that differs from what is found in mature B cells. In class II cells, the other heavy chain locus may be in embryonic configuration, whereas it is deleted in class III cells. The first of these classes may represent the earliest stage of the lymphoid lineage yet encountered among virus-transformed cells, whereas the second and third classes represent an apparently anomalous rearrangement whose origin remains unknown.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Gerondakis S., Webb E., Corcoran L. M., Cory S. Cellular myc oncogene is altered by chromosome translocation to an immunoglobulin locus in murine plasmacytomas and is rearranged similarly in human Burkitt lymphomas. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1982–1986. doi: 10.1073/pnas.80.7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandrini A., Pierce J. H., Baltimore D., Desiderio S. V. Continuing rearrangement of immunoglobulin and T-cell receptor genes in a Ha-ras-transformed lymphoid progenitor cell line. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1799–1803. doi: 10.1073/pnas.84.7.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., Rosenberg N., Enea V., Siden E., Baltimore D. Multiple immunoglobulin heavy-chain gene transcripts in Abelson murine leukemia virus-transformed lymphoid cell lines. Mol Cell Biol. 1982 Apr;2(4):386–400. doi: 10.1128/mcb.2.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., Müller H., Grieser S., Doederlein G., Graf T. Hematopoietic cells transformed in vitro by REVT avian reticuloendotheliosis virus express characteristics of very immature lymphoid cells. Virology. 1981 Dec;115(2):295–309. doi: 10.1016/0042-6822(81)90112-4. [DOI] [PubMed] [Google Scholar]
- Chen I. S., Wilhelmsen K. C., Temin H. M. Structure and expression of c-rel, the cellular homolog to the oncogene of reticuloendotheliosis virus strain T. J Virol. 1983 Jan;45(1):104–113. doi: 10.1128/jvi.45.1.104-113.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. C., Courtneidge S. A., Bishop J. M. Immunological phenotype of lymphomas induced by avian leukosis viruses. Mol Cell Biol. 1983 Jun;3(6):1077–1085. doi: 10.1128/mcb.3.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D., Balaton A. M. T-cell receptor and immunoglobulin genes are rearranged together in Abelson virus-transformed pre-B and pre-T cells. Mol Cell Biol. 1987 Jan;7(1):266–272. doi: 10.1128/mcb.7.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Purchase H. G., Bockman D. E., Gathings W. E. Studies on the nature of the abnormality of B cell differentiation in avian lymphoid leukosis: production of heterogeneous IgM by tumor cells. J Immunol. 1974 Oct;113(4):1210–1222. [PubMed] [Google Scholar]
- Dahan A., Reynaud C. A., Weill J. C. Nucleotide sequence of the constant region of a chicken mu heavy chain immunoglobulin mRNA. Nucleic Acids Res. 1983 Aug 25;11(16):5381–5389. doi: 10.1093/nar/11.16.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. B., Maldonado R. L., Bose H. R. Isolation and characterization of reticuloendotheliosis virus transformed bone marrow cells. Intervirology. 1974;3(5-6):342–352. doi: 10.1159/000149771. [DOI] [PubMed] [Google Scholar]
- Gilmore T. D., Temin H. M. Different localization of the product of the v-rel oncogene in chicken fibroblasts and spleen cells correlates with transformation by REV-T. Cell. 1986 Mar 14;44(5):791–800. doi: 10.1016/0092-8674(86)90845-7. [DOI] [PubMed] [Google Scholar]
- Hagiya M., Davis D. D., Takahashi T., Okuda K., Raschke W. C., Sakano H. Two types of immunoglobulin-negative Abelson murine leukemia virus-transformed cells: implications for B-lymphocyte differentiation. Proc Natl Acad Sci U S A. 1986 Jan;83(1):145–149. doi: 10.1073/pnas.83.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzer J. D., Lewis R. B., Wasmuth C. R., Bose H. R., Jr Hematopoietic cell transformation by reticuloendotheliosis virus: characterization of the genetic defect. Virology. 1980 Jan 30;100(2):462–474. doi: 10.1016/0042-6822(80)90536-x. [DOI] [PubMed] [Google Scholar]
- Honjo T. Immunoglobulin genes. Annu Rev Immunol. 1983;1:499–528. doi: 10.1146/annurev.iy.01.040183.002435. [DOI] [PubMed] [Google Scholar]
- Lewis R. B., McClure J., Rup B., Niesel D. W., Garry R. F., Hoelzer J. D., Nazerian K., Bose H. R., Jr Avian reticuloendotheliosis virus: identification of the hematopoietic target cell for transformation. Cell. 1981 Aug;25(2):421–431. doi: 10.1016/0092-8674(81)90060-x. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Reth M. G., Alt F. W. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. 1984 Nov 29-Dec 5Nature. 312(5993):418–423. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- Reth M. G., Ammirati P., Jackson S., Alt F. W. Regulated progression of a cultured pre-B-cell line to the B-cell stage. 1985 Sep 26-Oct 2Nature. 317(6035):353–355. doi: 10.1038/317353a0. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Grimal H., Weill J. C. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell. 1987 Feb 13;48(3):379–388. doi: 10.1016/0092-8674(87)90189-9. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Dahan A., Weill J. C. Complete sequence of a chicken lambda light chain immunoglobulin derived from the nucleotide sequence of its mRNA. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4099–4103. doi: 10.1073/pnas.80.13.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Keath E. J., Piccoli S. P., Cole M. D. Novel myc oncogene RNA from abortive immunoglobulin-gene recombination in mouse plasmacytomas. Cell. 1982 Dec;31(2 Pt 1):443–452. doi: 10.1016/0092-8674(82)90137-4. [DOI] [PubMed] [Google Scholar]
- Shibuya T., Chen I., Howatson A., Mak T. W. Morphological, immunological, and biochemical analyses of chicken spleen cells transformed in vitro by reticuloendotheliosis virus strain T. Cancer Res. 1982 Jul;42(7):2722–2728. [PubMed] [Google Scholar]
- Theilen G. H., Zeigel R. F., Twiehaus M. J. Biological studies with RE virus (strain T) that induces reticuloendotheliosis in turkeys, chickens, and Japanese quail. J Natl Cancer Inst. 1966 Dec;37(6):731–743. [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Ortiz S. Retroviruses as mutagens: insertion and excision of a nontransforming provirus alter expression of a resident transforming provirus. Cell. 1981 Jul;25(1):23–36. doi: 10.1016/0092-8674(81)90228-2. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Hirose T., Miyake T., Kawashima E. H., Itakura K. The use of synthetic oligonucleotides as hybridization probes. II. Hybridization of oligonucleotides of mixed sequence to rabbit beta-globin DNA. Nucleic Acids Res. 1981 Feb 25;9(4):879–894. doi: 10.1093/nar/9.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill J. C., Reynaud C. A., Lassila O., Pink J. R. Rearrangement of chicken immunoglobulin genes is not an ongoing process in the embryonic bursa of Fabricius. Proc Natl Acad Sci U S A. 1986 May;83(10):3336–3340. doi: 10.1073/pnas.83.10.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S. F., Treiman L. J., Witte O. N. kappa gene diversity among the clonal progeny of pre-B lymphocytes. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1529–1533. doi: 10.1073/pnas.81.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]