Abstract
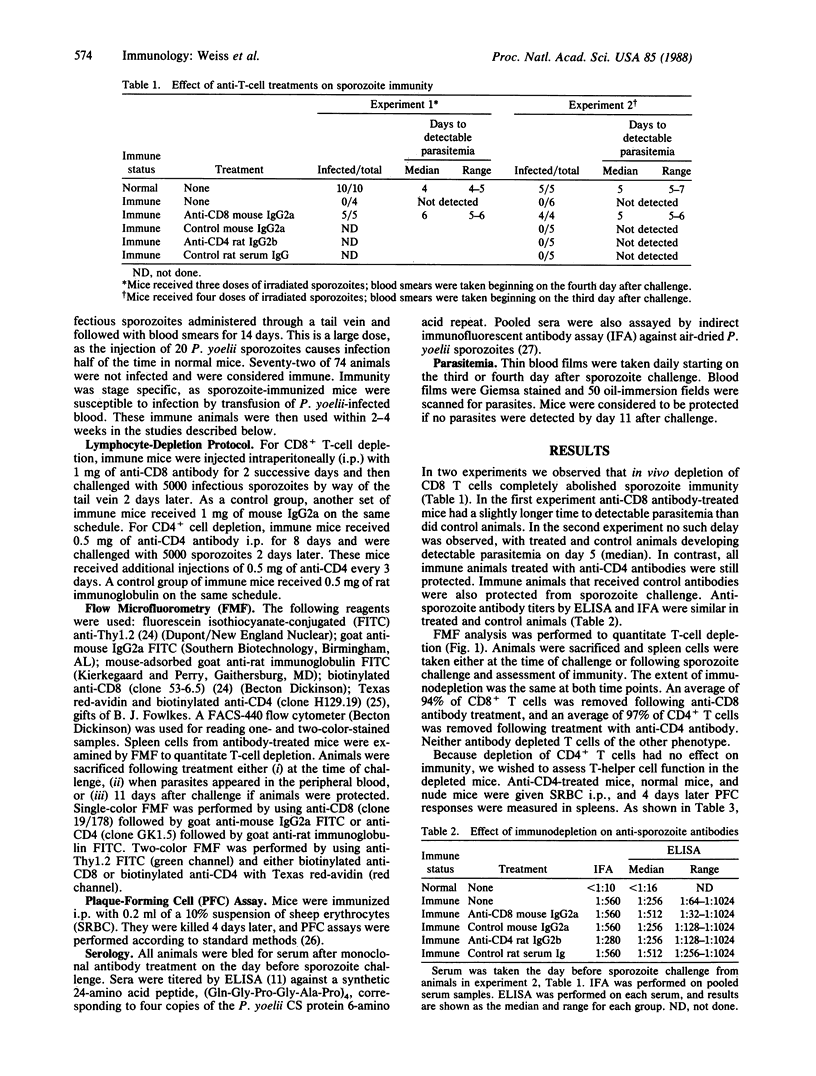
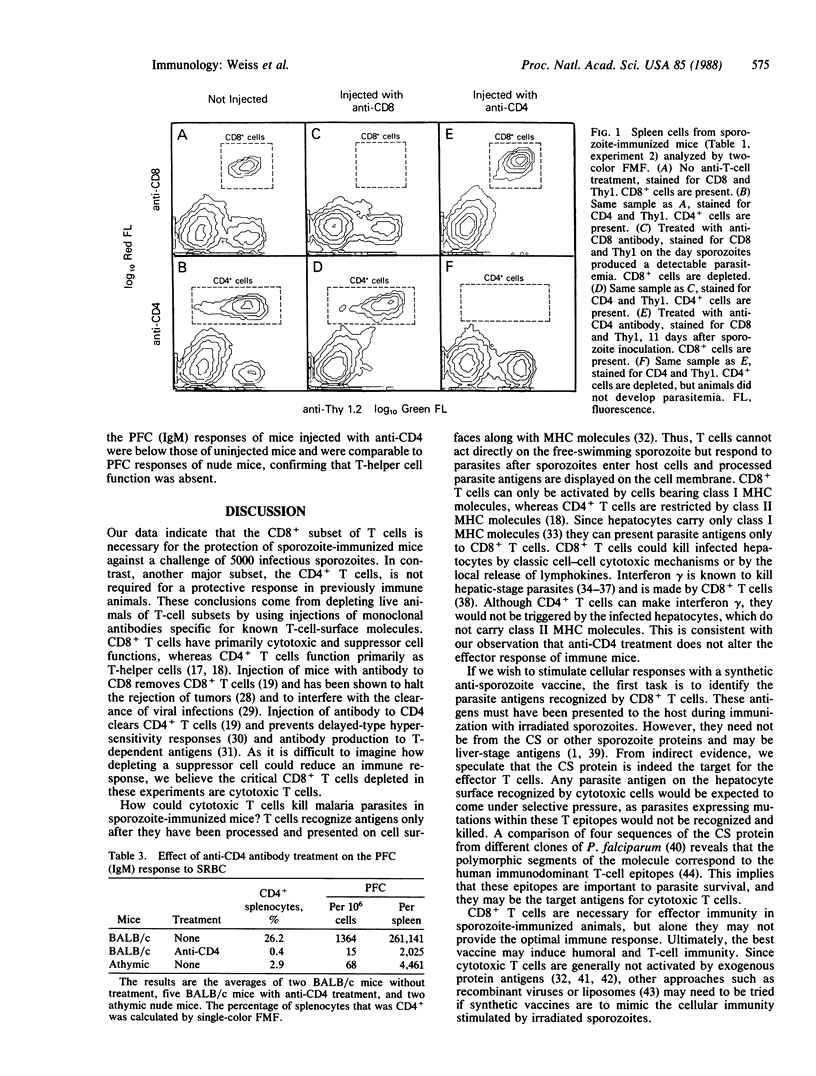
In recent malaria sporozoite vaccine trials in humans and mice, antibodies to the sporozoite coat protein have given only modest protection against sporozoite challenge. In contrast, irradiated sporozoites can protect mice against massive sporozoite infections. Evidence suggests that immunity in these mice is mediated by T cells. To identify the mechanism of immunity, we used monoclonal antibodies specific for either the CD4 or CD8 molecule to selectively deplete sporozoite-immunized mice of T-cell subsets. Though in vivo depletion of CD4+ T cells did not reduce immunity, depletion of CD8+ T cells abolished protection. Monoclonal antibody treatment did not affect anti-sporozoite antibody levels. Our data indicate that cytotoxic T cells are critical for immunity to large numbers of sporozoites and suggest that vaccine development should be reoriented toward stimulating cellular as well as humoral immunity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M., Yoshida N., Nussenzweig R. S., Nussenzweig V. The protective antigen of malarial sporozoites (Plasmodium berghei) is a differentiation antigen. J Immunol. 1981 Jun;126(6):2494–2495. [PubMed] [Google Scholar]
- Ballou W. R., Hoffman S. L., Sherwood J. A., Hollingdale M. R., Neva F. A., Hockmeyer W. T., Gordon D. M., Schneider I., Wirtz R. A., Young J. F. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987 Jun 6;1(8545):1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Ballou W. R., Rothbard J., Wirtz R. A., Gordon D. M., Williams J. S., Gore R. W., Schneider I., Hollingdale M. R., Beaudoin R. L., Maloy W. L. Immunogenicity of synthetic peptides from circumsporozoite protein of Plasmodium falciparum. Science. 1985 May 24;228(4702):996–999. doi: 10.1126/science.2988126. [DOI] [PubMed] [Google Scholar]
- Charoenvit Y., Leef M. F., Yuan L. F., Sedegah M., Beaudoin R. L. Characterization of Plasmodium yoelii monoclonal antibodies directed against stage-specific sporozoite antigens. Infect Immun. 1987 Mar;55(3):604–608. doi: 10.1128/iai.55.3.604-608.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D. H., Tigelaar R. E., Weinbaum F. I. Immunity to sporozoite-induced malaria infeciton in mice. I. The effect of immunization of T and B cell-deficient mice. J Immunol. 1977 Apr;118(4):1322–1327. [PubMed] [Google Scholar]
- Clyde D. F., Most H., McCarthy V. C., Vanderberg J. P. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci. 1973 Sep;266(3):169–177. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Coulie P. G., Coutelier J. P., Uyttenhove C., Lambotte P., Van Snick J. In vivo suppression of T-dependent antibody responses by treatment with a monoclonal anti-L3T4 antibody. Eur J Immunol. 1985 Jun;15(6):638–640. doi: 10.1002/eji.1830150620. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Egan J. E., Weber J. L., Ballou W. R., Hollingdale M. R., Majarian W. R., Gordon D. M., Maloy W. L., Hoffman S. L., Wirtz R. A., Schneider I. Efficacy of murine malaria sporozoite vaccines: implications for human vaccine development. Science. 1987 Apr 24;236(4800):453–456. doi: 10.1126/science.3551073. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Schofield L., Enea V., Schellekens H., van der Meide P., Collins W. E., Nussenzweig R. S., Nussenzweig V. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science. 1986 May 16;232(4752):881–884. doi: 10.1126/science.3085218. [DOI] [PubMed] [Google Scholar]
- Germain R. N. Immunology. The ins and outs of antigen processing and presentation. Nature. 1986 Aug 21;322(6081):687–689. doi: 10.1038/322687a0. [DOI] [PubMed] [Google Scholar]
- Glasebrook A. L., Kelso A., MacDonald H. R. Cytolytic T lymphocyte clones that proliferate autonomously to specific alloantigenic stimulation. II. Relationship of the Lyt-2 molecular complex to cytolytic activity, proliferation, and lymphokine secretion. J Immunol. 1983 Apr;130(4):1545–1551. [PubMed] [Google Scholar]
- Herrington D. A., Clyde D. F., Losonsky G., Cortesia M., Murphy J. R., Davis J., Baqar S., Felix A. M., Heimer E. P., Gillessen D. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature. 1987 Jul 16;328(6127):257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- Hämmerling G. J., Hämmerling U., Flaherty L. Qat-4 and Qat-5, new murine T-cell antigens governed by the Tla region and identified by monoclonal antibodies. J Exp Med. 1979 Jul 1;150(1):108–116. doi: 10.1084/jem.150.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A. A., de la Cruz V. F., Good M. F., Weiss W. R., Lunde M., Maloy W. L., Welsh J. A., McCutchan T. F. In vivo testing of subunit vaccines against malaria sporozoites using a rodent system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8647–8651. doi: 10.1073/pnas.84.23.8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Maheshwari R. K., Czarniecki C. W., Dutta G. P., Puri S. K., Dhawan B. N., Friedman R. M. Recombinant human gamma interferon inhibits simian malaria. Infect Immun. 1986 Sep;53(3):628–630. doi: 10.1128/iai.53.3.628-630.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Endres R., Shimonkevitz R., Zlotnik A., Dialynas D., Fitch F., Kappler J. The major histocompatibility complex-restricted antigen receptor on T cells. II. Role of the L3T4 product. J Exp Med. 1983 Oct 1;158(4):1077–1091. doi: 10.1084/jem.158.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. D., Jenkins M. K. In vivo effects of GK1.5 (anti-L3T4a) monoclonal antibody on induction and expression of delayed-type hypersensitivity. Cell Immunol. 1985 May;92(2):414–426. doi: 10.1016/0008-8749(85)90022-x. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskophidis D., Cobbold S. P., Waldmann H., Lehmann-Grube F. Mechanism of recovery from acute virus infection: treatment of lymphocytic choriomeningitis virus-infected mice with monoclonal antibodies reveals that Lyt-2+ T lymphocytes mediate clearance of virus and regulate the antiviral antibody response. J Virol. 1987 Jun;61(6):1867–1874. doi: 10.1128/jvi.61.6.1867-1874.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama E., Uenaka A. Effect of in vivo administration of Lyt antibodies. Lyt phenotype of T cells in lymphoid tissues and blocking of tumor rejection. J Exp Med. 1985 Feb 1;161(2):345–355. doi: 10.1084/jem.161.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig R. S., Vanderberg J., Most H., Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967 Oct 14;216(5111):160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- Ojo-Amaize E. A., Vilcek J., Cochrane A. H., Nussenzweig R. S. Plasmodium berghei sporozoites are mitogenic for murine T cells, induce interferon, and activate natural killer cells. J Immunol. 1984 Aug;133(2):1005–1009. [PubMed] [Google Scholar]
- Ozaki L. S., Gwadz R. W., Godson G. N. Simple centrifugation method for rapid separation of sporozoites from mosquitoes. J Parasitol. 1984 Oct;70(5):831–833. [PubMed] [Google Scholar]
- Pierres A., Naquet P., Van Agthoven A., Bekkhoucha F., Denizot F., Mishal Z., Schmitt-Verhulst A. M., Pierres M. A rat anti-mouse T4 monoclonal antibody (H129.19) inhibits the proliferation of Ia-reactive T cell clones and delineates two phenotypically distinct (T4+, Lyt-2,3-, and T4-, Lyt-2,3+) subsets among anti-Ia cytolytic T cell clones. J Immunol. 1984 Jun;132(6):2775–2782. [PubMed] [Google Scholar]
- Rieckmann K. H., Carson P. E., Beaudoin R. L., Cassells J. S., Sell K. W. Letter: Sporozoite induced immunity in man against an Ethiopian strain of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1974;68(3):258–259. doi: 10.1016/0035-9203(74)90129-1. [DOI] [PubMed] [Google Scholar]
- Spitalny G. L., Verhave J. P., Meuwissen J. H., Nussenzweig R. S. Plasmodium berghei: T cell dependence of sporozoite-induced immunity in rodents. Exp Parasitol. 1977 Jun;42(1):73–81. doi: 10.1016/0014-4894(77)90063-7. [DOI] [PubMed] [Google Scholar]
- Sprent J., Schaefer M., Lo D., Korngold R. Functions of purified L3T4+ and Lyt-2+ cells in vitro and in vivo. Immunol Rev. 1986 Jun;91:195–218. doi: 10.1111/j.1600-065x.1986.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Sprent J., Schaefer M. Properties of purified T cell subsets. I. In vitro responses to class I vs. class II H-2 alloantigens. J Exp Med. 1985 Dec 1;162(6):2068–2088. doi: 10.1084/jem.162.6.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L. Significance of Lyt phenotypes: Lyt2 antibodies block activities of T cells that recognize class 1 major histocompatibility complex antigens regardless of their function. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7101–7105. doi: 10.1073/pnas.78.11.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderberg J. P., Nussenzweig R. S., Most H., Orton C. G. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. II. Effects of radiation on sporozoites. J Parasitol. 1968 Dec;54(6):1175–1180. [PubMed] [Google Scholar]
- Vergara U., Ferreira A., Schellekens H., Nussenzweig V. Mechanism of escape of exoerythrocytic forms (EEF) of malaria parasites from the inhibitory effects of interferon-gamma. J Immunol. 1987 Jun 15;138(12):4447–4449. [PubMed] [Google Scholar]
- Verhave J. P., Strickland G. T., Jaffe H. A., Ahmed A. Studies on the transfer of protective immunity with lymphoid cells from mice immune to malaria sporozoites. J Immunol. 1978 Sep;121(3):1031–1033. [PubMed] [Google Scholar]
- Watari E., Dietzschold B., Szokan G., Heber-Katz E. A synthetic peptide induces long-term protection from lethal infection with herpes simplex virus 2. J Exp Med. 1987 Feb 1;165(2):459–470. doi: 10.1084/jem.165.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman D., Parker D. J., Rothstein T. L., Marshak-Rothstein A. Methods for the production of xenogeneic monoclonal antibodies in murine ascites. J Immunol. 1985 Aug;135(2):1001–1003. [PubMed] [Google Scholar]
- Yamada A., Ziese M. R., Young J. F., Yamada Y. K., Ennis F. A. Influenza virus hemagglutinin-specific cytotoxic T cell response induced by polypeptide produced in Escherichia coli. J Exp Med. 1985 Aug 1;162(2):663–674. doi: 10.1084/jem.162.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. F., Hockmeyer W. T., Gross M., Ballou W. R., Wirtz R. A., Trosper J. H., Beaudoin R. L., Hollingdale M. R., Miller L. H., Diggs C. L. Expression of Plasmodium falciparum circumsporozoite proteins in Escherichia coli for potential use in a human malaria vaccine. Science. 1985 May 24;228(4702):958–962. doi: 10.1126/science.2988125. [DOI] [PubMed] [Google Scholar]
- Zavala F., Tam J. P., Hollingdale M. R., Cochrane A. H., Quakyi I., Nussenzweig R. S., Nussenzweig V. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science. 1985 Jun 21;228(4706):1436–1440. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]
- de la Cruz V. F., Lal A. A., McCutchan T. F. Sequence variation in putative functional domains of the circumsporozoite protein of Plasmodium falciparum. Implications for vaccine development. J Biol Chem. 1987 Sep 5;262(25):11935–11939. [PubMed] [Google Scholar]



