Abstract
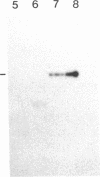
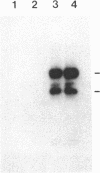
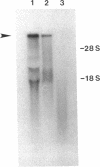
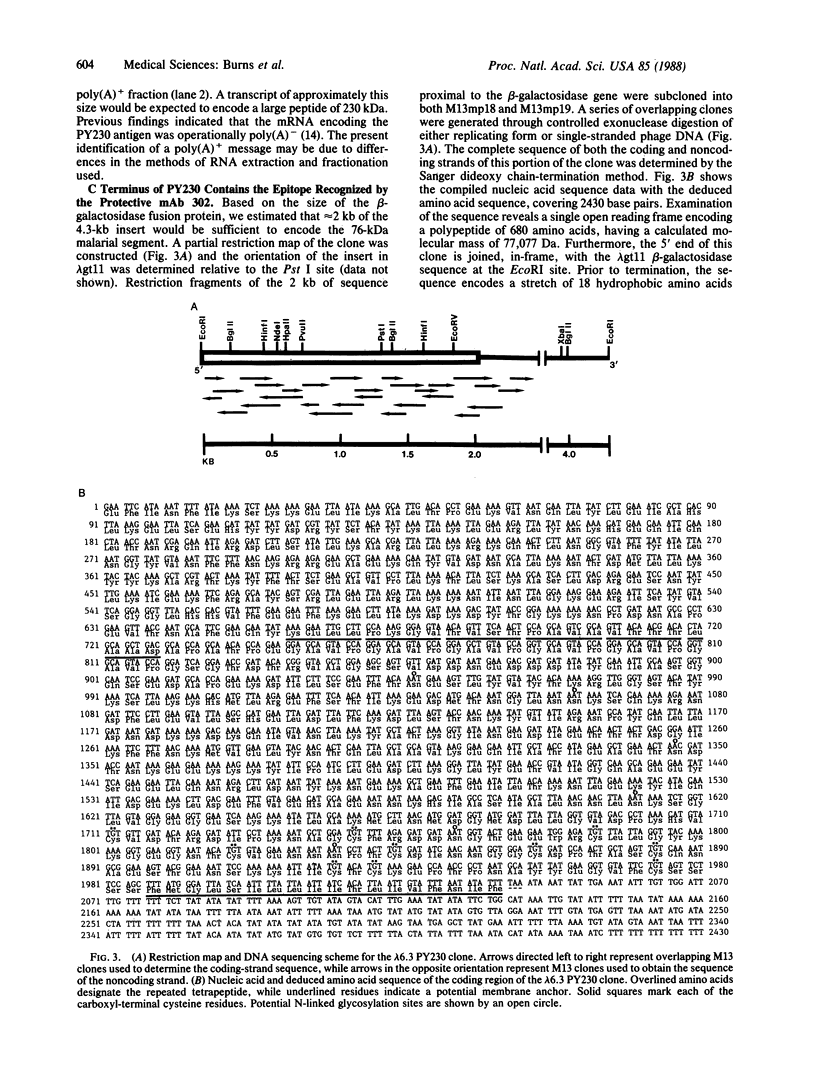
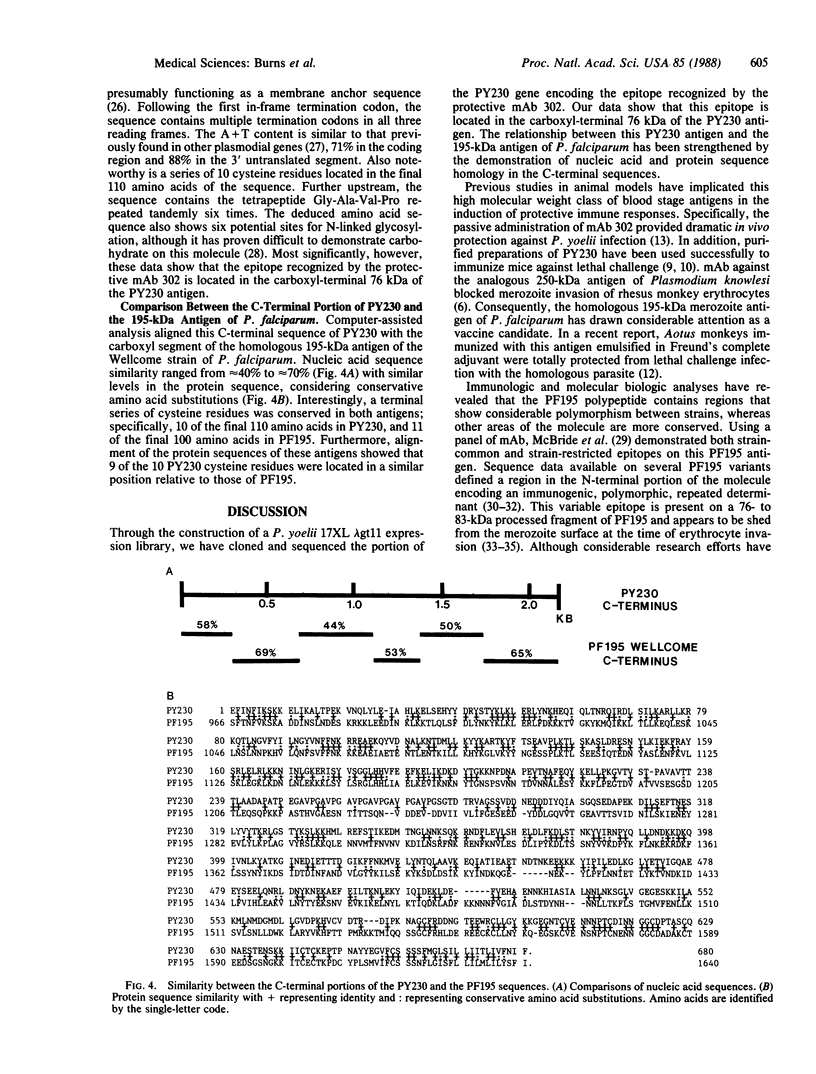
The 230-kDa merozoite antigen of the murine malarial parasite Plasmodium yoelii provides a potential model system for the development of a protective erythrocytic stage vaccine. To characterize this antigen at the molecular level, isolated P. yoelii 17XL DNA was used to construct a genomic library in the expression vector lambda gt11. A monoclonal antibody, mAb 302, which passively protected mice against P. yoelii challenge infection, was used to identify a lambda gt11 recombinant clone encoding a portion of the 230-kDa antigen of this parasite. Using this clone as a probe, we identified an mRNA of 7.6 kilobases by RNA blot analysis. Nucleic acid sequence analysis of the clone showed that the epitope recognized by the protective mAb 302 is encoded by the 3' portion of the gene for the 230-kDa antigen. The deduced amino acid sequence revealed that this antigen also contains the tandemly repeated tetrapeptide Gly-Ala-Val-Pro, a series of 10 cysteine residues located within the terminal 110 amino acids, and a potential membrane anchor of 18 hydrophobic residues. Comparison of this C-terminal sequence with the carboxyl segment of the 195-kDa merozoite antigen of Plasmodium falciparum revealed nucleic acid and amino acid sequence similarities ranging from 40% to 70%. The localization of a B-cell epitope recognized by the protective mAb 302 to this carboxyl region of the P. yoelii antigen, combined with the limited strain variability in this region of the homologous 195-kDa antigen of P. falciparum, has implications for the development of an effective erythrocytic stage malarial vaccine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Eling W. Ficoll fractionation for the separation of parasitized erythrocytes from malaria infected blood. Bull World Health Organ. 1977;55(1):105–114. [PMC free article] [PubMed] [Google Scholar]
- Epstein N., Miller L. H., Kaushel D. C., Udeinya I. J., Rener J., Howard R. J., Asofsky R., Aikawa M., Hess R. L. Monoclonal antibodies against a specific surface determinant on malarial (Plasmodium knowlesi) merozoites block erythrocyte invasion. J Immunol. 1981 Jul;127(1):212–217. [PubMed] [Google Scholar]
- Freeman R. R., Holder A. A. Characteristics of the protective response of BALB/c mice immunized with a purified Plasmodium yoelii schizont antigen. Clin Exp Immunol. 1983 Dec;54(3):609–616. [PMC free article] [PubMed] [Google Scholar]
- Freeman R. R., Holder A. A. Surface antigens of malaria merozoites. A high molecular weight precursor is processed to an 83,000 mol wt form expressed on the surface of Plasmodium falciparum merozoites. J Exp Med. 1983 Nov 1;158(5):1647–1653. doi: 10.1084/jem.158.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R. R., Trejdosiewicz A. J., Cross G. A. Protective monoclonal antibodies recognising stage-specific merozoite antigens of a rodent malaria parasite. Nature. 1980 Mar 27;284(5754):366–368. doi: 10.1038/284366a0. [DOI] [PubMed] [Google Scholar]
- Hadley T. J. Invasion of erythrocytes by malaria parasites: a cellular and molecular overview. Annu Rev Microbiol. 1986;40:451–477. doi: 10.1146/annurev.mi.40.100186.002315. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Biosynthesis and processing of a Plasmodium falciparum schizont antigen recognized by immune serum and a monoclonal antibody. J Exp Med. 1982 Nov 1;156(5):1528–1538. doi: 10.1084/jem.156.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Characterization of a high molecular weight protective antigen of Plasmodium yoelii. Parasitology. 1984 Apr;88(Pt 2):211–219. [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature. 1981 Nov 26;294(5839):361–364. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R., Newbold C. I. Serological cross-reaction between high molecular weight proteins synthesized in blood schizonts of Plasmodium yoelii, Plasmodium chabaudi and Plasmodium falciparum. Mol Biochem Parasitol. 1983 Nov;9(3):191–196. doi: 10.1016/0166-6851(83)90096-8. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Lockyer M. J., Odink K. G., Sandhu J. S., Riveros-Moreno V., Nicholls S. C., Hillman Y., Davey L. S., Tizard M. L., Schwarz R. T. Primary structure of the precursor to the three major surface antigens of Plasmodium falciparum merozoites. Nature. 1985 Sep 19;317(6034):270–273. doi: 10.1038/317270a0. [DOI] [PubMed] [Google Scholar]
- Howard R. F., Stanley H. A., Campbell G. H., Langreth S. G., Reese R. T. Two Plasmodium falciparum merozoite surface polypeptides share epitopes with a single Mr 185 000 parasite glycoprotein. Mol Biochem Parasitol. 1985 Oct;17(1):61–77. doi: 10.1016/0166-6851(85)90128-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lyon J. A., Geller R. H., Haynes J. D., Chulay J. D., Weber J. L. Epitope map and processing scheme for the 195,000-dalton surface glycoprotein of Plasmodium falciparum merozoites deduced from cloned overlapping segments of the gene. Proc Natl Acad Sci U S A. 1986 May;83(9):2989–2993. doi: 10.1073/pnas.83.9.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon J. A., Haynes J. D., Diggs C. L., Chulay J. D., Haidaris C. G., Pratt-Rossiter J. Monoclonal antibody characterization of the 195-kilodalton major surface glycoprotein of Plasmodium falciparum malaria schizonts and merozoites: identification of additional processed products and a serotype-restricted repetitive epitope. J Immunol. 1987 Feb 1;138(3):895–901. [PubMed] [Google Scholar]
- Mackay M., Goman M., Bone N., Hyde J. E., Scaife J., Certa U., Stunnenberg H., Bujard H. Polymorphism of the precursor for the major surface antigens of Plasmodium falciparum merozoites: studies at the genetic level. EMBO J. 1985 Dec 30;4(13B):3823–3829. doi: 10.1002/j.1460-2075.1985.tb04154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majarian W. R., Daly T. M., Weidanz W. P., Long C. A. Passive immunization against murine malaria with an IgG3 monoclonal antibody. J Immunol. 1984 Jun;132(6):3131–3137. [PubMed] [Google Scholar]
- McBride J. S., Heidrich H. G. Fragments of the polymorphic Mr 185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol Biochem Parasitol. 1987 Feb;23(1):71–84. doi: 10.1016/0166-6851(87)90189-7. [DOI] [PubMed] [Google Scholar]
- McBride J. S., Newbold C. I., Anand R. Polymorphism of a high molecular weight schizont antigen of the human malaria parasite Plasmodium falciparum. J Exp Med. 1985 Jan 1;161(1):160–180. doi: 10.1084/jem.161.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Howard R. J., Carter R., Good M. F., Nussenzweig V., Nussenzweig R. S. Research toward malaria vaccines. Science. 1986 Dec 12;234(4782):1349–1356. doi: 10.1126/science.2431481. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Merkli B., Loche M., Chizzolini C., Smart J., Richle R. Antimalarial immunity in Saimiri monkeys. Immunization with surface components of asexual blood stages. J Exp Med. 1984 Aug 1;160(2):441–451. doi: 10.1084/jem.160.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirson P. J., Perkins M. E. Characterization with monoclonal antibodies of a surface antigen of Plasmodium falciparum merozoites. J Immunol. 1985 Mar;134(3):1946–1951. [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. T., Riveros-Moreno V., Lockyer M. J., Nicholls S. C., Davey L. S., Hillman Y., Sandhu J. S., Freeman R. R., Holder A. A. Structural diversity of the major surface antigen of Plasmodium falciparum merozoites. Mol Cell Biol. 1986 Mar;6(3):964–968. doi: 10.1128/mcb.6.3.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui W. A., Tam L. Q., Kramer K. J., Hui G. S., Case S. E., Yamaga K. M., Chang S. P., Chan E. B., Kan S. C. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci U S A. 1987 May;84(9):3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K., Mackay M., Goman M., Scaife J. G. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J Mol Biol. 1987 May 20;195(2):273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya A. B., Arasu P. Tandemly arranged gene clusters of malarial parasites that are highly conserved and transcribed. Mol Biochem Parasitol. 1987 Jan 15;22(2-3):249–257. doi: 10.1016/0166-6851(87)90056-9. [DOI] [PubMed] [Google Scholar]
- Vaidya A. B., Schleif W. A., Majarian W. R., Daly T. M., Taylor D. W., Long C. A. Analysis of mRNA coding for blood-stage antigens of a rodent malarial parasite, Plasmodium yoelii: mRNA coding for a possible protective antigen purify as poly A-. J Immunol. 1984 Jun;132(6):3126–3130. [PubMed] [Google Scholar]
- Weber J. L. Analysis of sequences from the extremely A + T-rich genome of Plasmodium falciparum. Gene. 1987;52(1):103–109. doi: 10.1016/0378-1119(87)90399-4. [DOI] [PubMed] [Google Scholar]
- Weber J. L., Leininger W. M., Lyon J. A. Variation in the gene encoding a major merozoite surface antigen of the human malaria parasite Plasmodium falciparum. Nucleic Acids Res. 1986 Apr 25;14(8):3311–3323. doi: 10.1093/nar/14.8.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]