Abstract
Whether selective attention affects C1, the first (earliest) visual cortical component of the event-related potential (ERP), remains controversial. We used a cued, involuntary attention task requiring discrimination of targets under low and high levels of perceptual load to examine early attentional modulation in visual cortex. Potential confounds due to physical stimulus differences between load conditions and cue-target sensory interaction were minimized. An interaction between perceptual load and involuntary attention was observed for the P1m component (peak latency between 100 and 140 ms). Furthermore, the parieto-central C1 component (peak latency 80 ms) was modulated by attention, but only under the high-load condition. Thus, whereas attention typically modulates the later P1 component, attentional modulation of C1 is possible under optimal conditions. Specifically, a high perceptual load is necessary for eliciting this earliest attentional effect on cortical processing.
Keywords: perceptual load, involuntary attention, event-related potentials (ERPs), peripheral cueing, C1, P1m
Introduction
Attention is known to influence visual cortical processing but the level at which this effect occurs is controversial. FMRI evidence suggests that area V1 is activated by attention [9, 21], but because of the low temporal resolution of fMRI, it is unclear whether this reflects an early, direct modulation or a later reactivation of V1 via feedback from higher cortical areas. The excellent temporal resolution of event-related potentials (ERP) provides a means for identifying the timing of attentional processing in cortex. Specifically, the first visual cortical component, C1, is thought to originate from V1, and has been used as an index to investigate whether V1 is directly modulated by attention [1, 8]. The evidence to date suggests that C1, which peaks at approximately 80 ms, is not modulated by attention [14], but that a later attentional effect occurs at about 150 ms that can be localized to V1 [15, 16, 18]. This is consistent with a feedback hypothesis [15, 16, 18, 22] of the role of V1 in visual attention the initial processing in V1, as indexed by visual C1 component, is not modulated by attention, whereas V1 activity is modulated by attention in a later processing stage via reentrant feedback from higher visual cortex.
Although the reentrant feedback model represents the current view on attentional processing in cortex, emerging evidence suggests that attention can directly modulate the amplitude of C1 [10, 11, 20, 24]. Selective attention effects on the MEG equivalent of C1 have also been reported [19]. These findings suggest that V1 activity can be directly modulated by attention at the initial stage of cortical visual processing, but perhaps only under certain stimulus and task conditions.
One such condition could be the perceptual load associated with the attention task, because early selection occurs only under high perceptual load condition [12, 13]. In a previous study we manipulated the perceptual load of the stimuli and found that an ERP component at the C1 time range can be modulated by attention under specific experimental conditions [6]. In that study, stimuli were presented to the left or right visual field, and participants were required to respond to the orientation of a diagonal line that appeared with different distractors. The perceptual load of the stimuli was low, medium, or high, as determined by the complexity of the background distractors. Consistent with Lavie’s perceptual load theory that early attentional selection is required only for high load stimuli [12, 13], we found that the ERPs in the C1 time range were modulated by attention, when and only when the perceptual load of the stimuli was high. This suggests that C1 might be modulated by attention under optimal conditions that include a high perceptual load, involuntary attention, and a cluttered visual field. Moreover, a perceptual load by involuntary attention interaction was observed for P1m (the midline P1 component), indicating an earlier locus for attentional modulation and different underlying mechanism for the interaction between perceptual load and involuntary attention, relative to that for voluntary attention.
However, one limitation of that study was a potential confound between cue-target sensory interaction and perceptual load. The former refers to the fact that since the cue appears in the same location as the target in an involuntary attention task, the two may interact (either positively or negatively) at a sensory level, so that any influence on C1 and P1m may reflect sensory as well as attention effects. The sensory interaction effect under the high-load condition could have differed from those under the medium- and low-load conditions, given that there were physical differences among the stimulus arrays associated with the different load levels. To address this issue, possible approaches are: (a) balancing the physical properties between different load conditions, (b) systematically manipulating the physical strength of the cues as an experimental factor, and (c) minimizing the interaction between the cue and the target array.
The present study addressed this issue of cue-target sensory interaction by using a new set of stimuli that better controlled for the difference between stimulus physical properties and cue-target interaction between the low- and high-load stimuli. The physical properties (such as orientation, luminance, and spatial frequency, etc.) and cue-target sensory interaction were balanced across low and high perceptual load conditions. This allowed us to determine whether the load by attention interaction on C1 and P1m and the earliest attentional modulation on the C1 component in the previous study [6] could be replicated in the absence of sensory interaction.
Method
Participants
21 healthy participants from the student population at George Mason University took part in this study as paid volunteers. 2 participants’ data were excluded because of a data collection issue. The remaining 19 participants (5 male) were between 18 and 26 years of age (mean age 20.3 years), right-handed, had normal or corrected to normal vision, and had no reported history of neurological illness. Informed consent was obtained from all participants.
Stimuli
A black fixation cross (0.31° × 0.31°) was presented on a white background at the center of the monitor throughout the entire recording session. Each trial consisted of a cue (3.53° × 3.53°) and a target array (Figure 1). The target array (2.87° × 2.87°) for diamond-shaped and 2.29° × 2.29° for square-shaped target) appeared randomly on the left or right side, with its center 7.07° to the side and 2.05° above the fixation cross. The location of the cues (consisting of 4 brackets, 0.57° × 0.57° in the four corners of the stimulus location, Figure 1) was valid, invalid, or neutral (bilateral) with equal probability in predicting the location of the subsequent stimulus array. In the bilateral cuing condition, the cue appeared simultaneously in both the left and right visual field. The durations of the cue and the target array were 50 ms and 100 ms, respectively. The SOAs between the cue and the target array was randomized between 100 and 300 ms, and the ITI (inter-trial interval) between the offset of the target array and the next cue ranged between 1100 and 1600 ms. The participants’ task was to discriminate the orientation of the bar in the center of the target array. The perceptual load of the stimuli was high or low with equal probability. In the high load condition, distractors sharing characteristic features surrounded the central bar -- a central horizontal or vertical bar surrounded by crosses, or a central forward or backward bar surrounded by Xs. In the low load condition, the central bar did not share any features with the surrounding distractor -- a central horizontal or vertical bar surrounded by Xs, or a central forward or backward bar surrounded by crosses (Figure 1). Note that the location of the four distractors was designed to avoid collinearity between the central bar and the distractors.
Figure 1.
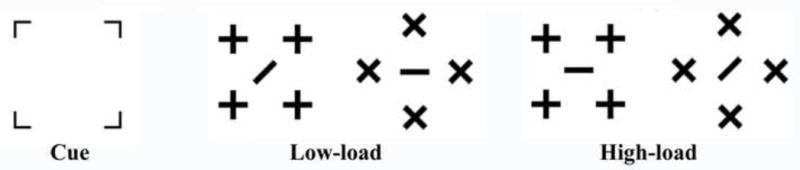
Illustrations of the cue, and the target stimuli with low and high perceptual load in the present study. The two high-load stimuli were developed by exchanging the central line of the low-load stimuli. Therefore, the overall physical property was comparable between the low and high load stimuli, and the possible interaction between the cue and the target was minimized.
Procedure
Participants were instructed to keep their eyes on the central fixation through the experiment. This reduced eye movements. Half of the participants were required to press “n” when they saw a backward or forward central bar and to press “z” when they saw a horizontal or vertical central bar. For the other half of the participants, the response keys were reversed. The participants were instructed to respond as fast as possible while maintaining high accuracy. During the 96-trial practice session, the experiment operator watched participants’ eyes to ensure their fixation maintaining. The trial sequence was randomized for each participant. In total 1920 trials were presented. Participants were given short breaks after each block of 60 trials.
EEG Recording
EPrime (Psychology Software Tools, Pittsburgh, PA) and SCAN 4.3.3 software packages (Compumedics, Texas, USA) were used to present stimuli and to record and analyze EEG. Twenty channels of EEG and EOG were recorded from the scalp with an electrode cap. Standard 10–20 sites were F3, F4, C3, C4, PZ, P3, P4, O1, O2, T5, and T6. Additional sites were CPZ, POZ, OZ, PO5, PO6, left and right mastoid (LM and RM). The physical reference electrode was approximately 2 cm posterior to CZ, and the EEG data were re-referenced to the average of LM and RM. Horizontal eye movements (HEOG) were monitored by placing two electrodes lateral to the left and right orbits. The impedances of all electrodes were kept below 5 KΩ throughout the recording session. Vertical eye movements (VEOG) and eye blinks were measured by placing two electrodes, with one 1.5 cm below and the other 1.5 cm above the left eye. The EEG from each electrode site was digitized at 500 Hz and was filtered with a band-pass of 0.1 to 40 Hz. The EEG for the 200 ms preceding target onset was used as baseline.
Data analysis
For behavioral data analysis, median RT was used for statistical analysis. For ERP analysis, the ADJAR algorithm [23] was used to remove ERP overlap due to short SOAs (100–300 ms) between the cues and the targets. The target-elicited ERPs were then averaged for the high- and low- load conditions, respectively. For C1 component, we analyzed the data on POZ site that had largest C1 amplitude, as well as on two midline sites, PZ and CPZ.
Results
Behavioral measures
The data for the valid and invalid trials were analyzed. The overall accuracy for target discrimination was 86.43%. Participants responded faster to the low-load stimuli relative to the high-load stimuli, as suggested by a significant main effect of load [553 vs. 594 ms, F (1, 18) = 171.344, p < .0005] (Figure 2). Participants also responded faster to valid trials relative to invalid [566 vs. 581 ms, F (1, 18) = 19.731, p < .0005]. No other main effects or interactions related to load and validity were significant.
Figure 2.
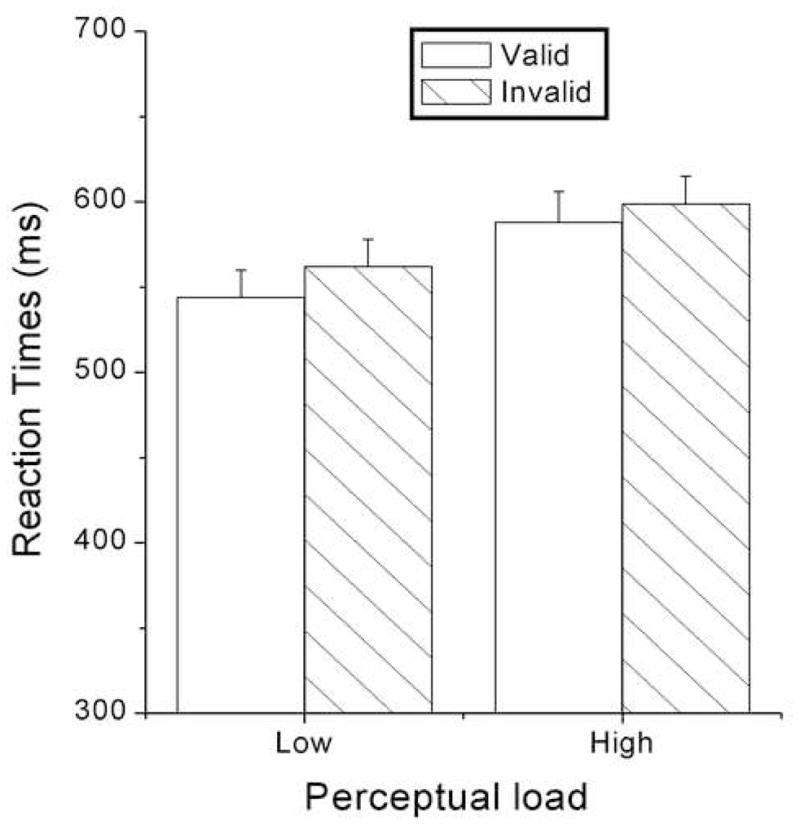
Reaction times (RTs) as a function of perceptual load and cue validity.
Electrophysiological measures
Figure 3 shows the target ERPs from valid and invalid trials for low-load and high-load stimuli. There is a clear negative C1 component over the parietal area about 80 ms after target onset, and a midline P1 component (peak latency approximately 108 ms for valid and 140 ms for invalid trials). It is also evident that there are effects of cue validity and perceptual load on the P1m component.
Figure 3.
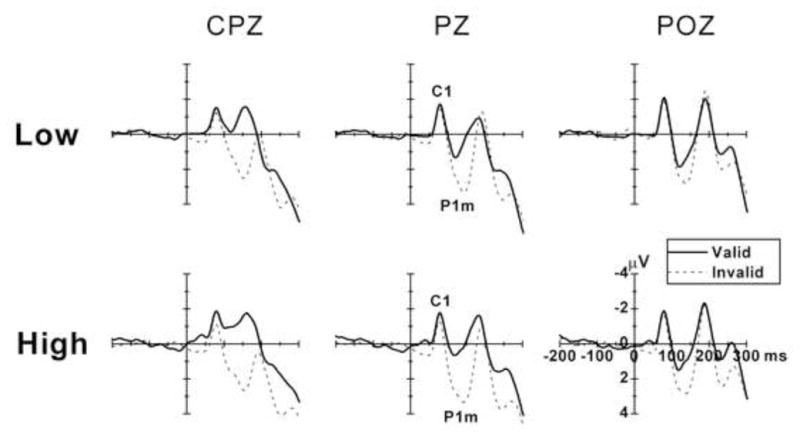
The grand averaged ERPs across all participants for the valid and invalid trials under low- and high-load conditions for three midline electrodes (CPZ, PZ, and POZ). Data were averaged across visual fields. The attentional effect on P1m increased as a function of perceptual load. The amplitude of C1 was larger for valid relative to invalid trials under the high-load condition, but only on CPZ site.
C1
The C1 component was seen over parietal cortex with its largest amplitude at the POZ site (Figure 3). For C1 amplitude, the validity and load main effects and all the interactions related to validity and load were not significant at POZ. Interestingly, the validity effect was significant on both PZ and CPZ sites [PZ: F (1, 18) = 4.909, p < 0.040; CPZ: F (1, 18) = 7.458, p < 0.014], with the valid trials eliciting larger C1 relative to invalid trials. The validity effect on CPZ remained significant after stringent Bonferroni correction for multiple comparisons (corrected p = 0.05/3 = 0.0167). Further separate analysis of low- and high-load conditions showed that the validity main effect was significant only for high-load stimuli [CPZ: F (1, 18) = 7.063, p < 0.016]. No significant load by attention interaction was found for all 3 electrode sites. For the peak latency of C1, the validity and load main effects were not significant, but the load by attention interaction was significant [POZ: F (1, 18) = 7.124, p < 0.016]. Further separate analysis showed that the validity main effect was only significant for the low-load stimuli [F (1, 18) = 8.594, p < 0.009], with a later C1 on valid relative to invalid trials (79 vs. 75 ms).
P1m
The amplitude of the midline P1 component (P1m) was larger for invalid relative to valid trials at the parietal area [PZ: F (1, 18) = 28.478, p < 0.0005], and this attentional effect increased with perceptual load, seen in the significant load × validity interaction [F (1, 18) = 4.750, p < 0.043]. The visual field by validity interaction was significant [F (1, 18) = 9.377, p < 0.007], suggesting that the validity effect was more pronounced for LVF relative to RVF stimuli. The peak latency of P1m was earlier for valid relative to invalid trials [F (1, 18) = 20.533, p < 0.0005]. No other main effects or interactions were significant for the latency of P1m.
P1l
For the lateral P1 amplitude, the visual field × validity interaction was significant [PO5/PO6: F (1, 18) = 7.256, p < 0.015]. Separate analysis showed a validity main effect for LVF stimuli [F (1, 18) = 4.413, p < 0.05], which, however, was not significant after a Bonferroni correction for multiple comparisons (corrected threshold: p = 0.05/2 = 0.025). No other main effects and interactions related to load and validity were significant. For the peak latency of P1l, the validity by visual field by hemisphere interaction was significant [F (1, 18) = 10.735, p < 0.004].
N1l
For the lateral N1 amplitude, No main effects and interactions related to load and validity were significant. The visual field by hemisphere interaction was significant [F (1, 18) = 43.527, p < 0.0005], suggesting that the N1 was larger on the contralateral relative to the ipsilateral hemisphere. For the peak latency of N1l, the visual by hemisphere interaction was significant [F (1, 18) = 39.324, p < 0.0005], and the validity by visual field by hemisphere interaction was significant [F (1, 18) = 11.295, p < 0.003].
Discussion
The perceptual load theory postulates that selective attention operates at an early stage of processing only when the perceptual load associated with target array is high [12, 13]. Meanwhile, several studies have shown that the time course of involuntary or reflexive attention effects is faster than that of voluntary attention [4, 17]. We therefore hypothesized that the earliest attentional selection may occur in the first cortical processing area V1 under conditions of high perceptual load and involuntary attention. We defined the term “involuntary attention” as the attention type that was elicited by peripheral stimulus onset, which presumably attracts attention automatically [4]. The present results support that hypothesis. Other important contributory factors may include the use of a cluttered visual field and stimuli that are tuned to V1 processing features (e.g., such as orientation).
A direct modification of Lavie’s experimental paradigm to ERP study is problematic and thus we have a different operational definition of perceptual load relative to Lavie’s original definition [7]. This is because of the ERP overlap between the central stimulus array and the peripheral testing stimulus in Lavie’s task. However, our definition of perceptual load is consistent with Lavie’s definition in terms of distractor filtering, which we believe is a key aspect in defining perceptual load of the stimuli. That is, for the high load stimuli, more distractors need to be rejected in Lavie’s design, whereas the distractors are more difficult to be rejected in our design.
Our results generally support the hypothesis that earliest attentional selection may occur in V1, given optimal experimental conditions. The visual C1 component, which has been localized to V1 [1, 3, 15], was modulated by attention under high load condition. This suggests that the initial processing in V1 may be affected by attention. Consistent with the main results of our previous study [6], invalid trials elicited a larger and later P1m relative to valid trials, and this attentional effect on P1m was larger for high-load relative to low-load stimuli. Therefore, these findings of a previous study were replicated in the absence of target stimulus confounds and cue-target sensory interactions. These results suggest (a) involuntary attention may affect stimulus processing as early as in the C1 time range (approximately 80 ms after stimulus onset) and (b) perceptual load interacts with involuntary attention at the posterior midline areas, starting at the P1m time range (approximately 140 ms for invalid trials).
It should be noted that the C1 attentional effect was found for a more parieto-centrally located site (CPZ) relative to more posterior sites such as POZ [1, 6] in previous studies. Interestingly, in the present study, the POZ site showed the largest C1 amplitude, but the attentional effect on that site was not significant. This raises a concern of whether the present attentional modulation in the C1 time range is a “real” C1 effect that arises from V1. One possibility is that, as proposed in a previous study [5], the early part of C1 (perhaps at the POZ site) is not modulated by attention, but a later part of C1 (perhaps at the CPZ site) is attention sensitive. This account seems less likely because the C1 peak latencies at CPZ and POZ shows no such timing difference (all around 80 ms). Alternatively, the C1 difference might be caused by ERP overlap from the subsequent P1m [7]. The later C1 peak latency of valid trials relative to invalid trials is consistent with this possibility. That is, the more negative C1 for valid relative to invalid trials might be caused by a larger P1m overlap in the C1 time range. Decisive evidence of the C1 origin requires source localization methods [1, 3, 7, 15, 20]. While those possibilities need further work to clarify, the present study used the term “C1 component” to refer to the ERP components in the C1 time range, as if it was a single ERP component without any sub-components and/or overlap. Consequently, “early modulation” or “early interaction” in the present study is between the C1 and P1 components but not between the early and late parts of the C1 component [5].
The attentional effect on P1m was robust in both the present study and our previous study [6]. As we discussed in that study [6], both the timing and the scalp distribution of this P1m component suggest that its underlying brain source(s) are different from the previously reported lateral P1 component and perhaps involves the dorsal parieto-frontal network (e.g., superior parietal lobule and frontal eye field) that is involved of involuntary attention [2]. Moreover, the posterior to parieto-central moving of the scalp distribution map of this P1m suggests that it occurs in the feed-forward processing of visual information. Furthermore, the perceptual load by attention interaction was reliable for P1m; however, the perceptual load by attention interaction for C1 component was observed in our previous study but was absent in the present study. Overall, the attentional effect and the perceptual load by attention interaction were more robust and consistent on P1m relative to C1. Whether this is only due to the amplitude size (and thus perhaps statistical power) difference between these two components, or this is due to other unknown mechanisms, deserves further research work.
Acknowledgments
This research was supported by NIH grant AG19653 to RP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clark VP, Hillyard SA. Spatial selective attention affects early extrastriate but not striate components of the visual evoked potential. Journal of Cognitive Neuroscience. 1996;8:387–402. doi: 10.1162/jocn.1996.8.5.387. [DOI] [PubMed] [Google Scholar]
- 2.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 3.Di Russo F, Martinez A, Hillyard SA. Source analysis of event-related cortical activity during visuo-spatial attention. Cerebral cortex. 2003;13:486–499. doi: 10.1093/cercor/13.5.486. [DOI] [PubMed] [Google Scholar]
- 4.Eimer M. The time course of spatial orienting elicited by central and peripheral cues: evidence from event-related brain potentials. Biological Psychology. 2000;53:253–258. doi: 10.1016/s0301-0511(00)00049-1. [DOI] [PubMed] [Google Scholar]
- 5.Foxe JJ, Simpson GV. Flow of activation from V1 to frontal cortex in humans. A framework for defining “early” visual processing. Experimental Brain Research. 2002;142:139–150. doi: 10.1007/s00221-001-0906-7. [DOI] [PubMed] [Google Scholar]
- 6.Fu S, Huang Y, Luo Y, Wang Y, Fedota J, Greenwood PM, Parasuraman R. Perceptual load interacts with involuntary attention at early processing stages: event-related potential studies. NeuroImage. 2009;48:191–199. doi: 10.1016/j.neuroimage.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu S, Zinni M, Squire P, Kumar R, Caggiano DM, Parasuraman R. When and where perceptual load interacts with voluntary visuospatial attention: an event-related potential and dipole modeling study. NeuroImage. 2008;39:1345–1355. doi: 10.1016/j.neuroimage.2007.09.068. [DOI] [PubMed] [Google Scholar]
- 8.Heinze HJ, Mangun GR, Burchert W, Hinrichs H, Scholz M, Munte TF, Gos A, Scherg M, Johannes S, Hundeshagen H. Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature. 1994;372:543–546. doi: 10.1038/372543a0. [DOI] [PubMed] [Google Scholar]
- 9.Hopf JM, Noesselt T, Tempelmann C, Braun J, Schoenfeld A, Heinze HJ. Popout modulates focal attention in the primary visual cortex. NeuroImage. 2004;22:574–582. doi: 10.1016/j.neuroimage.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Kelly SP, Gomez-Ramirez M, Foxe JJ. Spatial attention modulates initial afferent activity in human primary visual cortex. Cerebral cortex. 2008;2008:2629–2636. doi: 10.1093/cercor/bhn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khoe W, Mitchell J, Reynolds J, Hillyard SA. Exogenous attentional selection of transparent superimposed surfaces modulates early event-related potentials. Vision Research. 2005;45:3004–3014. doi: 10.1016/j.visres.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Lavie N. Perceptual load as a necessary condition for selective attention. Journal of Experimental Psychology: Human Perception & Performance. 1995;21:451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- 13.Lavie N, Tsal Y. Perceptual load as a major determinant of the locus of selection in visual attention. Perception & Psychophysics. 1994;56:183–197. doi: 10.3758/bf03213897. [DOI] [PubMed] [Google Scholar]
- 14.Mangun GR, Buonocore M, Girelli M, Jha A. ERP and fMRI measures of visual spatial selective attention. Human Brain Mapping. 1998;6:383–389. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<383::AID-HBM10>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez A, Anllo-Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, Wong EC, Hinrichs H, Heinze HJ, Hillyard SA. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nature Neuroscience. 1999;2:364–369. doi: 10.1038/7274. [DOI] [PubMed] [Google Scholar]
- 16.Martinez A, DiRusso F, Anllo-Vento L, Sereno MI, Buxton RB, Hillyard SA. Putting spatial attention on the map: timing and localization of stimulus selection processes in striate and extrastriate visual areas. Vision Research. 2001;41:1437–1457. doi: 10.1016/s0042-6989(00)00267-4. [DOI] [PubMed] [Google Scholar]
- 17.Muller HJ, Rabbitt PM. Reflexive and voluntary orienting of visual attention: time course of activation and resistance to interruption. Journal of Experimental Psychology: Human Perception and Performance. 1989;15:315–330. doi: 10.1037//0096-1523.15.2.315. [DOI] [PubMed] [Google Scholar]
- 18.Noesselt T, Hillyard SA, Woldorff MG, Schoenfeld A, Hagner T, Jancke L, Tempelmann C, Hinrichs H, Heinze HJ. Delayed striate cortical activation during spatial attention. Neuron. 2002;35:575–587. doi: 10.1016/s0896-6273(02)00781-x. [DOI] [PubMed] [Google Scholar]
- 19.Poghosyan V, Shibata T, Loannides AA. Effects of attention and arousal on early responses in striate cortex. European Journal of Neuroscience. 2005;22:225–234. doi: 10.1111/j.1460-9568.2005.04181.x. [DOI] [PubMed] [Google Scholar]
- 20.Rauss KS, Pourtois G, Vuilleumier P, Schwartz S. Attentional load modifies early activity in human primary visual cortex. Human Brain Mapping. 2009;30:1723–1733. doi: 10.1002/hbm.20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Somers DC, Dale AM, Seiffert AE, Tootell RB. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1663–1668. doi: 10.1073/pnas.96.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Super H, Spekreijse H, Lamme VA. Two distinct modes of sensory processing observed in monkey primary visual cortex (V1).[see comment] Nature Neuroscience. 2001;4:304–310. doi: 10.1038/85170. [DOI] [PubMed] [Google Scholar]
- 23.Talsma D, Woldorff MG. Methods for the estimation and removal of artifacts and overlap in ERP waveforms. In: Handy TC, editor. Event-related Potentials: a Method Handbook. Cambridge, MA: MIT Press; 2005. pp. 115–148. [Google Scholar]
- 24.Wu Y, Chen J, Han S. Neural mechanisms of attentional modulation of perceptual grouping by collinearity. Neuroreport. 2005;16:567–570. doi: 10.1097/00001756-200504250-00010. [DOI] [PubMed] [Google Scholar]


