Abstract
TNF-related apoptosis-inducing ligand (TRAIL) is a potent inducer of cell death in several cancer cells, but many cells are resistant to TRAIL. The mechanism that determines sensitivity to TRAIL-killing is still elusive. Here we report that deletion of TAK1 kinase greatly increased activation of caspase-3 and induced cell death following TRAIL stimulation in keratinocytes and fibroblasts as well as cancer cells. Although TAK1 kinase is involved in NF-κB pathway, ablation of NF-κB did not alter sensitivity to TRAIL. We found that TRAIL could induce accumulation of reactive oxygen species (ROS) when TAK1 was deleted. Furthermore, we found that TAK1 deletion induces TRAIL-dependent downregulation of cIAP, which enhances activation of caspase-3. These results demonstrate that TAK1 deletion facilitates TRAIL-induced cell death by activating caspase through ROS and downregulation of cIAP. Thus, inhibition of TAK1 can be an effective approach to increase TRAIL sensitivity.
Keywords: TAK1, TRAIL, reactive oxygen species, cIAP, apoptosis
INTRODUCTION
TNF-related apoptosis inducing ligand (TRAIL) is a member of the TNF family cytokines that can selectively kill several cancer cells, while non-transformed cells are resistant to TRAIL (Takeda et al, 2007). TRAIL initiates cell death signaling through the binding of TRAIL to death receptor 4 (DR4) and death receptor 5 (DR5) (Ashkenazi, 2002; LeBlanc & Ashkenazi, 2003; Wang & El-Deiry, 2003). TRAIL binding to its receptors leads to the assembly of Fas-associated death domain (FADD) and caspases-8 at the C-terminus of the receptors to form the death inducing signaling complex (DISC). Activation of caspase-8 in the DISC is then followed by the activation of executioner caspases, such as caspases-3. In TNF-induced signaling pathways, TNF not only initiates cell death signaling but also up-regulates anti-apoptotic genes such as cellular FLICE-inhibitory protein (c-FLIP) through a transcription factor NF-κB (Aggarwal, 2003; Hayden & Ghosh, 2008; Micheau et al, 2001; Wang et al, 1998). Because this anti-apoptotic pathway normally overrides the cell death pathway in most cell types, TNF does not kill cells (Aggarwal, 2003). Inhibition of NF-κB pathway increases the sensitivity to TNF-induced cell death (Karin & Lin, 2002). In contrast to TNF, involvement of NF-κB in the TRAIL sensitivity has not been established. In several types of cells, TRAIL activates NF-κB resulting in upregulation of anti-apoptotic genes such as Mcl-1 (Kim et al, 2008b; Meng et al, 2007; Ricci et al, 2007) and c-FLIP (Kim et al, 2002; Siegmund et al, 2001; Xiao et al, 2002), which have been implicated in TRAIL resistance. In other reports, NF-κB signaling is suggested to be not critical for TRAIL resistance (Kurbanov et al, 2007; Varfolomeev et al, 2005). Thus, involvement of NF-κB in TRAIL sensitivity is still undetermined.
TAK1 kinase is a member of the mitogen activated protein kinase kinase kinase (MAPKKK) family and is activated by innate immune stimuli including bacterial components and proinflammatory cytokines such as IL-1 and TNF (Chen et al, 2006; Ninomiya-Tsuji et al, 1999; Sato et al, 2005). Recently, Choo et al has demonstrated that TAK1 is important for blocking TRAIL-induced cancer cell death (Choo et al. 2006). However, the mechanism of how TAK1 regulates TRAIL-killing is still elusive. TAK1 plays an essential role in innate immune signaling by activating both IKK-NF-κB and MAPK pathways. TAK1 is an indispensable intermediate of NF-κB activation. In addition to this well-established TAK1 pathway, we have recently identified that the TAK1 modulates the levels of reactive oxygen species (ROS), which is important for preventing TNF-induced cell death in keratinocytes (Omori et al, 2006; Omori et al, 2008). It has been reported that ROS plays an essential role in TNF-induced cell death (Chang et al, 2006; Kamata et al, 2005; Ventura et al, 2004). We found that TAK1 is essential for preventing accumulation of TNF-induced ROS and subsequent activation of ROS-mediated death pathways (Omori et al, 2008). Thus, TAK1 is involved in two pathways that potentially affect sensitivity to cell death signaling; one is NF-κB pathway; and another is a pathway through ROS regulation. In this study, we investigated the mechanism by which TAK1 regulates sensitivity to TRAIL-induced cell death. We asked whether TAK1-NF-κB or TAK1-ROS pathway is involved in TRAIL sensitivity.
RESULTS AND DISCUSSION
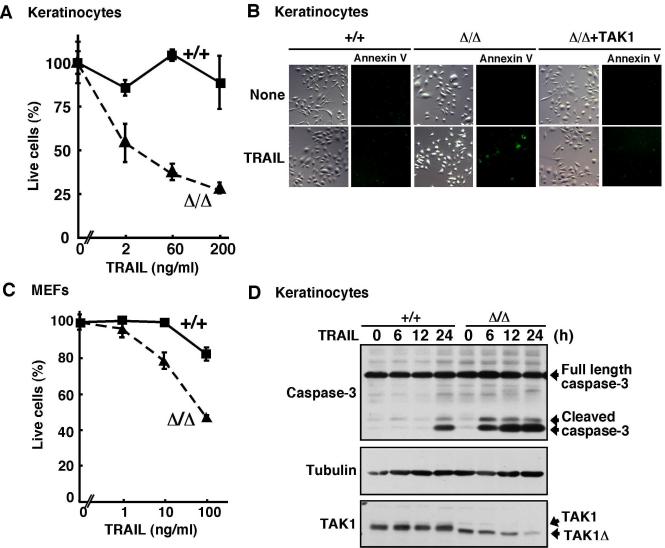
TAK1 is essential for cell survival against TRAIL-induced cell death
We investigated the role of TAK1 signaling in TRAIL-induced cell death. We used wild-type and TAK1Δ/Δ mouse keratinocytes (Omori et al, 2006) and embryonic fibroblasts (MEFs) (Sato et al, 2005) to determine the essential role of TAK1 in TRAIL-killing pathway. Wild-type and TAK1Δ/Δ keratinocytes were isolated from the dorsal skin of TAK1-floxed (TAK1flox/flox) or epidermis-specific TAK1 deletion (K5-Cre TAK1flox/flox) mice, respectively. In this floxed TAK1 system, Cre recombinase catalyzes the deletion of the TAK1 ATP binding site, amino acid 41-77, resulting in the generation of a truncated form of TAK1 (TAK1Δ) (Fig. 1D, bottom panel). We treated wild-type and TAK1Δ/Δ keratinocytes with TRAIL and cell viability was determined. While wild-type keratinocytes were completely resistant to TRAIL, TRAIL could induce cell death in TAK1Δ/Δ keratinocytes in a dose dependent manner (Fig. 1A). To verify whether this cell death is associated with apoptosis, we examined annexin V binding (Fig. 1B). TRAIL increased annexin V binding positive cells in TAK1Δ/Δ keratinocytes. To confirm whether this cell death is due to TAK1 deficiency, we generated TAK1-restored TAK1Δ/Δ keratinocytes by infecting TAK1 expressing retrovirus and performed the annexin V binding assay. Restoration of TAK1 completely inhibited TRAIL-induced apoptosis, suggesting that TAK1 ablation is the cause of hypersenisitivty to TRAIL (Fig. 1B). We next asked whether this hypersensitivity to TRAIL is specific for keratinocytes, and we treated wild-type and TAK1Δ/Δ MEFs with TRAIL stimulation. The earlier study reported that TAK1 ablation does not increase sensitivity to TRAIL-induced cell death in MEFs (Choo et al. 2006). However, we found that TRAIL also induced cell death in TAK1Δ/Δ MEFs, although the sensitivity to TRAIL-induced cell death is less compared with that in keratinocytes (Fig. 1C). Our data suggest that TAK1 is essential for preventing TRAIL-induced cell death in non-transformed cells including keratinocytes and MEFs. TRAIL binding to TRAIL receptor initiates activation of the caspase cascade consisting of caspase-8 and caspase-3, which is the major pathway to TRAIL-induced cell death (Aggarwal, 2003). Thus, we examined TRAIL-induced activation of the downstream caspase, caspase-3. Whereas TRAIL weakly induced caspase-3 activation at 24 h in wild-type keratinocytes, TRAIL highly induced caspase-3 activation within 6 h in TAK1Δ/Δ keratinocytes (Fig. 1D). Thus, TAK1 prevents TRAIL-induced cell death through inhibiting caspase activation.
Fig. 1. TAK1 deletion causes hypersensitivity to TRAIL.
(A) Wild-type (+/+) and TAK1Δ/Δ keratinocytes were stimulated with TRAIL. At 24 h post-TRAIL stimulation, live cells were measured by crystal violet staining. Data are the means and SD of three samples.
(B) Wild-type (+/+), TAK1Δ/Δ and TAK1-restored TAK1Δ/Δ (Δ/Δ+TAK1) keratinocytes were stimulated with TRAIL (200 ng/ml) for 18 h. Annexin V-Alexa Fluor 488 binding assay was performed. Light microscope images show cell morphology.
(C) Wild-type (+/+) and TAK1Δ/Δ MEFs were stimulated with TRAIL. At 24 h post-TRAIL stimulation, live cells were measured by crystal violet staining. Data are the means and SD of three samples.
(D) Wild-type (+/+) and TAK1Δ/Δ keratinocytes were stimulated with TRAIL (100 ng/ml). Activation of caspase-3 was analyzed by immunoblots. α-tubulin was used as a loading control. The proteins of TAK1 (full length) and TAK1Δ were detected by immunoblots.
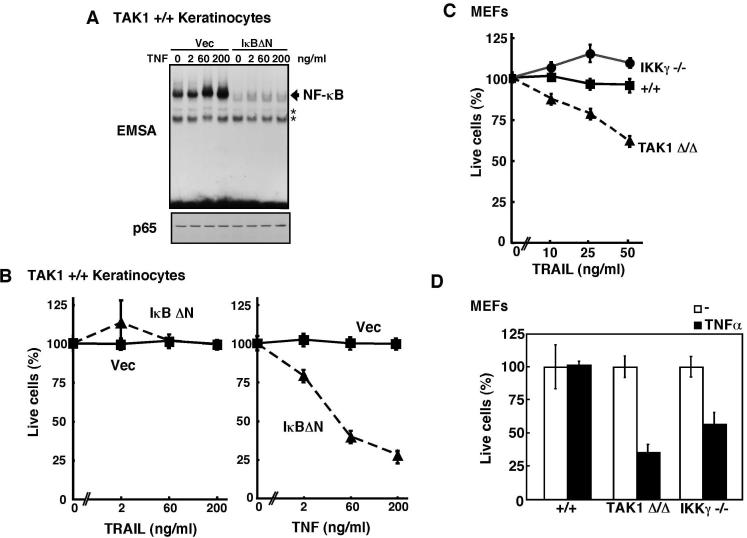
NF-κB does not contribute to cell survival in TRAIL-dependent cell death
NF-κB is one of the major cell survival factors that upregulates a number of anti-apoptotic molecules including caspase inhibitors, c-FLIP, Mcl-1 and BclxL. TAK1 is the major upstream regulator of NF-κB in proinflammatory cytokine signaling pathways including TNF pathway (Shim et al, 2005; Takaesu et al, 2003). It has been reported that TRAIL activates NF-κB in several cell types (Varfolomeev et al, 2005). Therefore, one likely mechanism by which TAK1 prevents TRAIL-induced cell death is that TAK1 activates NF-κB in response to TRAIL stimulation and promotes cell survival. The earlier report proposed that lack of NF-κB activation might be the cause of cell death in TAK1-deficient cells (Choo et al. 2006). However, we found that TNF but not TRAIL could activate TAK1 pathways including activation of NF-κB, JNK, and p38 (Supplementary Fig. S1), suggesting that NF-κB may not be the major pathway to determine TRAIL sensitivity. To further examine whether the NF-κB pathway contributes to TRAIL resistance in keratinocytes, we generated wild-type keratinocytes ectopically expressing a dominant negative type of IκB (IκBΔN) that lacks the N-terminal region including the phosphorylation sites for its degradation (Brockman et al, 1995). In these cells, TNF-induced NF-κB activation was completely abolished (Fig. 2A). Furthermore, the basal level of NF-κB was also significantly reduced in the IκBΔN expressing keratinocytes. Therefore, the IκBΔN expressing keratinocytes may be considered as NF-κB-deficient cells. We examined the susceptibility of these cells to TRAIL-killing. We found that abrogation of NF-κB pathway did not sensitize cells to TRAIL (Fig. 2B). In contrast, TNF could induce cell death in the IκBΔN expressing keratinocytes to some extent (Fig. 2B) as we showed previously (Omori et al, 2008), which is consistent with earlier studies that used IKK deficient MEFs (Li et al, 1999; Rudolph et al, 2000). To further examine whether downregulation of NF-κB does not cause hypersensitivity to TRAIL-induced cell death, we utilized IKKγ/NEMO knockout MEFs (Makris et al, 2000) and examined the TRAIL sensitivity. IKKγ/NEMO knockout MEFs were sensitive to TNF-induced cell death as previously reported (Rudolph et al, 2000) (Fig. 2D), however, we found that they were completely resistant to TRAIL-induced cell death (Fig. 2C). These results demonstrate that NF-κB pathway is not the major pathway to prevent TRAIL-induced cell death. Therefore, TAK1-mediated prevention of TRAIL-induced cell death should be through non-NF-κB mechanisms.
Fig. 2. Inhibition of NF-κB does not alter TRAIL sensitivity.
(A) Wild-type keratinocytes infected retrovirus expressing IκBΔN or control vector were stimulated with TNF for 30 min and the level of NF-κB activation was analyzed by EMSA (upper panal). The amount of p65 NF-κB was analyzed by immunoblot (lower panel). Asterisks indicate non-specific bands.
(B) Wild-type keratinocytes infected retrovirus expressing IκBΔN or control vector were stimulated with TRAIL (left panel) or TNF (right panel). At 24 h post-TRAIL or -TNF stimulation, live cells were measured by crystal violet staining. Data are the means and SD of three samples.
(C and D) Wild-type (+/+), TAK1Δ/Δ or IKKγ-/- MEFs were stimulated with TRAIL (C) or TNF (20 ng/ml) (D). At 24 h post TRAIL or TNF stimulation, live cells were measured by crystal violet staining. Data are the means and SD of three samples.
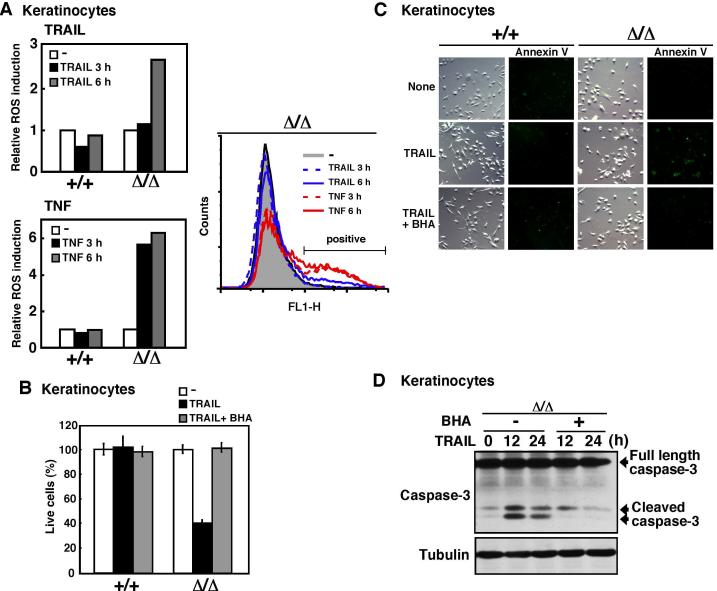
Reactive oxygen species were accumulated upon TRAIL stimulation in TAK1 deficient keratinocytes and cancer cells
Apoptosis is induced by activation of the caspase cascade and it has been known that reactive oxygen species (ROS) facilitate this apoptotic signaling (Chang et al, 2006). Moreover, we have recently reported that TAK1 deficiency causes increased ROS in TNF-treated TAK1-deficient cells (Omori et al, 2008). Therefore, we speculated that TRAIL might increase ROS in TAK1-deficient cells, and that TAK1-dependent ROS regulation might contribute to TRAIL sensitivity. We examined the level of ROS in TRAIL- and TNF-treated keratinocytes (Fig. 3A). We found that ROS were increased in TAK1Δ/Δ but not wild-type keratinocytes upon both TNF and TRAIL stimulation. TNF greatly increased the level of ROS within 3 h after stimulation. TRAIL was a less potent inducer of ROS compared with TNF. TRAIL did not increase the level of ROS at 3 h, but the level of ROS was increased 6 h after stimulation. We next asked whether this increased ROS causes hypersensitivity to TRAIL. We pretreated cells with the antioxidant butylated hydroxyanisole (BHA) and stimulated cells with TRAIL. BHA treatment completely blocked TRAIL-induced cell death (Fig. 3B) and annexin V binding (Fig. 3C). TRAIL-induced caspase-3 activation was also inhibited by BHA treatment (Fig. 3D). These results suggest that TRAIL increased ROS when TAK1 is deleted, which promotes apoptotic signaling pathway.
Fig. 3. ROS are involved in TRAIL-induced cell death in TAK1 deficient cells.
(A) Wild-type (+/+) and TAK1Δ/Δ keratinocytes were stimulated with TRAIL (100 ng/ml) or TNF (20ng/ml), subsequently incubated with CM-H2DCFDA for 30 min, and cells were analyzed by flow cytometry and FlowJo software (Tree Star Inc.). The fluorescence units relative to that of unstimulated cells are shown (left graphs). Data are representative of two independent experiments with similar results.
(B-D) TAK1Δ/Δ keratinocytes were pretreated with BHA (100 μ-M) or vehicle for 1 h and subsequently stimulated with TRAIL (200 ng/ml). At 24 h post-TRAIL stimulation, live cells were measured by crystal violet staining. Data are the means and SD of three samples (B). At 18 h post-TRAIL stimulation, apoptotic cells were determined by an annexin V-binding assay (C). Activation of caspase-3 was analyzed by immunoblots and α-tubulin was use as a loading control (D).
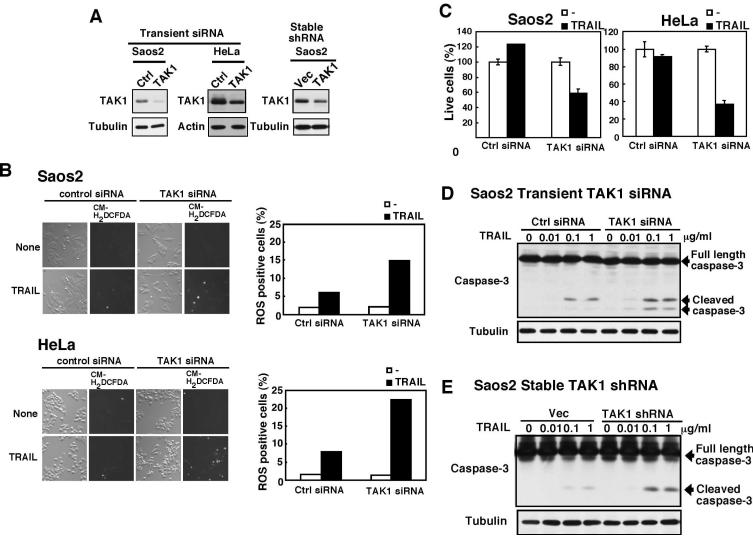
TRAIL treatment is one of the most anticipated anti-cancer therapies. However, many cancer cells are TRAIL resistant. We postulate that downregulation of TAK1 can increase in sensitivity to TRAIL through ROS in cancer cells. We downregulated TAK1 expression in human osteosarcoma Saos2 cells and HeLa cells by transient transfection of a siRNA and also by stable transfection of a shRNA targeted against TAK1, and examined for ROS accumulation and TRAIL-induced cell death (Fig. 4). Saos2 cells were resistant to TRAIL-induced cell death, while HeLa cells were moderately sensitive to TRAIL-induced cell death. We treated Saos2 and HeLa cells with 100 ng/ml and 10 ng/ml TRAIL, respectively, which did not kill control cells with normal TAK1 expression (Fig. 4C). We found that TAK1 knockdown greatly increased TRAIL-induced ROS accumulation (Fig. 4B) and concomitantly induced cell death in both Saos2 and HeLa cells (Fig. 4C). We used two different siRNA or shRNA targeted sequences of TAK1 and obtained the similar results. To further assess whether this cell death is through the same mechanism as that in keratinocytes, we examined activation of caspase-3 (Fig. 4D and E). TRAIL-induced activation of caspase-3 was enhanced in TAK1 knockdown Saos2 cells. These results indicate that inhibition of TAK1 can sensitize cancer cells to TRAIL through increasing ROS.
Fig. 4. TAK1 down regulation accumulates ROS and sensitizes cancer cells to TRAIL.
(A) TAK1 or non-targeting (Ctrl) siRNA was transfected into Saos2 or HeLa cells using the electroporation method, and cells were incubated for 3 days (left and middle panels). Saos2 cells were infected a retrovirus expressing TAK1 shRNA or control vector, and infected cells were selected as described in Supplementary information (right panels). The amounts of TAK1 were analyzed by immunoblots, and α-tubulin or β-actin was used as a loading control.
(B) Non-targeting (Ctrl) and TAK1 siRNA transfected Saos2 cells were stimulated with TRAIL (100 ng/ml) for 18 hours. Non-targeting (control) and TAK1 siRNA transfected HeLa cells were stimulated with TRAIL (10 ng/ml) for 6 hours. Subsequently, cells were incubated with CM-H2DCFDA for 30 min, and 3-5 randomly selected areas were photographed with the same exposure time. The images were processed using the fixed threshold in all samples in each experiment, and more than 1000 cells were counted for each sample.
(C) Saos2 cells were stimulated with TRAIL (100 ng/ml) for 24 hours. HeLa cells were stimulated with TRAIL (10 ng/ml) for 18 hours. Live cells were measured by crystal violet staining. Data are the means and SD of three samples.
(D, E) Activation of caspase-3 was analyzed by immunoblots in TAK1 siRNA transiently transfected (D) or TAK1 shRNA stably infected (E) Saos2 cells. α-tubulin was used as a loading control.
Ablation of TAK1 downregulates cIAP
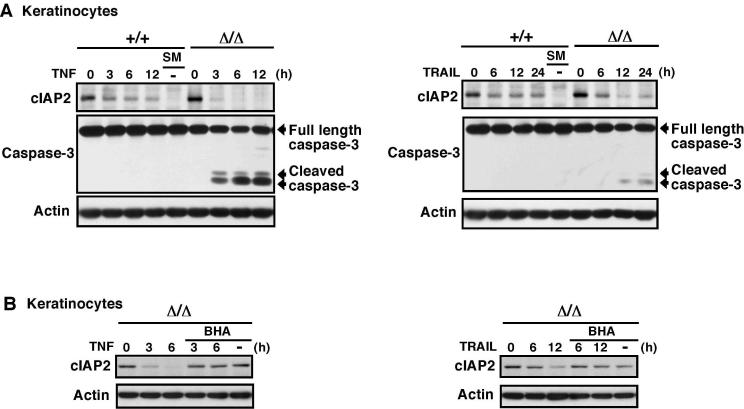
We next attempted to determine the mechanism of how TRAIL-induced ROS facilitates activation of caspases in TAK1 deficient cells. Because TRAIL activates caspase through DISC, we speculated that ROS might enhance activation of DISC. Two pathways have been known to enhance DISC activation. One is c-FILP downregulation, which is through inhibition of NF-κB (Chang et al, 2006). Recently, a new pathway to activate DISC, which is independent of inhibition of NF-κB, has been revealed (Wang et al, 2008). In this pathway, smac, an inhibitor of IAP, induces degradation of cIAPs which results in activation of DISC-induced caspases (Petersen et al, 2007; Vince et al, 2007). Smac mimetics, small molecules mimicking smac binding to IAP, induce DISC-dependent apoptosis through this pathway (Petersen et al, 2007; Vince et al, 2007; Wang et al, 2008). Because NF-κB is not involved in TAK1 deletion-induced TRAIL sensitivity as described above, we speculated that TAK1 deletion might facilitate this smac-cIAP pathway. We treated cells with TNF and TRAIL, and examined the levels of cIAPs in wild-type and TAK1Δ/Δ keratinocytes (Fig. 5A). The smac mimetic was used as a positive control. We found that the amount of cIAP2 somewhat decreased upon treatment of TNF and TRAIL in both wild-type and TAK1Δ/Δ keratinocytes, and that the reduction was augmented by TAK1 deletion. At 6-12 h post-TNF treatment and at 12-24 h post-TRAIL treatment, the amount of cIAP2 was greatly diminished in TAK1Δ/Δ keratinocytes. This decreased cIAP2 level was correlated with activation of caspase 3 (Fig. 5A). The smac mimetic alone did not induce activation of caspase 3 in keratinocytes, which suggests that cIAP degradation alone is not sufficient to induce apoptosis in keratinocyte. This is consistent with the previously reported effect of smac mimetics in several cell types (Petersen et al, 2007; Vince et al, 2007; Wang et al, 2008). Both DISC activation and cIAP degradation are required to trigger apoptosis. We note that we were not able to detect cIAP1 in keratinocytes. To examine whether ROS mediate this decrease of cIAP2, we treated keratinocytes with the antioxidants BHA and L-N-acetylcysteine (L-NAC). BHA could inhibit TNF- and TRAIL-induced reduction of cIAP2 (Fig. 5B). L-NAC could also block TNF-induced reduction of cIAP2 and activation of caspase 3 (Supplementary Fig. S2). These results suggest that ablation of TAK1 downregulates cIAP in response to TNF and TRAIL treatment, which facilitates caspase activation.
Fig. 5. TRAIL downregulates cIAP in TAK1 deficient keratinocytes.
(A) Wild-type (+/+) and TAK1Δ/Δ keratinocytes were stimulated with TNF (20 ng/ml) or TRAIL (100 ng/ml). Smac mimetic (SM) (1 μ-M) was used as a control. cIAP and caspase-3 were detected by immunobots. β-actin was used as a loading control.
(B) TAK1Δ/Δ keratinocytes was pretreated with or without 100 μ-M BHA for 1 h and stimulated with TNF (20 ng/ml) or TRAIL (100 ng/ml) or left unstimulated (BHA alone). cIAP was detected by immunobots. β-actin was used as a loading control.
Choo et al. has previously reported that TAK1 knockdown sensitizes HeLa cells to TRAIL-induced cell death, and discussed that impaired activation of NF-κB might be the cause of this hypersensitivity (Choo et al, 2006). However, here we show that TAK1 but not NF-κB regulates TRAIL sensitivity. We further demonstrate that TRAIL induces ROS accumulation and cIAP degradation in TAK1 deficient keratinocytes. Our results revealed a novel mechanism that determines sensitivity to TRAIL. TAK1 regulates TRAIL sensitivity through the ROS-cIAP pathway.
Our findings raise several important questions. One is the mechanism by which ROS induce cIAP degradaion. We speculate that ROS damage mitochondria resulting in mitochondrial permeabilization, which could release smac, a major inducer of cIAP degaradation (Wang et al, 2008). Another important question is the mechanism by which TAK1 regulates ROS. We have shown that TRAIL does not detectably activate TAK1 at least in keratinocytes. In contrast, Herrero-Martin et al. has recently reported that TAK1 is activated by TRAIL in human breast epithelial cells, and that TAK1 activation of AMP-activated protein kinase is involved in TRAIL sensitivity (Herrero-Martin et al, 2009). The difference in TAK1 activation by TRAIL may be due to cell type difference. In regard to ROS regulation, we have recently reported that the basal level of c-Jun is regulated by TAK1, which is critically involved in ROS metabolism (Omori et al. 2008). Furthermore, we have recently observed that the basal levels of several redox enzymes including catalase, NAD(P)H:quinone oxidoreductase 1 (NQO1) and glutathione S-transferases were downregulated in TAK1-deficient cells (SM, EO, RK-S and JN-T unpublished observation). These suggest that TAK1 modulates redox enzymes possibly through cJun under basal conditions, which prevents accumulation of ROS following TRAIL stimulation. We propose that the basal activity of TAK1 is important for determination of TRAIL sensitivity. In some cell types, TAK1 may be further activated by TRAIL and prevents accumulation of ROS.
TRAIL can selectively kill several cancer cells but not the majority of normal cells. Therefore, TRAIL is one of the most anticipated anti-cancer drugs (Ashkenazi, 2002; Reed, 2006; Takeda et al, 2007). However, some cancer cells are resistant to TRAIL treatment. A number of trials have been conducted to sensitize these cancer cells to TRAIL-killing (Kim et al, 2008b). We here demonstrate that deletion of TAK1 effectively sensitizes cells to TRAIL-killing even in non-transformed keratinocytes and fibroblasts, whereas NF-κB deletion is not capable of sensitizing those cells to TRAIL-killing. This raises the possibility that a combination of inhibition of TAK1 with the TRAIL treatment could effectively kill cancer cells, although this combination may kill normal cells. In summary, our finding provides a novel strategy to sensitize cells to TRAIL. The TAK1-ROS-cIAP degradation axis may be a new target for cancer therapy.
METHODS
Cells
Wild-type and TAK1Δ/Δ keratinocytes were isolated from TAK1flox/flox, K5-Cre TAK1 flox/flox mice described previously and cultured as described previously (Omori et al, 2006). Wild-type and TAK1Δ/Δ MEFs were gifts from Dr. Akira, Osaka University (Sato et al, 2005). IKKγ-/- MEFs were gifts from Dr. Karin, University of San Diego (Makris et al, 2000). MEFs, 293 cells, Saos2 and HeLa cells were cultured in DMEM with 10% bovine growth serum (Hyclone), and penicillin-streptomycin at 37°C in 5% CO2.
Reagents
TRAIL (human recombinant; Peprotech) and TNFα (human recombinant; Roche) were used. Smac mimetic was a gift from Dr. Xiaodong Wang, University of Texas Southwestern Medical Center at Dallas (Li et al, 2004). Butylated Hydroxy-Anisole (BHA) and L-N-acetylcysteine (L-NAC) were purchased from Sigma and Calbiochem, respectively. Polyclonal Antibodies used were TAK1 described previously (Ninomiya-Tsuji et al, 1999), JNK1 (FL; Santa Cruz), p65 (F-6; Santa Cruz), caspase-3 (Cell Signaling), phospho-p38 (Thr-180/Tyr-182; Cell Signaling), and p-38 (N-20; Santa Cruz). Monoclonal antibodies were tubulin (Santa Cruz), βactin (Sigma), IκBα (H-4; Santa Cruz), phospho-JNK (Thr-183/Tyr-185; Cell signaling) and cIAP (R&D Systems).
Plasmids
Retroviral vector for TAK1 (pMxpuro-TAK1) was described previously (Kim et al, 2008a). Retroviral vector for IκBΔN (pQCXIP-IκBΔN) was generated by inserting IκBΔN cDNA into retroviral vector pQCXIP (Clontech). IκBΔN cDNA was a gift from Dr. D. Ballard, Vanderbilt University (Brockman et al, 1995). TAK1 siRNA target sequence corresponded to nucleotides 88-106 of the TAK1 cording region was used to generate a retrovirus vector expressing shRNA against TAK1. The region containing the TAK1 shRNA in the BS/H1 vector for TAK1 siRNA (Kajino et al, 2007) was subcloned into pSUPERRetro vector (OligoEngine).
Cytotoxicity Assay
The viable adherent cells were fixed with 10% formalin and stained with 0.1% crysatal violet. The stain was solubilized by adding 50% ethanol containing 50 mM sodium citrate, and the absorbance of each plate was determined at 595 nm.
Electrophoresis mobility shift assay (EMSA)
The binding reaction contained radiolabeled 32P-NF-κB oligonucleotide probe (Promega), 10 μg cell extracts, 4% glycerol, 1mM MgCl2, 0.5mM EDTA, 0.5 mM DTT, 50 mM NaCl, 10 mM Tris-HCl (pH 7.5), 500 ng of poly (dI-dC) (GE Healthcare), and 10 μg of bovine serum albumin to a final concentration of 15 μl. The reaction mixture were incubated at 25°C for 30 min, separated by 5% (w/v) polyacrylamide gel, and visualized by autoradiography.
Immunoblotting
Whole cell extracts were prepared using a lysis buffer containing 20 mM HEPES (pH 7.4), 150 mM NaCl, 12.5 mM β-glycerophospate, 1.5 mM MgCl2, 2 mM EGTA, 10 mM NaF, 2 mM DTT, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 20 μM aprotinin, 0.5% Triton X-100. Cell extracts were resolved on SDS-PAGE and transferred to Hypond-P membranes (GE Healthcare). The membranes were immunoblotted with various antibodies, and the bound antibodies were visualized with horseradish peroxidase-conjugated antibodies against rabbit or mouse IgG using the ECL Western blotting system (GE Healthcare).
Retroviral infection
EcoPack 293 cells (BD Biosciences) were transiently transfected with pQCXIP-IκBΔN. After 48 h culture, growth medium containing the retrovirus was collected and filtered with 0.45 μm cellulose acetate membrane to remove packaging cells. Keratinocytes were incubated with the collected virus-containing medium with 8 μg/ml polybrane for 24 h. Uninfected cells were removed by puromycin selection. GP-293 cells (BD Biosciences) were transiently transfected with pSUPERRetro-TAK1shRNA. After 48 h culture, growth medium containing the retrovirus was collected and filtered with 0.45 μm cellulose acetate membrane to remove packaging cells. Saos2 or Hela cells were incubated with the collected virus-containing medium with 8 μg/ml polybrane for 24 h. Uninfected cells were removed by puromycin selection.
Annexin V-binding assay
To determine apoptotic cells, Annexin V-Alexa Fluor 488 binding was performed according to the manufacturer's protocol (Invitrogen), and fluorescence was detected with fluorescent microscope (Olympus).
ROS measurement
Keratinocytes were stimulated with TRAIL and incubated with 10 μM CM-H2DCFDA (Invitrogen) for 30 min at 37°C, harvested and analyzed by flow cytometry or fluorescence was detected by microscope.
Transient siRNA system
TAK1 and non-targeting control siRNAs were obtained from Dharmacon (TAK1 siRNA, 5′-GAGUGAAUCUGGACGUUUA-3′; Non-targeting siRNA#1). SAOS2 or Hela cells were tripsinized and collected. Cells were sustained in OPTI-MEM medium (Invitrogen) containing 1 μM siRNA oligos and subjected to electroporation using Gene Pulser Xcell (BIO-RAD).
Supplementary Material
Acknowledgements
We thank Drs. Akira, Karin, Ballard and Wang for materials. This work was supported by a grant (GM068812) from NIH to J. N-T.
REFERENCES
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- Brockman JA, Scherer DC, McKinsey TA, Hall SM, Qi X, Lee WY, Ballard DW. Coupling of a signal response domain in IκB alpha to multiple pathways for NF-kB activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M. The E3 ubiquitin ligase itch couples JNK activation to TNFα-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Bhoj V, Seth RB. Ubiquitin, TAK1 and IKK: is there a connection? Cell Death Differ. 2006;13:687–692. doi: 10.1038/sj.cdd.4401869. [DOI] [PubMed] [Google Scholar]
- Choo MK, Kawasaki N, Singhirunnusorn P, Koizumi K, Sato S, Akira S, et al. Blockade of transforming growth factor-beta-activated kinase 1 activity enhances TRAIL-induced apoptosis through activation of a caspase cascade. Mol Cancer Ther. 2006;5:2970–6. doi: 10.1158/1535-7163.MCT-06-0379. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Herrero-Martin G, Hoyer-Hansen M, Garcia-Garcia C, Fumarola C, Farkas T, Lopez-Rivas A, et al. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 2009;28:677–85. doi: 10.1038/emboj.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajino T, Omori E, Ishii S, Matsumoto K, Ninomiya-Tsuji J. TAK1 MAPK kinase kinase mediates transforming growth factor-β signaling by targeting SnoN oncoprotein for degradation. J Biol Chem. 2007;282:9475–9481. doi: 10.1074/jbc.M700875200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A. NF-κB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Kim J-Y, Omori E, Matsumoto K, Nunez G, Ninomiya-Tsuji J. TAK1 is a central mediator of NOD2 signaling in epidermal cells. J Biol Chem. 2008a;283:137–144. doi: 10.1074/jbc.M704746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Ricci MS, El-Deiry WS. Mcl-1: a gateway to TRAIL sensitization. Cancer Res. 2008b;68:2062–2064. doi: 10.1158/0008-5472.CAN-07-6278. [DOI] [PubMed] [Google Scholar]
- Kim Y, Suh N, Sporn M, Reed JC. An inducible pathway for degradation of FLIP protein sensitizes tumor cells to TRAIL-induced apoptosis. J Biol Chem. 2002;277:22320–22329. doi: 10.1074/jbc.M202458200. [DOI] [PubMed] [Google Scholar]
- Kurbanov BM, Fecker LF, Geilen CC, Sterry W, Eberle J. Resistance of melanoma cells to TRAIL does not result from upregulation of antiapoptotic proteins by NF-κB but is related to downregulation of initiator caspases and DR4. Oncogene. 2007;26:3364–3377. doi: 10.1038/sj.onc.1210134. [DOI] [PubMed] [Google Scholar]
- LeBlanc HN, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10:66–75. doi: 10.1038/sj.cdd.4401187. [DOI] [PubMed] [Google Scholar]
- Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule smac mimic potentiates TRAIL- and TNFα-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. The IKKβ subunit of IκB kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris C, Godfrey VL, Krahn-Senftleben G, Takahashi T, Roberts JL, Schwarz T, Feng L, Johnson RS, Karin M. Female mice heterozygous for IKKγ/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol Cell. 2000;5:969–979. doi: 10.1016/s1097-2765(00)80262-2. [DOI] [PubMed] [Google Scholar]
- Meng XW, Lee SH, Dai H, Loegering D, Yu C, Flatten K, Schneider P, Dai NT, Kumar SK, Smith BD, Karp JE, Adjei AA, Kaufmann SH. Mcl-1 as a buffer for proapoptotic Bcl-2 family members during TRAIL-induced apoptosis: a mechanistic basis for sorafenib (Bay 43-9006)-induced TRAIL sensitization. J Biol Chem. 2007;282:29831–29846. doi: 10.1074/jbc.M706110200. [DOI] [PubMed] [Google Scholar]
- Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-κB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21:5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- Omori E, Matsumoto K, Sanjo H, Sato S, Akira S, Smart RC, Ninomiya-Tsuji J. TAK1 is a master regulator of epidermal homeostasis involving skin inflammation and apoptosis. J Biol Chem. 2006;281:19610–19617. doi: 10.1074/jbc.M603384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori E, Morioka S, Matsumoto K, Ninomiya-Tsuji J. TAK1 regulates reactive oxygen species and cell death in keratinocytes, which Is essential for skin integrity. J Biol Chem. 2008;283:26161–26168. doi: 10.1074/jbc.M804513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, Harran P, Wang X. Autocrine TNFα signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JC. Drug insight: cancer therapy strategies based on restoration of endogenous cell death mechanisms. Nat Clin Pract Oncol. 2006;3:388–398. doi: 10.1038/ncponc0538. [DOI] [PubMed] [Google Scholar]
- Ricci MS, Kim SH, Ogi K, Plastaras JP, Ling J, Wang W, Jin Z, Liu YY, Dicker DT, Chiao PJ, Flaherty KT, Smith CD, El-Deiry WS. Reduction of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or sorafenib sensitizes resistant human cancer cells to TRAIL-induced death. Cancer Cell. 2007;12:66–80. doi: 10.1016/j.ccr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Rudolph D, Yeh WC, Wakeham A, Rudolph B, Nallainathan D, Potter J, Elia AJ, Mak TW. Severe liver degeneration and lack of NF-κB activation in NEMO/IKKγ-deficient mice. Genes Dev. 2000;14:854–862. [PMC free article] [PubMed] [Google Scholar]
- Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- Shim J-H, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee K-Y, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund D, Mauri D, Peters N, Juo P, Thome M, Reichwein M, Blenis J, Scheurich P, Tschopp J, Wajant H. Fas-associated death domain protein (FADD) and caspase-8 mediate up-regulation of c-Fos by Fas ligand and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) via a FLICE inhibitory protein (FLIP)-regulated pathway. J Biol Chem. 2001;276:32585–32590. doi: 10.1074/jbc.M100444200. [DOI] [PubMed] [Google Scholar]
- Takaesu G, Surabhi RM, Park KJ, Ninomiya-Tsuji J, Matsumoto K, Gaynor RB. TAK1 is Critical for IkB Kinase-mediated Activation of the NF-κB Pathway. J Mol Biol. 2003;326:105–115. doi: 10.1016/s0022-2836(02)01404-3. [DOI] [PubMed] [Google Scholar]
- Takeda K, Stagg J, Yagita H, Okumura K, Smyth MJ. Targeting death-inducing receptors in cancer therapy. Oncogene. 2007;26:3745–3757. doi: 10.1038/sj.onc.1210374. [DOI] [PubMed] [Google Scholar]
- Varfolomeev E, Maecker H, Sharp D, Lawrence D, Renz M, Vucic D, Ashkenazi A. Molecular determinants of kinase pathway activation by Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand. J Biol Chem. 2005;280:40599–40608. doi: 10.1074/jbc.M509560200. [DOI] [PubMed] [Google Scholar]
- Ventura JJ, Cogswell P, Flavell RA, Baldwin AS, Jr., Davis RJ. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev. 2004;18:2905–2915. doi: 10.1101/gad.1223004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, Brink R, Leverkus M, Tergaonkar V, Schneider P, Callus BA, Koentgen F, Vaux DL, Silke J. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- Wang L, Du F, Wang X. TNF-α induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003;22:8628–8633. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- Xiao C, Yang BF, Asadi N, Beguinot F, Hao C. Tumor necrosis factor-related apoptosis-inducing ligand-induced death-inducing signaling complex and its modulation by c-FLIP and PED/PEA-15 in glioma cells. J Biol Chem. 2002;277:25020–25025. doi: 10.1074/jbc.M202946200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.