Abstract
Purpose
Extracellular matrix metalloprotease inducer (EMMPRIN) is a tumor surface protein that promotes growth and is overexpressed in head and neck cancer. These features make it a potential therapeutic target for monoclonal antibody (mAb) – based therapy. Because molecular therapy is considered more effective when delivered with conventional cytotoxic agents, anti-EMMPRIN therapy was assessed alone and in combination with external beam radiation.
Experimental Design
Using a murine flank model, loss of EMMPRIN function was achieved by transfection with a small interfering RNA against EMMPRIN or treatment with a chimeric anti-EMMPRIN blocking mAb. Cytokine expression was assessed for xenografts, tumor cells, fibroblasts, and endothelial cells.
Results
Animals treated with anti-EMMPRIN mAb had delayed tumor growth compared with untreated controls, whereas treatment with combination radiation and anti-EMMPRIN mAb showed the greatest reduction in tumor growth (P = 0.001). Radiation-treated EMMPRIN knockdown xenografts showed a reduction in tumor growth compared with untreated knockdown controls (P = 0.01), whereas radiation-treated EMMPRIN – expressing xenografts did not show a delay in tumor growth. Immunohistochemical evaluation for Ki67 and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotin nick end labeling (TUNEL) resulted in a reduction in proliferation (P = 0.007) and increased apoptosis in anti-EMMPRIN mAb – treated xenografts compared with untreated controls (P = 0.087). In addition, we provide evidence that EMMPRIN suppression results in decreased interleukin 1β (IL-1β), IL-6, and IL-8 cytokine production, in vitro and in vivo.
Conclusions
These data suggest that anti-EMMPRIN antibody inhibits tumor cell proliferation in vivo and may represent a novel targeted treatment option in head and neck squamous cell carcinoma.
Head and neck squamous cell carcinoma affects >40,000 people in the United States annually, with a 5-year survival between 56% (oral cavity) and 65% (larynx). Despite advances in surgical and medical therapies, the survival rate has remained unchanged for the last 30 years (1, 2). Recent recognition that the tumor microenvironment may modulate tumor cell growth, invasion, and metastasis has generated enthusiasm for targeted therapeutic options (3–5).
Extracellular matrix metalloprotease inducer (EMMPRIN), also known as CD147, is a membrane-bound glycoprotein found on the surface of tumor cells (6). EMMPRIN is overexpressed to varying degrees in most tumor types, with head and neck squamous cell carcinoma having some of the highest levels (7). Elevated EMMPRIN expression has been shown to correlate with lymphatic metastasis and tumor progression in tumors of the oral cavity (8) and larynx (9), as well as other non – head and neck squamous cell carcinoma tumor types (10, 11). Although the exact mechanism by which EMMPRIN promotes tumor growth is unclear (12–14), it has been well established that EMM-PRIN modulates the tumor microenvironment by stimulating matrix metalloproteinase (MMP)–1, MMP-2, and MMP-3 production from stromal tissue (15–17). EMMPRIN-stimulated collagen degradation in vitro and tumor growth in vivo have been shown to be largely dependent on the presence of fibroblasts (14). EMMPRIN has also been shown to promote neovascularization through the expression of vascular endothelial growth factor (VEGF) in murine models of breast cancer (12, 18).
Targeted therapy has gained traction for its potential to selectively inhibit neoplastic cells while minimizing collateral damage to neighboring healthy tissue and, when combined with cytotoxic therapies, provides additional survival benefit without overlapping toxicity. EMMPRIN is expressed on the tumor surface, which makes it a novel target for monoclonal antibody (mAb) – based therapy. In this study, we evaluate EMMPRIN as a therapeutic target through siRNA knockdown and the development of an anti-EMMPRIN mAb (CNTO3899). Because recent studies have shown that elevated EMMPRIN expression confers resistance to radiation therapy (19), we also evaluate anti-EMMPRIN therapy in combination with radiation (20, 21).
Translational Relevance
This study evaluates extracellular matrix metalloprotease inducer (EMMPRIN) as a novel target for head and neck squamous cell carcinoma. Targeted therapy has become a new strategy in the treatment of head and neck cancer, primarily through monoclonal based therapy. As a tumor surface protein that is highly expressed in head and neck squamous cell carcinoma and has been shown to be associated with lymphatic metastasis, EMMPRIN may represent an excellent target for future treatment of head and neck cancer. We present preclinical data that show that anti-EMMPRIN therapy inhibits tumor growth and proliferation and provides further growth inhibition when used in combination with radiotherapy. Furthermore, we provide evidence that EMMPRIN function is associated with cytokine production of proinflammatory and proangiogenic factors interleukin 1β (IL-1β), IL-6, IL-8, and vascular endothelial growth factor, which are elevated in head and neck cancer patients.
Materials and Methods
Cell culture
FaDu (ATCC), FaDu/siE, SCC-1 (University of Michigan), and normal dermal fibroblast cells were maintained in DMEM and supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin solution. Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2. Human umbilical vein endothelial cells (University of Alabama at Birmingham) were maintained in M199 media with 0.1 g/L heparin, 0.1 g/L endothelial cell growth factor, and 10% fetal bovine serum with antibiotics, as previously described (13). Normal dermal fibroblast isolation from primary culture and construction of the FaDu/siE cell lines by siRNA knockdown were also as described previously (13, 22).
Reagents
The anti-EMMPRIN mAb CNTO3899, which was obtained from Centocor, Inc., was derived from the following procedure outlined below and then chimerized, via V-region cloning, into a human immunoglobulin G1 isotype using standard procedures.
Generation and selection of CNTO3899
BALB/c mice were immunized with a recombinant form of human EMMPRIN extracellular domain, and hybridomas were derived from subsequent splenic fusions. From a total of 52 clones identified as immunogen binders to plate-bound EMMPRIN extracellular domain, a subset was chosen for cell-based bioactivity characterization that led to the identification of the original CNTO3899 hybridoma. Following cloning, the binding and in vitro assays were repeated with the human immunoglobulin G1–chimerized CNTO3899 mAb. For binding, human EMMPRIN extracellular domain (R&D Systems; ref. 23) was immobilized in an ELISA plate, and CNTO3899 mAb binding was detected using an appropriate secondary antibody. For in vitro activity, two assays were used: First, normal human lung fibroblasts (Clonetics) were stimulated, in the presence and absence of CNTO3899 mAb, with EMMPRIN extracellular domain, and MMP-1 generation was monitored using the extracellular domain ELISA. Secondly, normal human dermal fibroblasts (Clonetics) were cocultured, in the presence and absence of CNTO3899 mAb, with the human melanoma G361 tumor cell line, and MMP-2 was monitored using a zymogram (Biorad; ref. 12). For in vitro testing of EMMPRIN blockade with radiation, mouse anti–human EMMPRIN mAb (Fitzgerald Industries International) was used.
Cell proliferation assay
SCC-1 and FaDu cells (5 × 104) were grown for 24 h with or without normal dermal fibroblasts (2.5 × 104) and treated with anti-EMMPRIN mAb CNTO3899 at increasing concentrations (0, 50, 100, and 200 μg/mL; day 0). To evaluate anti-EMMPRIN tumor specificity, normal dermal fibroblast cells were also cultured alone with CNTO3899. Two hours following antibody treatment, cells in radiation treatment groups were exposed to 4 Gy. On day 3 cells were trypsinized and counted with a hemacytometer.
Collagen degradation assay
Collagen assays were done as previously described (13) using type I collagen (BD Biosciences). FaDu, FaDu/siE, and normal dermal fibroblast cells were plated in 25 μL of DMEM with 0.1% bovine serum albumin in the center of each well and allowed to adhere for 2 h. Additional media with or without anti-EMMPRIN mAb, CNTO3899, was added to each well, and cells were incubated for 4 d. At the end of treatment, cells were removed and wells were stained with Coomassie blue (0.2%). The extent of collagenolysis was determined by densitometry using Image J (NIH) following digital capture (Coolpix 4500, Nikon USA) and compared with the surrounding undegraded matrix.
Preparation of cell membrane extracts and xenograft lysates
FaDu and FaDu/siE cell membrane extracts were prepared as described previously (22) and cultured alone or with normal dermal fibroblasts and human umbilical vascular endothelial cells to assess the influence of EMMPRIN on paracrine cytokine production. Serum-free media was collected from all samples after 18 h for ELISA analysis.
SCC-1 xenografts were harvested, minced, and lysed via sonification (Branson digital sonifier) in radioimmunoprecipitation assay buffer solution to evaluate the effect of anti-EMMPRIN mAb on cytokine production.
ELISA quantification of cytokine expression
OptEIA human inter-leukin 1β (IL-1β), IL-6, and IL-8 ELISA kits (II; BD Biosciences) and a Quantikine ELISA kit for human VEGF (R&D Systems) were used according to manufacturer’s instructions for quantification of cytokine expression. Each sample was examined in duplicate.
Animal models
Severe combined immunodeficient female mice with the age of 4 to 6 wk (Charles River Laboratories and National Cancer Institute–Frederick) were obtained and housed in accordance with our institution’s Institutional Animal Care and Use Committee (IACUC) guidelines. To determine if EMMPRIN silencing provides a reduction in radiation-induced tumor growth, mice were injected with FaDu or FaDu/siE cells (2 × 106) and divided into control and radiation treatment groups (n = 8 per group). Tumors were measured biweekly using calipers to approximate surface area, and treatment was initiated after FaDu and FaDu/siE xenograft size was equal to ~ 45 mm2 (on days 10 and 17, respectively). Radiation treatment was delivered via a 60Co therapy unit (Picker) using a lead shield to provide isolated flank xenograft irradiation (24). Animals received a total of 12 Gy of radiation divided into six 2 Gy increments on days 10, 13, 17, 20, 24, and 27 (FaDu tumors) and on days 17, 20, 24, 27, 31, and 34 (FaDu/siE tumors).
To assess anti-EMMPRIN antibody in combination with radiation, mice were xenografted with SCC-1 (2 × 106) tumor cells and divided into control, anti-EMMPRIN antibody, radiation, or combination anti-EMMPRIN antibody and radiation groups (n = 7 per group). Tumors were measured trice weekly, and once average size was equal to 45 mm2, treatment began. Two groups of mice received a total of 1.2 mg of anti-EMMPRIN mAb divided into 200 μg i.p. doses on days 13, 16, 20, 23, 27, and 30. In addition, two groups received radiation therapy with 12 Gy 60Co given over six 2 Gy fractions on days 14, 17, 21, 24, 28, and 31. Anti-EMMPRIN mAb was given 24 h before radiation for the combined treatment group. A dose of 12 Gy 60Co was chosen because previous experiments have shown inhibition of tumor growth in the same cell line with the use of 8 Gy in combination with chemotherapy (25).
Immunohistochemistry
Tumors were harvested at the end of the study and paraffin embedded for immunohistochemical analysis. Ki67 and TUNEL labeling and analysis were done as previously described (22).
Statistical analysis
FaDu, FaDu/siE, and SCC-1 xenograft data was transformed to reflect tumor size as a percent change from baseline, wherein percent change = (tumor surface area/tumor surface area at the beginning of treatment) * 100%. A polynomial linear regression, including days after baseline and square term of days after baseline, was fitted for the tumor growth of each animal, and time to tumor doubling and tumor tripling was calculated based on the regression model. Because a few animals did not have tumor doubling, the log-rank test was also applied to compare doubling and tripling times between groups.
Bias between caliper, immunohistochemistry, and cytokine expression measurements were expressed as SE. Data analysis for cytokine expression, immunohistochemistry, collagen degradation assays, and in vitro cell growth was done using GraphPad Prism software (Graph-Pad Software, Inc.). P < 0.05 was considered significant in unpaired t test analysis.
Results
Human immunoglobulin G1 chimerized CNTO3899 binds to and inhibits EMMPRIN
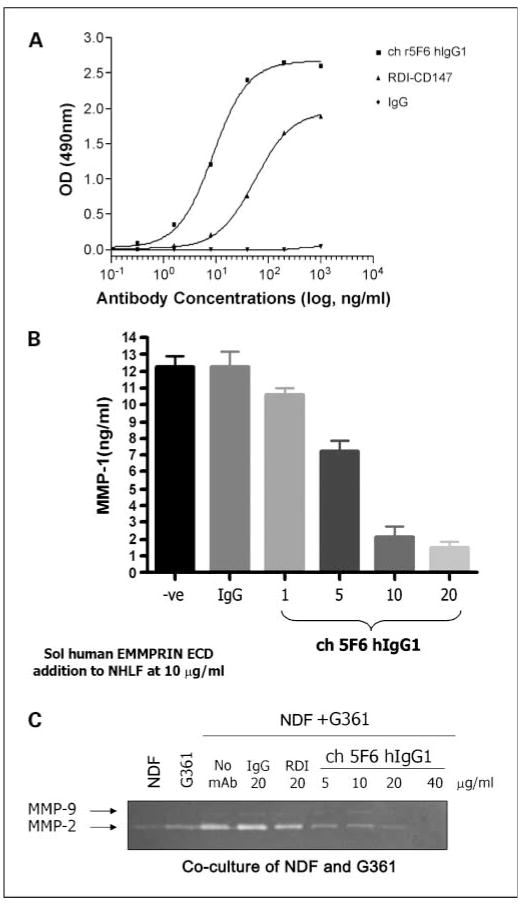
Anti-EMMPRIN mAb, CNTO3899, was generated because of its potential use in targeted therapy, confirmation that the cloned version of CNTO3899 mAb bound to EMMPRIN was evaluated via a simple ELISA plate assay. Chimeric CNTO3899 mAb binds in a dose-dependent manner to immobilized human EMMPRIN and with greater affinity than commercially available anti–human EMMPRIN mAb (RDI-CD147). No binding was observed with the human immunoglobulin G1 isotype control (Fig. 1A). Previous studies have shown EMMPRIN stimulates MMP-1 and MMP-2 production from normal human lung fibroblasts (12). To confirm that bound chimerized CNTO3899 results in neutralization of EMMPRIN activity, normal human lung fibroblast cells were cultured in the presence of anti-EMMPRIN mAb and MMP-1 levels were assessed. CNTO3899 was found to inhibit MMP-1 release in a dose-dependent manner (Fig. 1B). To show that the activity of CNTO3899 extended to neutralization of cell-surface EMMPRIN, the mAb was assessed in a fibroblast/tumor cell coculture assay. Figure 1C shows elevation in MMP-2 release above background, when normal dermal fibroblasts and G361 tumor cells are cocultured. When cells are incubated with CNTO3899 mAb, a dose-dependent decrease in MMP-2 is observed by diminishing zymogram intensity.
Fig. 1.
A, CNTO3899 binds to immobilized human EMMPRIN extracellular domain. The EC50 for mAb binding in the ELISA format is ~ 10 ng/mL. B, CNTO3899 inhibits soluble EMMPRIN – induced MMP-1generation. The expression of MMP-1 by EMMPRIN extracellular domain stimulated normal human lung fibroblast cells is inhibited in a dose-dependent manner by CNTO3899. The IC50 for CNTO3899 in this assay format is between 5 and 10 mg/mL. C, CNTO3899 inhibits MMP-2 expression induced by cocultured normal dermal fibroblast/G361 cells. MMP-2 levels are elevated above background when normal dermal fibroblast and G361cells are cocultured, as indicated in the intensity of the band observed by zymogram. Intensity decreases in response to increasing concentrations of CNTO3899 mAb, showing bioactivity without the addition of exogenously added soluble EMMPRIN. No change in intensity is observed with the isotype control.
Anti-EMMPRIN antibody inhibits collagen degradation and cell growth in vitro
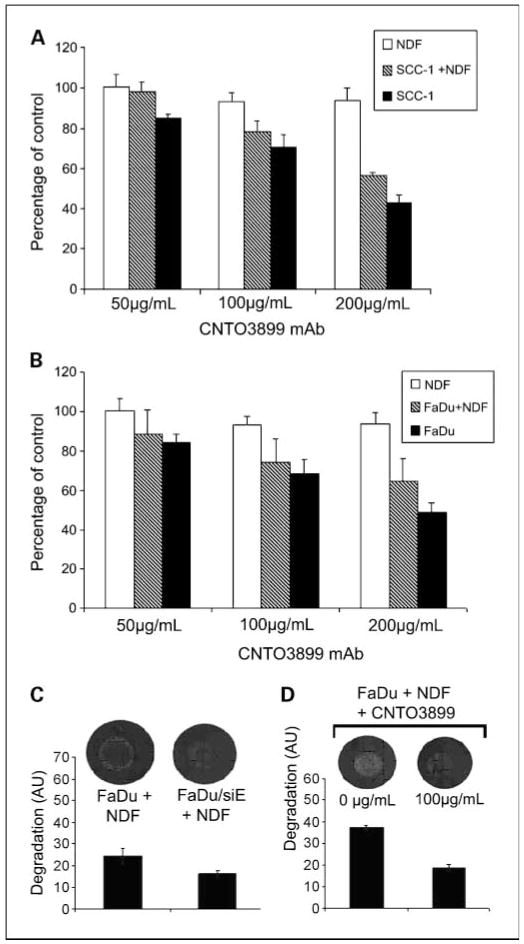
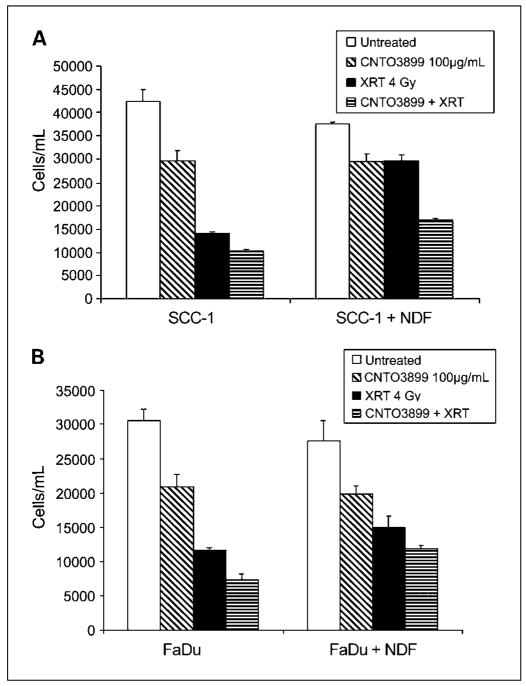
To determine the growth-suppressing effect of blocking EMMPRIN with anti-EMMPRIN mAb in vitro, SCC-1, FaDu, and normal dermal fibroblast cells were incubated with increasing concentrations of CNTO3899. Tumor cells were cocultured with normal dermal fibroblasts to evaluate the role of tumor-stromal interactions in EMMPRIN expression. Seventy-two hours after CNTO3899 treatment, SCC-1 cell proliferation was 15% (50 μg/mL), 30% (100 μg/mL), and 57% (200 μg/mL) less than control (Fig. 2A). Significant reductions in tumor cells were obtained by treatment with 100 μg/mL (P = 0.02) and 200 μg/mL of CNTO3899 (P < 0.001). No reduction in tumor cell proliferation was observed in normal dermal fibroblast cells treated with anti-EMMPRIN antibody. However, when SCC-1 cells were cocultured with normal dermal fibroblasts, a significant reduction in tumor growth with 100 and 200 μg/mL of CNTO3899 mAb was observed (P < 0.001). Similar results were obtained when FaDu and FaDu/normal dermal fibroblast cells were treated with anti-EMMPRIN mAb. FaDu cells showed a 16% (50 μg/mL), 32% (100 μg/mL), and 52% (200 μg/mL) reduction in cell proliferation following treatment with CNTO3899 compared with control (Fig. 2B). A significant reduction in FaDu cells was obtained with 100 (P = 0.01) and 200 μg/mL (P = 0.001) of CNTO3899.
Fig. 2.
A, SCC-1and normal dermal fibroblast cells were incubated with increasing concentrations of CNTO3899 alone and in combination. After 72 h, anti-EMMPRIN mAb – treated SCC-1cells were 15% (50 μg/mL), 30% (100 μg/mL), and 57% (200 μg/mL) less than control, with a significant reduction in tumor cell viability obtained with100 μg/mL (P = 0.02) and 200 μg/mL of CNTO3899 (P < 0.001). No reduction in tumor cell viability was observed in normal dermal fibroblast cells treated with CNTO3899. SCC-1cells cocultured with normal dermal fibroblasts showed a significant reduction in tumor growth with 100 and 200 μg/mL of CNTO3899 (P < 0.001). B, FaDu and normal dermal fibroblast cells were incubated with CNTO3899. A reduction in cell proliferation was seen with 100 μg/mL (P = 0.01) and 200 μg/mL (P = 0.001) of CNTO3899. C, FaDu and FaDu/siE cells were plated on type I collagen with normal dermal fibroblasts. A significant decrease in collagenolysis was observed in EMMPRIN knockdown cells compared with FaDu controls (P = 0.01). D, FaDu and normal dermal fibroblast cells (1 × 105 of each) were plated on type I collagen and treated with 100 μg/mL of CNTO3899. A significant inhibition in collagenolysis was observed (P = 0.005).
Because previous studies have shown that fibroblast-mediated collagen degradation is EMMPRIN dependent (13), we assessed FaDu and FaDu/siE cells atop type I collagen with normal dermal fibroblasts (Fig. 2C). A significant decrease in collagenolysis was observed in EMMPRIN knockdown cells compared with FaDu controls (P = 0.01). Similarly, when FaDu and normal dermal fibroblast cells were treated with anti-EMMPRIN mAb (100 μg/mL), a significant inhibition in collagenolysis was observed (P = 0.005; Fig. 2D).
CNTO3899 inhibits head and neck squamous cell carcinoma xenograft growth in vivo
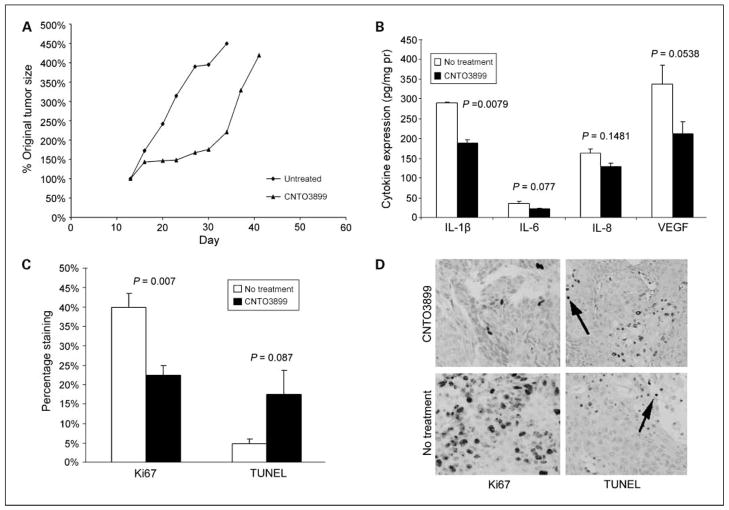
Severe combined immunodeficient mice bearing SCC-1 xenografts were divided into control and anti-EMMPRIN antibody groups (n = 7; Fig. 3A). Animals treated with CNTO3899 mAb showed significant tumor growth delay based on tumor doubling times (34.3 ± 0.9 days) when compared with untreated controls (21.1 ± 1.0 days; P = 0.004). However, CNTO3899 treated tumors rapidly regrew after treatment.
Fig. 3.
CNTO3899 inhibits head and neck squamous cell carcinoma xenograft growth. Monoclonal anti-EMMPRIN antibody (200 μg i.p. twice weekly for 3 wk) was administered to severe combined immunodeficient mice bearing SCC-1tumors (n = 7 per group). A, xenografts treated with CNTO3899 showed a significant reduction in tumor growth (tumor doubling time, 34.3 ± 0.9 d) when compared with untreated controls (21.1 ± 1.0 d; P = 0.004). B, lysates from treated and untreated xenografts were analyzed by ELISA. CNTO3899 treatment decreased expression of IL-1β (P = 0.0079), IL-6 (P = 0.077), IL-8 (P = 0.1481), and VEGF (P = 0.0538) when compared with animals receiving no treatment. C, analysis for Ki67 (cell proliferation) revealed that treated tumors had fewer proliferating cells compared with the untreated group (P = 0.007). TUNEL analysis of xenografts treated with CNTO3899 showed a higher percentage of positively stained cells compared with control tumors, although the difference was not significant (P = 0.087). Bars, SEM. *, raw values multiplied by 100 for scale. Original magnification, × 200.
To evaluate the role of CNTO3899 on cytokines known to induce proliferation in head and neck squamous cell carcinoma, mice that received two doses of CNTO3899 (200 μg i.p.) or no treatment were sacrificed and SCC-1 xenografts were harvested for ELISA (Fig. 3B) and Western blot analysis. Xenografts from mice treated with CNTO3899 showed lower levels of all proliferative cytokines measured compared with control. Differences in cytokine expression levels were significant for IL-1β (P = 0.0079).
The mechanisms by which CNTO3899 acts on tumor cell growth are still relatively unknown. Based on rapid regrowth of treated xenografts, we sought to determine if CNTO3899 suppressed tumor cell proliferation. To this end, xenografts were analyzed by Ki67 and TUNEL assays. The percentage of cells in treated xenografts (23%) positively stained for Ki67 was significantly less than control (40%, Fig. 3C and D; P = 0.007), suggesting that CNTO3899 treatment decreases tumor cell proliferation in vivo. Xenografts treated with CNTO3899 had a higher percentage of TUNEL positive cells (18%) compared with untreated control tumors (5%; Fig. 3C and D; P = 0.087).
Anti-EMMPRIN treatment correlates with suppression of multiple cytokines
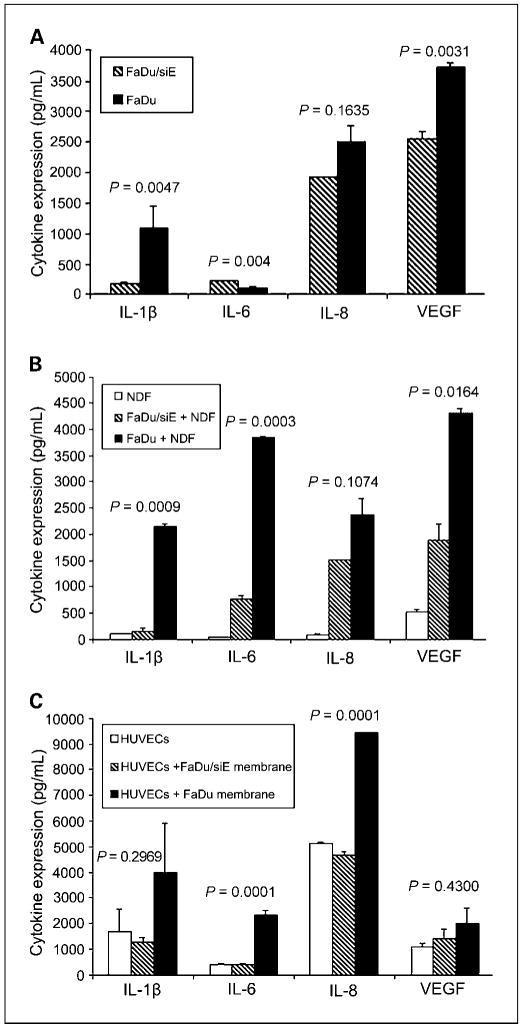
Because EMMPRIN has been shown to mediate its effects through tumor-stromal interactions (11, 14), we investigated the autocrine and paracrine stimulation of cytokines known to be important in head and neck squamous cell carcinoma. To evaluate autocrine cytokine production, FaDu and FaDu/siE tumor cells were cultured in DMEM and serum-free media was collected for ELISA analysis (Fig. 4A). IL-1β (P = 0.0047) and VEGF (P = 0.0031) production was significantly higher in FaDu versus FaDu/siE cells. IL-8 secretion was greater in FaDu cells, but not significant (P = 0.1635). The influence of EMMPRIN on paracrine cytokine production was assessed by stimulating normal dermal fibroblasts and human umbilical vascular endothelial cells with FaDu and FaDu/siE cells membrane extracts (Fig. 4B and C). Normal dermal fibroblasts showed low baseline expression of all cytokines, which increased with exposure to FaDu membranes and only slightly increased in the presence of FaDu/siE membranes (Fig. 4B). The differences between cytokine levels for FaDu and FaDu/siE membrane exposure were significant for IL-1β (P = 0.0009), IL-6 (P = 0.0003), and VEGF (P = 0.0164). Differences in IL-8 failed to reach significance (P = 0.1074). Cytokine production increased with EMMPRIN expression when human umbilical vascular endothelial cells were cultured with FaDu membranes (Fig. 4C). Cytokine production was significantly greater for FaDu versus FaDu/siE membrane exposure for IL-6 (P = 0.0001) and IL-8 (P = 0.0001).
Fig. 4.
EMMPRIN knockdown alters in vitro cytokine expression. To determine the role of EMMPRIN in autocrine and paracrine cytokine production. A, FaDu and FaDu/siE tumor cells were cultured in DMEM with 0.1% bovine serum albumin, serum-free media was collected after 18 h, and analyzed by ELISA for IL-1β, IL-6, IL-8, and VEGF cytokine levels. IL-1β (P = 0.0047) and VEGF (P = 0.0031) production by FaDu cells were significantly higher than from FaDu/siE cells. IL-8 production was greater in FaDu cells versus FaDu/siE, but the difference was not statistically significant (P = 0.1635), whereas IL-6 production was lower in FaDu versus FaDu/siE cells (P = 0.004). Normal dermal fibroblasts (B) and human umbilical vascular endothelial cells (C) were stimulated with cell membrane preparations from FaDu and FaDu/siE cells; serum-free media was collected after 18 h and analyzed for IL-1β, IL-6, IL-8, and VEGF cytokine levels. The differences between normal dermal fibroblast stimulation with FaDu and FaDu/siE were significant for IL-1β (B, P = 0.0009), IL-6 (P = 0.0003), and VEGF (P = 0.0164), but not IL-8 (P = 0.1074). C, the increases in cytokine expression after stimulation of human umbilical vascular endothelial cells with EMMPRIN-positive cell membranes were significant for IL-6 (P = 0.0001) and IL-8 (P = 0.0001), but not for IL-1β (P = 0.2969) or VEGF (P = 0.4300). Bars, SEM. *, raw values multiplied by 100 for scale.
Loss of EMMPRIN function and CNTO3899 augment radiation response
To evaluate the role of EMMPRIN in combination with radiation therapy in vitro, SCC-1 and FaDu cells were plated with and without normal dermal fibroblasts in the presence of anti-EMMPRIN antibody (100 μg/mL) and exposed to 4 Gy of 60Co radiation. A significant reduction in SCC-1 cell proliferation was observed with exposure to radiation (P = 0.0003) and radiation plus anti-EMMPRIN antibody (P = 0.0002) compared with control. The difference between radiation– and radiation plus CNTO3899–reduced cell proliferation was significant (P = 0.006, Fig. 5A). Similar results were seen in FaDu cells, wherein a reduction in proliferation was seen with radiation (P = 0.0003) and combination therapy (P = 0.0002, Fig. 5B). Cell proliferation was significantly reduced for cells treated with radiation and CNTO3899 when compared with radiation alone (P = 0.009). When SCC-1 and FaDu tumor cells were combined with normal dermal fibroblasts, a reduction in cell viability was observed for all treatment groups following treatment with anti-EMMPRIN mAb. A greater reduction in tumor cell proliferation was seen with combination treatment than with radiation alone for SCC-1/normal dermal fibroblast cells (P < 0.0001). FaDu/normal dermal fibroblast cell proliferation was not significantly reduced with combination therapy when compared with single-modality radiation treatment (P = 0.16). Loss of EMMPRIN expression results in tumor growth delay. Consistent with previous studies, we showed a reduction in tumor growth by siRNA down-regulation of EMMPRIN expression (22). Mice were injected with FaDu and FaDu/siE (EMMPRIN silenced) cell lines, and tumors were evaluated over the course of 6 weeks. Tumor doubling times were significantly different between FaDu (32 ± 2.1 days) and siRNA knockdown (FaDu/siE) xenografts (36 ± 1 days; P = 0.008; data not shown).
Fig. 5.
Anti-EMMPRIN mAb provides greater radiation response in vitro. SCC-1 and FaDu cells were plated with and without normal dermal fibroblast cells in the presence of CNTO3899 (100 μg/mL) and exposed to 4 Gy of 60Co radiation. A, after 72 h, SCC-1cells exposed to radiation were 68% less than control (P = 0.0003), whereas those that received anti-EMMPRIN and radiation were 76% less than control (P = 0.0002). A significant difference was observed between radiated groups treated with and without CNTO3899 (P = 0.006). When SCC-1 cells were combined with normal dermal fibroblasts, a greater reduction in tumor cell proliferation was seen with combination treatment than with radiation alone (P < 0.0001). B, FaDu cells exposed to radiation were 62% less than control (P = 0.0003), whereas cells treated with radiation and CNTO3899 were 76% less than control (P = 0.0002). Tumor reduction was greater for cells treated with radiation and CNTO3899 than radiation alone (P = 0.009). FaDu/normal dermal fibroblast tumor cell proliferation was not significantly reduced with combination therapy when compared with radiation alone (P = 0.16).
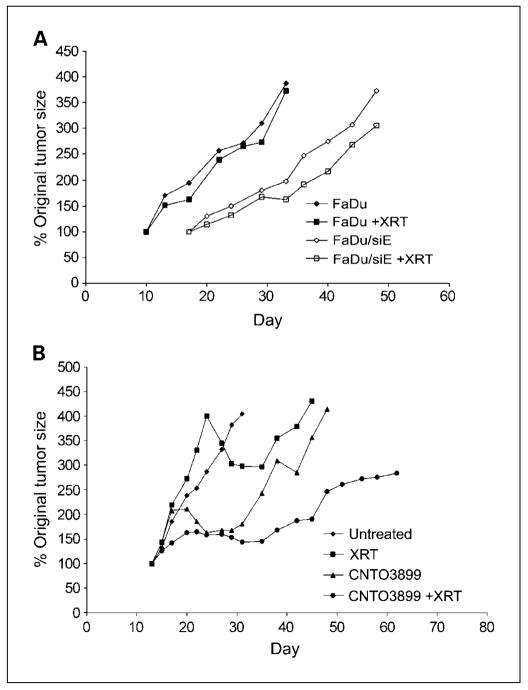
To determine if silencing EMMPRIN provides greater radiation sensitivity, mice xenografted with FaDu or FaDu/siE tumors and assigned to radiation or control groups (n = 8 per group). Treatment mice received 2 Gy fractions of 60Co over the course of 3 weeks and were followed for a total of 4 weeks. No significant difference was seen between tumor doubling time for FaDu control (29 ± 1.9 days) and FaDu radiation groups (29 ± 1.8 days; P = 0.61; Fig. 6A), whereas irradiated FaDu/siE xenografts showed significant growth delay (42 ± 2.1 days) in comparison with nonirradiated FaDu/siE controls (33 ± 1.9 days; P = 0.012).
Fig. 6.
Enhanced radiation response in knockdown or anti-EMMPRIN antibody treated xenografts. A, FaDuand FaDu/siE tumor cells (2 × 106)were injected in to the flanks of severe combined immunodeficient mice, and animals were divided into radiation and control groups (n = 8 per group). Mice in treatment groups received 12 Gy of 60Co radiation divided into six 2 Gy fractions. No significant difference was observed between FaDu control (tumor doubling time, 29 ± 1.9 d) and FaDu radiation groups (29 ± 1.8 d; P = 0.61), whereas irradiated FaDu/siE xenografts showed significant growth delay (42 ± 2.1d) in comparison with nonirradiated FaDu/siE controls (33 ± 1.9 d; P = 0.012). B, severe combined immunodeficient mice bearing SCC-1xenografts were divided into control, CNTO3899, radiation, and combined CNTO3899 and radiation groups (n = 7 per group). Treatment groups received 1.2 mg of antibody and/or 12 Gy of60 Co radiation. Combination therapy with CNTO3899 and radiation significantly inhibited tumor growth (tumor doubling time, 40 ± 8.3 d) compared with control (8 ±0.8 d; P = 0.001). CNTO3899 treated xenografts showed inhibition of tumor growth (tumor tripling time, 27 ±4.6 d) when compared with untreated xenografts (14 ± 1.2; P = 0.019), whereas radiation alone did not produce a significant reduction in tumor growth (22 ± 7.1; P = 0.510).
Mice (n = 7) were xenografted with SCC-1 cells and divided into control, anti-EMMPRIN antibody, radiation, or anti-EMMPRIN antibody and radiation groups. Animals were treated with 12 Gy 60Co and/or 1.2 mg of CNTO3899. Time to tumor doubling and tripling for each group was assessed (Fig. 6B). Combination therapy with CNTO3899 and radiation significantly inhibited tumor growth (tumor doubling time, 40 ± 8.3 days) when compared with control (8 ± 0.8 days; P = 0.001). Because tumors treated with single-modality therapy (radiation or CNTO3899) showed a delay in tumor reduction, time to tumor tripling was also compared between groups. Anti-EMMPRIN antibody treated xenografts showed inhibition of tumor growth (tumor tripling time, 27 ± 4.6 days) when compared with untreated xenografts (14 ± 1.2, P = 0.019), whereas radiation alone did not produce a significant reduction in tumor growth (22 ± 7.1; P = 0.510). Animals receiving radiation and anti-EMMPRIN antibody developed tumor ulceration. In compliance with our institution’s IACUC guidelines, these mice were sacrificed before reaching a size that was thrice greater than that of the original tumor size.
Discussion
The treatment of locoregional advanced head and neck squamous cell carcinoma has evolved over the last decade from surgery to multimodality therapy with radiation and chemotherapy. Toxicity associated with conventional chemotherapy-based regimens and radiation has impeded advances in improved control rates and thus survival (26). Anti–epidermal growth factor receptor targeted mAb therapy has shown survival benefits across a range of treatment settings and is currently the only targeted therapy approved for head and neck squamous cell carcinoma (20). Like epidermal growth factor receptor, EMM-PRIN is expressed in high levels in head and neck cancer, is located on the cell surface, and is known to promote tumor growth and lymphatic metastasis. We believe these attributes make EMMPRIN a potential molecular target for monoclonal-based therapy and the treatment of head and neck squamous cell carcinoma.
Assessment of a functional blocking antibody to EMMPRIN (CNTO3899) shows in vitro and in vivo antitumor activity. Tumor cells treated with anti-EMMPRIN antibody showed decreased cell proliferation that was augmented by radiation therapy. Given the 76% reduction seen with the addition of CNTO3899 to radiotherapy, it is likely that these effects are additive rather than sensitizing. Normal dermal fibroblast cells were relatively unaffected by anti-EMMPRIN mAb treatment, which is consistent with their low exogenous EMMPRIN expression. CNTO3899 was found to reduce cell proliferation in head and neck cancer cell lines examined in vitro similar to reductions observed when the same cell lines were treated with anti–epidermal growth factor receptor mAb (27). The head and neck squamous cell carcinoma cell lines SCC-1 and FaDu were chosen specifically for treatment with anti-EMMPRIN mAb in our studies because of high levels of EMMPRIN expression compared with other head and neck cell lines, as shown previously (28). Consistent with in vitro studies, inhibition of EMMPRIN with CNTO3899 resulted in a significant difference in tumor doubling time in vivo (P = 0.004). Based on Ki67 immunohistochemical results, this is likely due to a reduction in tumor cell proliferation. As a single-modality therapeutic agent, anti-EMMPRIN mAb stabilized tumor growth for the duration of treatment. However, upon discontinuing treatment, tumors rapidly regrew, consistent with the antiproliferative mechanism of action of the antibody.
When combined with radiation, the effect of anti-EMMPRIN mAb was enhanced and inhibition of growth was sustained even after cessation of treatment (P = 0.001). Further studies in which a knockdown model of EMMPRIN expression was combined with radiation therapy confirmed that loss of EMMPRIN function results in greater tumor growth delay in comparison with knockdown controls. A total dose of 12 Gy 60Co radiation given in 2 Gy fractions was chosen based on previous experiments, wherein 8 Gy showed inhibition of tumor growth when combined with chemotherapy (25). Although a decrease in tumor growth was observed for both SCC-1 and FaDu irradiated tumors, the reduction in tumor growth was not significant when compared with controls. When combined with radiation, anti-EMMPRIN mAb produced moderate SCC-1 tumor growth suppression. Previous studies have shown that EMMPRIN expression may promote resistance to radiation therapy (19, 21). Additional studies will be required to determine if anti-EMMPRIN mAb is a radiosensitizing agent.
Although EMMPRIN induced tumor growth and metastasis has been studied in detail, the mechanisms by which it exerts its effects are still not completely understood. Previous studies show that stimulation of matrix metalloproteases in the surrounding stroma provides a favorable microenvironment for tumor growth (13). Using a type I collagen degradation model, we confirm that loss of EMMPRIN expression through siRNA knockdown or treatment with CNTO3899 inhibits collagenolysis. These findings suggest that tumor-stromal interactions play a pivotal role in EMMPRIN-related tumor progression. In addition to MMP modulation, the recent discovery that EMMPRIN stimulates VEGF cytokine production suggests that it may have a more complex mechanism of action (12, 22). Cytokines IL-1β (29, 30), IL-6 (30), IL-8 (31, 32), and VEGF (31, 32) have all been shown to be overexpressed in head and neck squamous cell carcinoma. These cytokines are believed to exert their proinflammatory and proangiogenic effects through stimulation of stromal cells, including fibroblasts and macrophages, which is similar to the proposed mechanism of action of EMMPRIN.
Loss of EMMPRIN function seems to abrogate IL-1β and VEGF secretion in multiple cell types in vitro, whereas production of IL-6 and IL-8 was primarily reduced in stromal elements. Analysis of whole tumor xenografts treated with CNTO3899 showed similar findings, wherein all cytokine levels decreased. Because IL-1β has been shown to modulate IL-6 and VEGF production, we hypothesize that EMMPRIN-mediated IL-1β secretion stimulates multiple downstream protumorigenic factors (33). The diverse local and systemic inflammatory response that is observed in patients with head and neck squamous cell carcinoma is consistent with those reported for cytokines IL-6 and VEGF. Chronic elevation of IL-6 has been shown to result in immune unresponsiveness and induction of wasting and cachexia, symptoms, which are observed in head and neck cancer patients who have poor prognoses (32). IL-8 and VEGF stimulate neovascularization, and IL-8 promotes proliferation and chemotaxis of granulocytes and macrophages. These data suggests that anti-EMMPRIN therapy inhibits tumor growth through cytokine and MMP suppression. Because the expression of IL-1β, IL-6, and IL-8 is known to increase with radiation therapy, treatment with anti-EMMPRIN mAb may suppress the effects of radiation.
In conclusion, anti-EMMPRIN therapy inhibits head and neck squamous cell carcinoma tumor growth alone and in combination with radiotherapy in vitro and in vivo. Inhibition of EMMPRIN, by CNTO3899 mAb and siRNA knockdown, leads to decreases in IL-1β, IL-6, IL-8, and VEGF levels. Anti-EMMPRIN therapy may provide a unique and effective option for the future treatment of head and neck carcinoma.
Acknowledgments
We thank Renee’ Desmond, D.V.M., Ph.D., for her assistance with statistical data analysis.
Grant support: National Cancer Institute (NCIK08CA102154) and the NIH (2T32 CA091078-06; E.L. Rosenthal).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Leon X, Quer M, Orus C, del Prado Venegas M. Can cure be achieved in patients with head and neck carcinomas? The problem of second neoplasm. Expert Rev Anticancer Ther. 2001;1:125–33. doi: 10.1586/14737140.1.1.125. [DOI] [PubMed] [Google Scholar]
- 2.Casiglia J, Woo SB. A comprehensive review of oral cancer. Gen Dent. 2001;49:72–82. [PubMed] [Google Scholar]
- 3.McCawley LJ, Crawford HC, King LE, Jr, Mudgett J, Matrisian LM. A protective role for matrix metallopro-teinase-3 in squamous cell carcinoma. Cancer Res. 2004;64:6965–72. doi: 10.1158/0008-5472.CAN-04-0910. [DOI] [PubMed] [Google Scholar]
- 4.Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res. 2001;264:169–84. doi: 10.1006/excr.2000.5133. [DOI] [PubMed] [Google Scholar]
- 5.Seljelid R, Jozefowski S, Sveinbjornsson B. Tumor stroma. Anticancer Res. 1999;19:4809–22. [PubMed] [Google Scholar]
- 6.Biswas C, Zhang Y, DeCastro R, et al. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–9. [PubMed] [Google Scholar]
- 7.Riethdorf S, Reimers N, Assmann V, et al. High incidence of EMMPRIN expression in human tumors. Int J Cancer. 2006;119:1800–10. doi: 10.1002/ijc.22062. [DOI] [PubMed] [Google Scholar]
- 8.Bordador LC, Li X, Toole B, et al. Expression of emmprin by oral squamous cell carcinoma. Int J Cancer. 2000;85:347–52. [PubMed] [Google Scholar]
- 9.Rosenthal EL, Shreenivas S, Peters GE, Grizzle WE, Desmond R, Gladson CL. Expression of extracellular matrix metalloprotease inducer in laryngeal squamous cell carcinoma. Laryngoscope. 2003;113:1406–10. doi: 10.1097/00005537-200308000-00027. [DOI] [PubMed] [Google Scholar]
- 10.Sameshima T, Nabeshima K, Toole BP, et al. Glioma cell extracellular matrix metalloproteinase inducer (EMMPRIN) (CD147) stimulates production of membrane-type matrix metalloproteinases and activated gelatinase A in co-cultures with brain-derived fibroblasts. Cancer Lett. 2000;157:177–84. doi: 10.1016/s0304-3835(00)00485-7. [DOI] [PubMed] [Google Scholar]
- 11.Kanekura T, Chen X, Kanzaki T. Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int J Cancer. 2002;99:520–8. doi: 10.1002/ijc.10390. [DOI] [PubMed] [Google Scholar]
- 12.Tang Y, Nakada MT, Kesavan P, et al. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 2005;65:3193–9. doi: 10.1158/0008-5472.CAN-04-3605. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal EL, Vidrine DM, Zhang W. Extracellular matrix metalloprotease inducer stimulates fibroblast-mediated tumor growth in vivo. Laryngoscope. 2006;116:1086–92. doi: 10.1097/01.mlg.0000224368.58870.3c. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Matrisian LM, Holmeck K, Vick CC, Rosenthal EL. Fibroblast-derived MT1-MMP promotes tumor progression in vitro and in vivo. BMC Cancer. 2006;6:52. doi: 10.1186/1471-2407-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caudroy S, Polette M, Nawrocki-Raby B, et al. EMMPRIN-mediated MMP regulation in tumor and endothelialcells. Clin Exp Metastasis. 2002;19:697–702. doi: 10.1023/a:1021350718226. [DOI] [PubMed] [Google Scholar]
- 16.Braundmeier AG, Fazleabas AT, Lessey BA, Guo H, Toole BP, Nowak RA. Extracellular matrix metalloproteinase inducer regulates metalloproteinases in human uterine endometrium. J Clin Endocrinol Metab. 2006;91:2358–65. doi: 10.1210/jc.2005-0601. [DOI] [PubMed] [Google Scholar]
- 17.Dalberg K, Eriksson E, Enberg U, Kjellman M, Backdahl M. Gelatinase A, membrane type 1matrix metalloproteinase, and extracellular matrix metalloproteinase inducer mRNA expression: correlation with invasive growth of breast cancer. World J Surg. 2000;24:334–40. doi: 10.1007/s002689910053. [DOI] [PubMed] [Google Scholar]
- 18.Zucker S, Hymowitz M, Rollo EE, et al. Tumorigenic potential of extracellular matrix metalloproteinase inducer. Am J Pathol. 2001;158:1921–8. doi: 10.1016/S0002-9440(10)64660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ju X-Z, Yang J-M, Zhou X-Y, Li Z-T, Wu X-H. EMMPRIN expression as a prognostic factor in radiotherapy of cervical cancer. Clin Cancer Res. 2008;14:494–501. doi: 10.1158/1078-0432.CCR-07-1072. [DOI] [PubMed] [Google Scholar]
- 20.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 21.Feng FY, Lopez CA, Normolle DP, et al. Effect of epidermal growth factor receptor inhibitor class in the treatment of head and neck cancer with concurrent radiochemotherapy in vivo. Clin Cancer Res. 2007;13:2512–8. doi: 10.1158/1078-0432.CCR-06-2582. [DOI] [PubMed] [Google Scholar]
- 22.Newman JR, Bohannon IA, Zhang W, Skipper JB, Grizzle WE, Rosenthal EL. Modulation of tumor cell growth in vivo by extracellular matrix metalloprotease inducer. Arch Otolaryngol Head Neck Surg. 2008;134:1218–24. doi: 10.1001/archotol.134.11.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang Y, Kesavan P, Nakada MT, Yan L. Tumor-stroma interaction: positive feedback regulation of extracellular matrix metalloproteinase inducer (EMMPRIN) expression and matrix metalloproteinase-dependent generation of soluble EMMPRIN. Mol Cancer Res. 2004;2:73–80. [PubMed] [Google Scholar]
- 24.Saleh MN, Raisch KP, Stackhouse MA, et al. Combined modality therapy of A431 human epidermoid cancer using anti-EGFR antibody C225 and radiation. Cancer Biother Radiopharm. 1999;14:451–63. doi: 10.1089/cbr.1999.14.451. [DOI] [PubMed] [Google Scholar]
- 25.Skipper JB, McNally LR, Rosenthal EL, Wang W, Buchsbaum DJ. In vivo efficacy of marimastat and chemoradiation in head and neck cancer xenografts. ORL J Otorhinolaryngol Relat Spec. 2009;71:1–5. doi: 10.1159/000163217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang ES, Murphy BM, Chung CH, et al. Evolution of clinical trials in head and neck cancer. Crit Rev Oncol Hematol. 2008 doi: 10.1016/j.critrevonc.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:1935–40. [PubMed] [Google Scholar]
- 28.Rosenthal EL, Zhang W, Talbert M, Raisch KP, Peters GE. Extracellular matrix metalloprotease inducer-expressing head and neck squamous cell carcinoma cells promote fibroblast-mediated type I collagen degradation in vitro. Mol Cancer Res. 2005;3:195 –202. doi: 10.1158/1541-7786.MCR-04-0203. [DOI] [PubMed] [Google Scholar]
- 29.Knerer B, Hulla W, Martinek H, Formanek M, Temmel A, Kornfehl J. IL-1 and TNF-alpha but no IL-2expression is found in squamous cell carcinomas of the head and neck by RT-PCR. Acta Otolaryngol. 1996;116:132–6. doi: 10.3109/00016489609137726. [DOI] [PubMed] [Google Scholar]
- 30.Thomas GR, Chen Z, Leukinova E, Van Waes C, Wen J. Cytokines IL-1 alpha, IL-6, and GM-CSF constitutively secreted by oral squamous carcinoma induce down-regulation of CD80 costimulatory molecule expression: restoration by interferon gamma. Cancer Immunol Immunother. 2004;53:33–40. doi: 10.1007/s00262-003-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bancroft CC, Chen Z, Dong G, et al. Coexpression of proangiogenic factors IL-8 and VEGF by human head and neck squamous cell carcinoma involves coactivation by MEK-MAPK and IKK-NF-kappaB signal pathways. Clin Cancer Res. 2001;7:435–42. [PubMed] [Google Scholar]
- 32.Chen Z, Malhotra PS, Thomas GR, et al. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res. 1999;5:1369–79. [PubMed] [Google Scholar]
- 33.Saijo Y, Tanaka M, Miki M, et al. Proinflammatory cytokine IL-1 beta promotes tumor growth of Lewis lung carcinoma by induction of angiogenic factors: in vivo analysis of tumor-stromal interaction. J Immunol. 2002;169:469–75. doi: 10.4049/jimmunol.169.1.469. [DOI] [PubMed] [Google Scholar]